- 1Laboratory of Medical Investigation 37, Department of Gastroenterology, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil
- 2Division of Plastic and Reconstructive Surgery, Shizuoka Cancer Center Hospital, Shizuoka, Japan
- 3Department of Surgery, University of Pittsburgh, Pittsburgh, PA, United States
Background: Multivisceral transplantation of pelvic organs would be a potential treatment for severe pelvic floor dysfunction with fecal and urinary incontinence, extensive perineal trauma, or congenital disorders. Here, we describe the microsurgical technique of multivisceral transplantation of pelvic organs, including the pelvic floor, in rats.
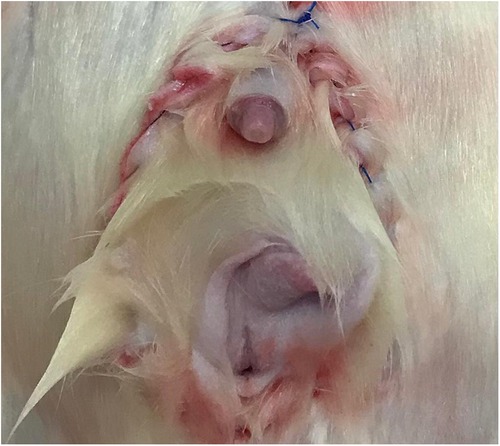
Donor operation: We performed a perineal (including the genitalia, anus, muscles, and ligaments) and abdominal incision. The dissection progressed near the pelvic ring, dividing ligaments, muscles, external iliac vessels, and pudendal nerves, allowing pelvic floor mobilization. The aorta and vena cava were isolated distally, preserving the internal iliac and gonadal vessels. The graft containing the skin, muscles, ligaments, bladder, ureter, rectum, anus and vagina, uterus and ovarian (female), or penile, testis and its ducts (male) was removed en bloc, flushed, and cold-stored.
Recipient operation: The infrarenal aorta and vena cava were isolated and donor/recipient aorta-aorta and cava-cava end-to-side microanastomoses were performed. After pelvic floor and viscera removal, we performed microanastomoses between the donor and the recipient ureter, and the rectum and pudenda nerves. The pelvic floor was repositioned in its original position (orthotopic model) or the abdominal wall (heterotopic model). We sacrificed the animals 2 h after surgery.
Results: We performed seven orthotopic and four heterotopic transplantations. One animal from the orthotopic model and one from the heterotopic model died because of technical failure. Six orthotopic and three heterotopic recipients survived up to 2 h after transplantation.
Conclusion: The microsurgical technique for pelvic floor transplantation in rats is feasible, achieving an early survival rate of 81.82%.
1. Introduction
Multivisceral transplantation of pelvic organs would be a potential treatment for complex pelvic fecal and urinary incontinence. This condition is also called “dual incontinence” (DI) and remains a critical complication of pelvic floor dysfunction. The prevalence rate in the general population ranges between 5.3% and 9.4%, and in women, it increases to 20%–30% (1–5). Patients with complex DI frequently experience feelings of shame, social isolation, silent desperation, and serious impairment in their quality of life (1–5). Furthermore, there is no effective treatment for severe DI (6–9). Another potential indication for multivisceral transplantation of pelvic organs would be complex perineal defects secondary to congenital disorders or those caused by extensive trauma or burn.
Vascularized composite tissue allotransplantation—covering the face, members (arm, leg, hand, etc.), trachea, and anorectal segment, among others—is a recent advancement in the field of transplantation. This innovation aims to improve the quality of life and individual function, rather than mere survival, defining a new trend for the treatment of many system dysfunctions (10–13).
We hypothesize that pelvic floor transplantation covering the skin, pelvic muscular complex, urethra, bladder, ureter, genitalia (vagina or penile), anorectal segment, neurovascular pedicle, and secondary genital organs is a potential treatment for severe DI and complex perineal congenital disorders or injuries following extensive trauma. We have already observed in another study that the surgical technique for pelvic floor transplantation in cadavers is feasible (14). In this report, we describe the surgical technique and anatomic details of pelvic floor transplantation in rats, aiming at translational research.
2. Method
2.1 Animal and anesthesia procedures
Twenty-two Lewis rats weighing 250–300 g were used as donors and recipients in 11 pelvic floor transplantations. All procedures were approved by the research ethics committee of the University of São Paulo School of Medicine and the anesthesia was performed intraperitoneally using ketamine (30 mg/kg) and xylazine (10 ml/kg).
