- Department of Visceral, Transplant and Thoracic Surgery, Center of Operative Medicine, Medical University of Innsbruck, Innsbruck, Austria
Background: Neurocrine neoplasms (NEN) of the small bowel (SBNEN) are a rare entity and mostly asymptomatic. The aim of this study was to explore trends in the clinical presentation, diagnostic workup, surgical approach and oncological outcome in patients with SBNEN at our surgical department.
Materials and methods: All patients who underwent surgical resection for SBNEN from 2004 to 2020 at our department were enrolled in this single center retrospective study.
Results: A total of 32 patients were included in this study. In most cases, the diagnosis was based on incidental findings during endoscopy or radiographic imaging (n = 23; 72%). Twenty cases had a G1 tumor and 12 cases a G2 tumor. The 1-, 3- and 5-year overall survival (OS) were 96%, 86% and 81%, respectively. Patients with a tumor more than 30 mm had a significantly lower OS (p = 0.01). For G1 tumors, the estimated disease-free survival (DFS) was 109 months. Again, the DFS was significantly lower when the tumor had more than 30 mm in diameter (p = 0.013).
Conclusion: Due to the mostly asymptomatic presentation, the diagnostic workup can be difficult. An aggressive approach and a strict follow-up seem to be important for the oncological outcome.
Introduction
Neuroendocrine neoplasms (NEN) can arise from neuroendocrine cells throughout the whole body (1), with the majority originating from neuroendocrine cells in the small bowel and pancreas (2).
The WHO classification and grading system for gastroenteropancreatic NEN was updated in 2017 and uses the Ki-67 Index to grade the tumors (3). Well-differentiated NEN are described as NET Grade 1 (G1) with a Ki-67 Index ≤3% and NET G2 with a Ki-67 Index 3%–20% and well-differentiated NET G3 with Ki-67 Index >20%. Poorly differentiated NEN are described as NEC G3 with a Ki-67 Index of more than 20% (3–5). Ki-67 and mitotic index are markers for cell-proliferation (6).
Small-bowel NEN (SBNEN) account for approximately 17% of all neuroendocrine tumors (7). The incidence of SBNEN has recently increased due to improved diagnosis of early stage disease and is now estimated to be 1.05 per 100,000 persons (8).
Clinically, SBNEN can present with the classical trial of flushing, diarrhea and wheezing due to hormone-active tumors especially in the presence of liver metastases (9, 10). These secretions are not metabolized by the liver, remain active and enter the systemic circulation (4). However, most SBNEN do not show any hormone-related symptoms and they are rather slow growing tumors, whose delayed clinical manifestation is mostly tumor-mass-related, e.g., intestinal obstruction or bleeding due to local invasion (10).
In SBNEN the diagnostic strategy strongly depends on the clinical presentation. In the case of a classical carcinoid syndrome, biochemical analysis can aid in confirming the diagnosis. One of the markers indicative of NEN is Chromogranin A (CgA), a protein secreted by cells with autocrine, paracrine or endocrine activity (4, 9). Although it has limited sensitivity and specificity (71% and 50%, respectively), CgA remains the only relevant diagnostic marker (11). Additionally, CgA has a role as tumor surveillance marker after resection (9).
For further work-up, imaging modalities can be used to evaluate anatomical location (CT-scan, ultrasound). Functional analysis using a radio-labeled somatostatin-analog in combination with radiological imaging, such as 68Gallium-DOTA-TOC PET CT scans, helps to determine disease extent throughout the whole body (5). Endoscopic strategies can be attempted if the primary tumor cannot be detected. As around 50% of SBNEN occur in the ileum, capsule endoscopy may facilitate the diagnostic work-up (7).
The therapeutic gold standard for SBNEN is a surgical treatment consisting of segmental small-bowel resection or right-sided hemicolectomy (for distal ileal tumors) with resection of regional mesenteric lymph nodes (7, 12). R0-resection should be attempted. To achieve this, no macroscopic nor microscopic tumor remains in the situs. Surgical treatment furthermore includes careful inspection of the peritoneal cavity to exclude potential peritoneal and liver metastasis (1). In the case of oligometastatic liver disease and limited to G1 and G2 differentiation, patients profit from simultaneous or two-stage surgical resection of those and/or local ablative strategies to achieve R0 situation (12–14).
In this study we attempt to evaluate the clinical presentation, the diagnostic workup and the surgical approach of SBNEN at our surgical department over a period of 16 years.