2.2 Surgical procedure
2.2.1 Donor operation
A combined perineal and abdominal incision was performed (Figures 1A,B). The dissection progressed externally between the pelvic floor and the structures of the legs, abdominal wall, and gluteus and internally near the pelvic ring bone, preserving the perineal muscles, anorectal segment, and genitourinary organs of male (Figure 1C) and female (Figure 1D) donors. The division of the pubis makes this dissection easier and facilitates the identification of the vascular and pudendal nerves. The entire pelvic floor was mobilized to inside the abdomen. The genital organs, urinary bladder, and rectum were mobilized by performing the abdominal incision. The abdominal aorta and vena cava were isolated up to the renal vessels and down to the iliac bifurcation, preserving the internal iliac vessels, including the rectal vessels (Figure 2A). The pudendal nerves and vessels were identified and divided far from the pelvis (Figure 2B). The aorta and vena cava were sectioned near the renal vessels to preserve the gonadal vessels. The rectum was sectioned 3 cm before the anus and the ureters were sectioned 2 cm proximally from the bladder. Finally, we removed the graft en bloc, containing the skin, muscular complex, ligaments, bladder, ureter, rectum, anus and vagina, uterus and ovarian in female (Figure 3A) or penile, and testis and its ducts in male (Figure 3B) and placed it in a recipient containing a cold lactate Ringer solution. Immediately after, a catheter was inserted in the aorta to flush the graft with the Ringer solution. Afterward, the back-table procedures were performed.
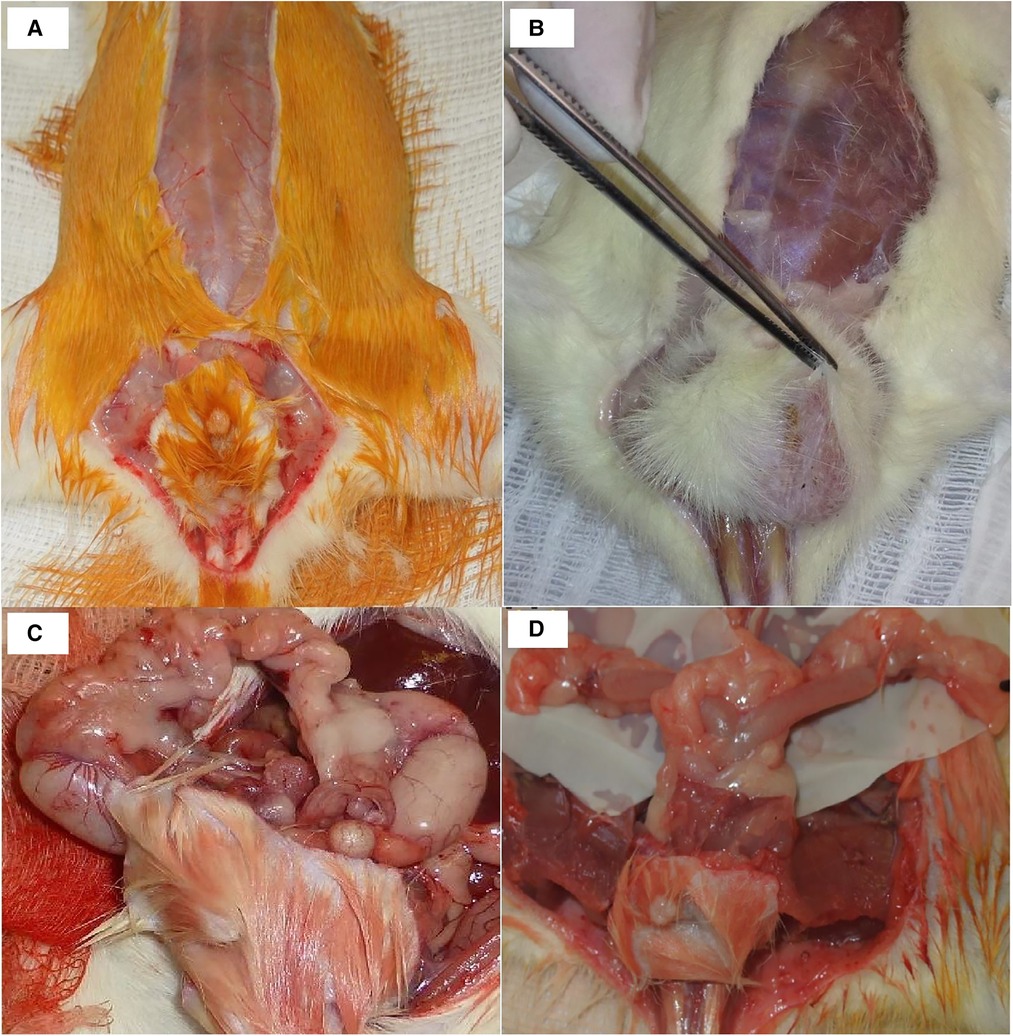
Figure 1. Incision details in male (A) and female (B) donors. Graft dissection in male (C) and female (D) rats.

Figure 2. (A) Vascular dissection details: AO, aorta; VC, vena cava; IIV, internal iliac vessels; EIV, external iliac vessels; CIV, common iliac vessels. (B) Pedicle vessels from the final dissection and the source of the pudendal nerves: AO, aorta; VC, vena cava; NP, pudendal nerves; GVP, graft vascular pedicle; G, graft.

Figure 3. (A) female graft: RO, right ovary; LO, left ovary; RGV, right gonadal vessels; LGV, left gonadal vessels; A/V, aorta and vena Cava; RU, right ureter; LU, left ureter; R, rectum; U, uterus; B, bladder; VA, vagina; AN, anus. (B) Male graft: RT, right testicle; LT, left testicle; RGV, right gonadal vessels; R, rectum; A, aorta; V, vena cava; B, bladder; PE, penis; AN, anus.
2.2.2 Recipient operation
We performed the same donor's anesthesia and abdominal incision and isolated a segment of 2 cm of the infrarenal aorta and vena cava for the anastomoses. We positioned the graft in the abdomen and performed end-to-side aorta-aorta and cava-cava microanastomoses using a 10.0 nylon suture. We removed the vascular clamps from the recipient's aorta and vena cava, allowing graft reperfusion. After that, we performed a similar perineal incision as that of the donor, and the internal iliac vessels were divided near the external vessels. We divided the pudendal nerves far from the sacrum, removed the native pelvic floor tissues, and performed end-to-end continuous anastomosis between the donor and the recipient rectum (7.0 polypropylene) and the pudendal nerves (10.0 nylon). For the ureter anastomosis, we used a polyethylene stent with a minimum ID of 0.5 mm inserted in both (donor and recipient) ureter ends and secured with two 7/0 silk ligatures. The pelvic floor was positioned in its original position (orthotopic) or in the lower part of the abdominal incision (heterotopic) and fixed by stitches between pelvic floor ligaments, muscles, and skin, completing the operation (Figure 4). We sacrificed the animals 2 h after surgery and removed the graft for histological analysis. The tissues were stained with hematoxylin and eosin for ischemia/reperfusion injury graduation.
3. Results
We performed a total of 11 consecutive pelvic floor transplantations in rats. Seven recipients were transplanted using the orthotopic model, whereas four were done with the heterotopic model. The reproductive organs were maintained in the graft, so the vascular pedicle included the gonadal vessels for their vascularization. Consequently, a long venous and arterial pedicle including the renal vessels was required. The donor/recipient size match is very important (4). Donor/recipient weight should be the same or the donor's weight should be slightly smaller to prevent compartmental syndrome. Furthermore, in this technique, we access the axis of the vascular (internal iliac vessels) and neuronal (pudendal nerves) pedicles, which allows larger vessels for anastomosis and provides higher chances of regeneration and functional reconstitution. The mean time for the donor's operation was 62.54 ± 11.45 min. For the recipient's operation, it was 108.14 ± 16.32 min for the orthotopic model and 68 ± 10.89 min for the heterotopic one. All grafts achieved normal color and good arterial pulse after reperfusion, and nine animals survived up to 2 h after the surgical procedure (experiment endpoint), with six in the orthotopic group and three in the heterotopic group. Two animals died before this period, one because of bleeding from perineal dissection (orthotopic fashion) and the other because of bleeding from arterial microanastomosis (heterotopic fashion). The histology assessment of the present investigation (normal = 0, mild = I, moderate = II, and severe = III) was based on the anorectal transplantation histological classification that searched for ischemic/reperfusion injury (mainly inflammatory cell infiltration and edema) in all organs of the composite tissue transplanted (15, 16). In the current research, ischemia/reperfusion injury was graded 0 in five grafts and 1 in four grafts. The small amount of lesion in the present investigation was probably due to the short period of cold ischemia. All animals that survived during the heterotopic model procedure recovered from anesthesia until the experiment endpoint, probably because of the shorter surgical time. In the orthotopic model, the three animals with the shortest surgical time also fully recovered from anesthesia. The heterotopic model of pelvic floor transplantation was conceptualized to develop a two-step technique in which the graft is implanted heterotopically first and then, after 2 days, another surgery is performed to remove the recipient's pelvic organs and to place the graft in orthotopic position. We believe that this two-step technique may improve the survival rate of patients undergoing this complex procedure.