Materials and methods
Study design and data collection
All patients who underwent surgical resection for small-bowel tumors at the Department of Visceral, Transplant and Thoracic Surgery Innsbruck from February 2004 until January 2020 were included into a retrospective study. The study protocol was approved by the local ethics committee (Vote number: 1435/2021). Exclusion criteria were defined as a benign disease and/or non-SBNET. After enforcing exclusion criteria, 32 patients were left for statistical analysis.
If the diagnosis was not due to an intestinal obstruction or bleeding leading to an emergency operation, all patients received a PET-CT scan. CgA was analyzed on laboratory testing prior to operation. All patients were discussed in the local interdisciplinary tumor board before and after surgical treatment (if not an emergency operation). If adjuvant therapy was not recommended, all patients received a strict follow-up regimen, which included an appointment every 3 months for the first 2 years. This was extended to a 6-monthly follow-up for further 3 years. Every follow-up included laboratory testing of CgA. Once a year, patients received a PET-CT scan.
At our institution a chemotherapeutic regime is only recommended in case of progression under other classical specific therapies, like somatostatine, PRRT and everolimus.
Demographic and clinical variables
Data collected from medical records included patient demographic data such as age, sex, Charlson comorbidity index, clinical data such as clinical presentation, imaging and biochemical evaluation results, history of pre- and/or postoperative chemotherapy, duration of hospital stay and surgical approach. Postoperative complications were graded according to the Clavien-Dindo-classification (15) and recorded as minor (grade I–II) or major complications (III–IV).
Tumor variables, such as tumor localization, lymph node and metastasis (TNM) classification, tumor grading, the diameter of the largest lesion, Ki-67 index and recurrence were analyzed. Biologic markers (serum CgA) were recorded.
Overall survival (OS) was calculated as the time from tumor resection to the date of death or last follow-up visit (FU). Disease-free survival (DFS) was defined as the time from initial clearance of all tumor deposits (primary, metastases) to the first recurrence at any site.
Statistical analysis
A descriptive analysis was performed for all study variables. Absolute and relative frequencies were reported for categorial data, median and range were reported for continuous variables. Differences in categorical variables were investigated by Chi-square- and t-test. Kaplan Meier Method was used for survival analysis. Log-rank test was conducted to measure differences in survival variables. Two-tailed p-values less than 0.05 were considered statistically significant. IBM SPSS Statistics 26 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis.
Results
Study population
The study population consists of 32 patients, 15 patients were female. The study population's median age at date of surgery was 64 years (range 26–87). The median age of female patients was 63 years, whereas the median age of male patients was 65 years. The clinical data are shown in Table 1.
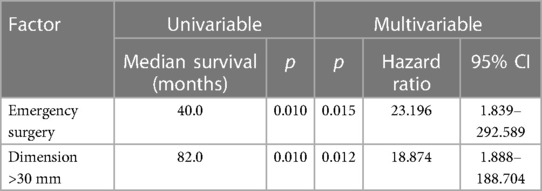
Table 2. Survival analysis for factors affecting patients' OS using a COX proportional hazard model.
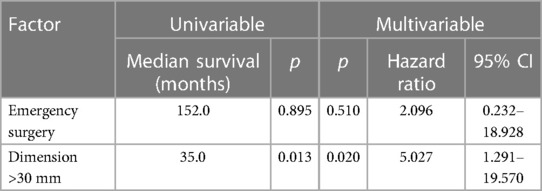
Table 3. Survival analysis for factors affecting patients' DSF using a Cox proportional hazard model.
Localization of the primary included ileum (n = 21; 66%), duodenum (n = 9; 28%) and jejunum (n = 2; 6%). The diagnosis was based on incidental findings in 23 cases (72%) during an endoscopic procedure or radiographic imaging. In the remaining patients, diagnostic evaluation was initiated upon clinical symptoms such as intestinal obstruction (n = 5; 16%), diarrhea (n = 2; 6%), B-symptoms (n = 1; 3%) and flushing syndrome (n = 1; 3%). In four cases (13%), an emergency operation had to be performed.
As per histopathological grading tumors were classified as G1 in 20 cases (63%) and G2 in 12 cases. There was no G3 tumor. Male patients displayed a higher percentage (88%) of G1 tumors when compared to female patients, whereas female patients displayed a higher rate (67%) of G2 tumors.