4. Discussion
Our group and others have investigated anorectal transplantation as a potential solution for severe fecal incontinence. The initial results of this procedure in rats, swine, and canines are promising, showing a convenient functional recovery of this composite graft transplantation. Thus, these experiments suggest that anorectal transplantation would be a promising solution for severe fecal incontinence and permanent colostomy (15–24).
The necessity of anatomic studies for our research in anorectal transplantation inspired us to develop pelvic floor transplantation. During pelvic floor dissection in cadavers and rats, we observed that the pelvic floor is a complex system working like a singular organ. They share the same vascular and neuronal pedicles that control the musculature, which promotes both urinary and fecal continence as well as sexual reproductive functions. Thus, we formulated the hypothesis that the entire structure could be transplanted as a composite graft.
Araki et al. suggest that the anastomosis of pudendal vessels by super-microsurgery would be important for the recovery of pudendal function after anal transplantation, affecting all pelvic organs (22, 23). Nevertheless, in a recent series of anorectal transplantations in rats, we observed adequate anorectal recovery without pudendal vessel anastomosis (16). Furthermore, we could observe satisfactory anal function in a heterotopic model of anal autotransplantation (19), suggesting a profound influence of intrinsic bowel innervation on the rat's anorectal function. These thought-provoking results demand further research studies for easy elucidation.
Many patients would benefit from this procedure, including those with complex perineal trauma and congenital pelvic deformation. We are currently designing new models to expand the indications of this composite graft, mainly for trauma. Another possible indication would be for sexual gender reassignment for patients with gender identity disorder (dysphoria). Gender reassignment surgeries have been indicated for these patients; however, these procedures create artificial organs and may cause intricate complications (25, 26). Pelvic floor transplantation research may explore new possibilities depending on the demand for the inclusion of cross-gender pelvic floor transplantation with the reproductive organs for dysphoria or inclusion in the graft of parts of the pelvic bone framework, gluteus, abdominal wall, and limbs, with their respective vascular and nervous pedicles, for complex trauma or congenital disorders.
Currently, we are designing in our laboratory new experiments to observe the long-term survival rates of rats in pelvic floor transplantation as well as the function of the graft. This is a highly complex procedure that requires high microsurgical skills and intensive peri- and postoperative care, including hemodynamic and biochemical monitoring, respiratory support, administration of antibiotics, fluid infusion, and potential blood transfusion.
The possibility of the genitalia and adnexa transfer in the present procedure may increase the bioethical and metaphysical concerns of patients; nevertheless, these anxieties would be similar to those that exist in gender reassignment surgeries, which are already well accepted as a current therapeutic option for gender identity disorder (25, 26). Furthermore, additional refined, basic, and preclinic translational research of this procedure and an intense and reflective community debate about this medical advancement would be necessary to prepare society for this innovation, mainly pertaining to the donation of these composite tissues by the deceased. Another concern would be the high amount of immunogenic tissues present in this graft like skin, which would importantly require permanent immunosuppression after transplantation. The current procedure the current procedure would request similar immunosuppression used for the face, arms, and lower-extremity transplantation (10, 12, 13), which also enclose high immunogenic graft and need treatment for rejection by specific immunosuppressive induction therapy and maintenance immunosuppression, mainly with tacrolimus.
Pelvic floor transplantation may be a relevant option for severe pelvic floor dysfunction caused by extensive trauma or complex congenital disorders. This procedure is feasible in rats and opens the door for meaningful ethical, biotechnological, anatomical, and surgical debate.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Comissão de Ética em Uso Animal da Faculdade de Medicina da Universidade de São Paulo (CEUA-FMUSP). It was aproved by the Ethical Committe for experimental animals from our institution, number: 1291/2019.
Author contributions
FG made the hypothesis. FG and JA conducted the experiment and looked after the management aspects. AF, CL, DW, EC, LN, MT, EM, and WA were responsible for providing technical support, participation in the experiments, article writing, and review and submission. LD’A oversaw the whole project in his capacity as the head of the department. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Genivaldo da Silva, Valcineia de Souza Andre Gaspar, Sandra Nassa Sampietre, and Genilton Serejo Mesquita for their help and support for this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roberts RO, Jacobsen SJ, Reilly WT, Pemberton JH, Lieber MM, Talley NJ. Prevalence of combined fecal and urinary incontinence: a community based study. J Am Geriatr Soc. (1999) 47:837–41. doi: 10.1111/j.1532-5415.1998.tb02468.x
2. Eva UF, Gun W, Preben K. Prevalence of urinary and fecal incontinence and symptoms of genital prolapse in women. Acta Obstet Gynecol Scand. (2003) 82:280–6. doi: 10.1034/j.1600-0412.2003.00103.x
3. Fialkow MF, Melville JL, Lentz GM, Miller EA, Fenner DE. The functional and psychosocial impact of fecal incontinence in women with urinary incontinence. Am J Obstet Gynecol. (2003) 189:127–9. doi: 10.1067/mob.2003.548
4. Meschia M, Buonaguidi A, Pifarotti P, Somigliana E, Spennacchio M, Amicarelli F. Prevalence of anal incontinence in women with symptoms of urinary incontinence and genital prolapse. Obstet Gynecol. (2002) 100:719–23. doi: 10.1016/s0029-7844(02)02215-9
5. Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. Women. Obstet Gynecol. (2014) 123:141–8. doi: 10.1097/AOG.0000000000000057
6. Gutierrez AB, Madoff RD, Lowry AC, Parker SC, Buie WD, Baxter NN. Long-term results of anterior sphincteroplasty. Dis Colon Rectum. (2004) 47:727–32. doi: 10.1007/s10350-003-0114-6
7. Matzel KE, Madoff RD, La Fontaine LJ, Baeten CG, Buie WD, Christiansen J, et al. Dynamic graciloplasty therapy study group. Complications of dynamic graciloplasty: incidence, management, and impact on outcome. Dis Colon Rectum. (2001) 44:1427–35. doi: 10.1007/BF02234593
8. Spencer M, Wong W, Congilosi S, Nogueras J. Artificial anal sphincter: preliminary results of a multicenter prospective trial. Dis Colon Rectum. (1998) 41:A15.
9. Maeda Y, Lundby L, Buntzen S, Laurberg S. Suboptimal outcome following sacral nerve stimulation for faecal incontinence. Br J Surg. (2011) 98:140–7. doi: 10.1002/bjs.7302
10. Washington KM, Solari MG, Sacks JM, Horibe EK, Unadkat JV, Carvell GE, et al. A model for functional recovery and cortical reintegration after hemifacial composite tissue allotransplantation. Plast Reconstr Surg. (2009) 123(2):26S–33S. doi: 10.1097/PRS.0b013e318191bca2
11. Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. (2010) 14:138. doi: 10.1056/NEJMoa0810653
12. Cavadas PC, Ibanez J, Thione A, Alfaro L. Bilateral trans-humer arm transplantation: result at 2 years. Am J T Ransplant. (2011) 11(5):1085. doi: 10.1111/j.1600-6143.2011.03503.x
13. Fattah A, Cypel T, Donner EJ, Wang F, Alman BA, Zuker RM. The first successful lower extremity transplantation: 6-year follow-up and implications for cortical plasticity. Am J Transplant. (2011) 11:2762. doi: 10.1111/j.1600-6143.2011.03782.x
14. Galvao FH, Baptista R, Seid VE, Waisberg DR, Rodrigues P, Chaib E, et al. Surgical technique for pelvic floor transplantation. Am J Transplant. (2014) 3(14):409. doi: 10.1097/00007890-201407151-01352
15. Galvão FH, Seid VE, Nunes dos Santos RM, Kitamura M, de Castro Galvão R, Ambar Pinto R, et al. Anorectal transplantation. Tech Coloproctol. (2009) 13(1):55–9. doi: 10.1007/pl00012129