Most patients had a T2 or T3 tumor (n = 9; 28%). In 23 patients, primary tumor lesions were smaller than 3 cm (72%) and distant metastases were diagnosed in 10 cases (31%). Considering tumor dimension larger than 3 cm, we could observe a significant correlation with local lymphatic-vessel invasion (p = 0.029) and perineural invasion (p = 0.08), but not with local vascular invasion (p = 0.409).
According to the guidelines, CgA was analyzed on laboratory testing before the surgical procedure (n = 21) and was elevated in 38% (n = 12). Our department's normal CgA levels range from 0.0 to 108 µg/L. This was only not performed in exceptional cases. For example, in emergency surgeries due to intestinal obstruction or CgA was not available for statistical analysis (missing n = 11).
The surgical approach was open in 30 cases. Laparoscopic resection was performed on only two patients. The majority of the patients received an oncological right-sided hemicolectomy (n = 13; 40%) or a segmental small bowel resection (n = 12; 38%) followed by tumor extirpation (n = 5; 16%), a pylorus-preserving pancreaticoduodenectomy (PPPD) and distal gastrectomy (n = 1; 3%) due to a tumor in the proximal duodenum. R0-resection was achieved in all but five patients (n = 27, 84%).
One patient received a platin-based neoadjuvant chemotherapy actually as a treatment for a concurrent histologically verified bronchial adenocarcinoma. Six patients received postoperative therapy: this included in three cases somatostatines and in three cases PRRT.
Post-operative complications occurred in 10 patients. Six cases were graded as mild and four as severe complications according to the Clavien–Dindo Classification. Severe complications included anastomotic leakage, postoperative hemorrhage, pleural effusion and postoperative cholecystitis with a consecutive operation (all n = 1).
Survival analysis and oncological outcome
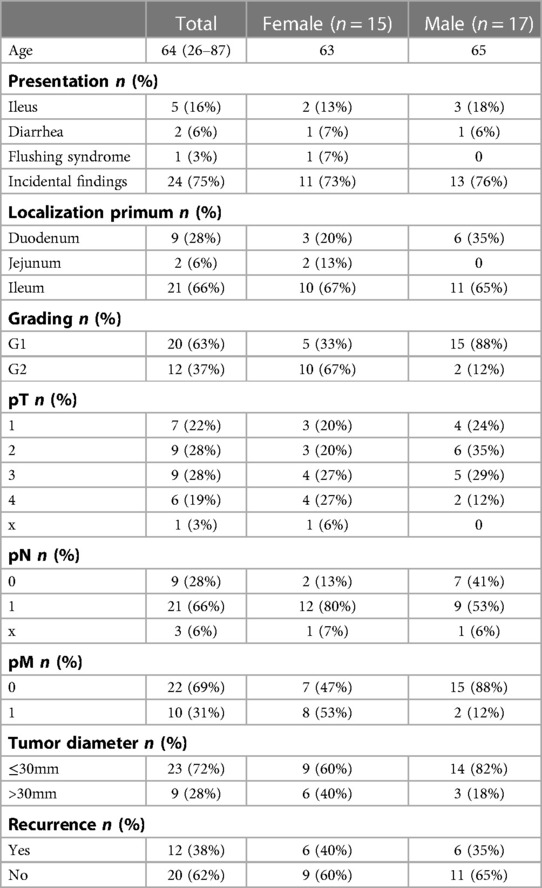
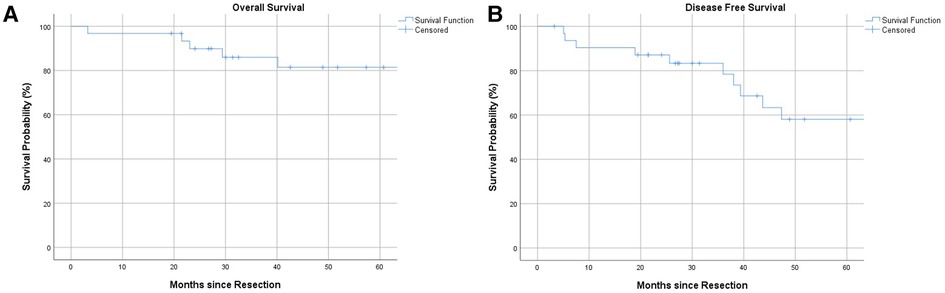
Overall 1-, 3- and 5-year survival were 96%, 86% and 81%, respectively (Figure 1A). Estimated survival for female patients was 136 vs. 142 months for male patients and did not show statistical significance (Figure 2A). We found no differences with respect to tumor grading (p = 0.47), nodal status (p = 0.72) and synchronous metastases (p = 0.87) regarding survival. Compared to the tumor size, the log-rank test revealed a significantly lower patient survival for patients with a tumor >30 mm (156 vs. 82 months; p = 0.01). Equally, patients undergoing an emergency surgery showed shorter OS (40 vs. 162 months; p = 0.01) as seen in Table 2.