16. Galvão FH, Waisberg DR, Seid VE, Costa AC, Chaib E, Baptista RR, et al. Allogeneic anorectal transplantation in rats: technical considerations and preliminary results. Sci Rep. (2016) 4(6):30894. doi: 10.1038/srep30894
17. Galvao FH, Seid VE, Waisberg DR, Cruz RJ, Hirano H, Catanozi S, et al. An innovative model of autologous anorectal transplantation with pudendal nerve reconstruction. Clinics (Sao Paulo). (2012) 67(8):971–2. doi: 10.6061)/clinics/2012(08)20
18. Galvão FH, Waisberg DR, Vianna RM, Galvão RC, Seid VE, Andraus W, et al. Intestinal transplantation including anorectal segment in the rat. Microsurgery. (2012) 32(1):77–9. doi: 10.1002/micr.20958
19. Seid VE, Galvão FH, Vaidya A, Waisberg DR, Cruz RJ, Chaib E, et al. Functional outcome of autologous anorectal transplantation in an experimental model. Br J Surg. (2015) 102:558–62. doi: 10.1002/bjs.9762
20. Galvao FH, Araki J, Seid VE, Waisberg DR, Traldi MC, Naito M, et al. Evidence that anorectal transplantation is the logical treatment for serious anorectal dysfunction and permanent colostomy. Transplant Proc. (2016) 48:497–8. doi: 10.1016/j.transproceed.2015.10.082
21. Araki J, Nishizawa Y, Sato T, Naito M, Akita K, Tashiro K, et al. Anorectal transplantation in human cadavers: mock anorectal allotransplantation. PLoS One. (2013) 8(7):e68977. doi: 10.1371/journal.pone.0068977
22. Araki J, Nishizawa Y, Nakamura T, Sato T, Naito M, Fujii S, et al. The development of a canine anorectal autotransplantation model based on blood supply: a preliminary case report. PLoS One. (2012) 7(9):e44310. doi: 10.1371/journal.pone.0044310
23. Araki J, Mihara M, Narushima M, Iida T, Sato T, Koshima I. Vascularized anal autotransplantation model in rats: preliminary report. Transplant Proc. (2011) 43(9):3552–6. doi: 10.1016/j.transproceed.2011.08.042
24. O'Bichere A, Shurey S, Sibbons P, Green C, Phillips RK. Experimental model of anorectal transplantation. Br J Surg. (2000) 87(11):1534–9. doi: 10.1046/j.1365-2168.2000.01557.x
25. Simonsen RK, Hald GM, Kristensen E, Giraldi A. Long-Term follow-up of individuals undergoing sex-reassignment surgery: somatic morbidity and cause of death. Sex Med. (2016) 4(1):e60–8. doi: 10.1016/j.esxm.2016.01.001
Keywords: pelvic floor disorders, fecal incontinence, urinary incontinence, intestinal transplantation, tissue transplantation, anal canal, gender reassignment, dysphoria
Citation: Galvao FHF, Araki J, Fonseca ABS, Cruz RJ, Lanchotte C, Waisberg DR, Chaib E, Nacif LS, Traldi Maria Clara de Camargo, Mello Estrella Bianco de, Andraus W and Carneiro-D'Albuquerque L (2023) Multivisceral transplantation of pelvic organs in rats. Front. Surg. 10:1086651. doi: 10.3389/fsurg.2023.1086651
Received: 1 November 2022; Accepted: 15 March 2023;
Published: 20 April 2023.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Nicolò Fabbri, Azienda Unità Sanitaria Locale di Ferrara, ItalySitaramaraju Adduri, University of Texas at Tyler, United States
Gennaro Selvaggi, University of Miami, United States
© 2023 Galvao, Araki, Fonseca, Cruz, Lanchotte, Waisberg, Chaib, Nacif, Traldi, Mello, Andraus and Carneiro-D'Albuquerque. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flavio Henrique Ferreira Galvao Av. Dr. Arnaldo 433, sala 3210. Sao Paulo-SP, Brazil. 01246-903ZmdhbHZhb0B1c3AuYnI=
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Flavio Henrique Ferreira Galvao
Flavio Henrique Ferreira Galvao Jun Araki
Jun Araki Ana Bruna Salles Fonseca1
Ana Bruna Salles Fonseca1 Ruy Jorge Cruz Jr
Ruy Jorge Cruz Jr