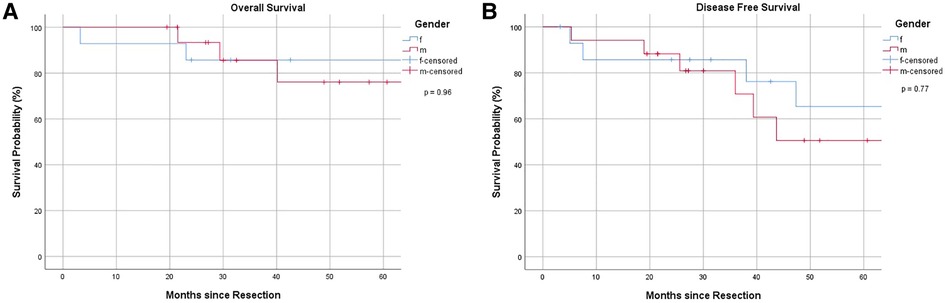
Figure 2. (A) Overall survival, stratified by gender. (B) Disease-free survival, stratified by gender.
The 1-, 3- and 5-year DFS were 90%, 78% and 58%, respectively with no statistical difference in female and male patients (p = 0.77) as Figures 1B, 2B show. The recurrence rate was 35.5% within the follow-up period. The estimated DFS for patients with G1 tumors was 109 months. For G2 tumors it was 94 months (p = 0.55). Again, when compared to the tumor size, patients with a tumor >30 mm had a significantly worse DFS (123 vs. 35 months; p = 0.013). We could not find a significant difference in nodal status (p = 0.21), synchronous metastases (p = 0.1) and performing emergency surgery (p = 0.895) regarding the DFS.
Local recurrence was observed in one out of 12 cases (8%), whereas the most frequent type of distant metastases were liver metastases (n = 6; 50%), followed by metastases in the mesentery (n = 2), pulmonic, ovarian and multiple metastases (all n = 1). In seven of these cases, CgA levels were elevated on laboratory testing. In 10 of these patients, mesenteric lymph node metastases were diagnosed at the time of resection.
Discussion
This retrospective study evaluated clinical presentation, surgical treatment, short-term surgical outcome and long-term oncological outcome in a cohort of patients who underwent surgical resection of SBNET with homogenous grading. In our study, the majority of diagnoses (n = 24, 75%) were based on incidental findings obtained, e.g., during a medical check-up. Presentations due to symptoms caused by hormone secretion were relatively uncommon. These findings are in accordance with other available data evaluation (16–18). Hormone-secretion related symptoms have been described by Terminassian et al. (12% diarrhea, 7% flushing syndrome), which supports the findings of this study (18). The histopathological evaluation found the majority of the tumor lesions to be ≤3 cm in diameter, which correlates with the literature (≤3 cm in 75.9% vs. >3 cm in 24.1%) (19).
Mesenteric lymph node metastases were detected in 21 patients (65.6%), of which ten presented with tumor recurrence. Landry et al. and Pasquer et al. demonstrated that resection of mesenteric lymph nodes improves survival by lowering disease recurrence, preventing complications like obstruction and ischemia and improving the survival rate (17, 20). This highlights the importance of a radical surgical regime with dissection of the mesenteric lymph nodes to achieve a better oncological outcome.
In our study, we observed only a small number of patients with an early tumor stage (four patients in UICC I and four patients in UICC II). Yet, despite the low percentage of early tumor stages and the higher percentages of stages III and IV in our cohort, we did not observe a higher recurrence rate, which seems to be similar to the available literature (35.5% in our cohort vs. 31% in the literature) (21). In contrast to the available literature, in our cohort, tumor grading similar to tumor stage had no significant impact on recurrence rate, where the risk of recurrence is described to increase with the stage of disease (18, 21). This can potentially be explained by the high number of well-differentiated G1 NET (n = 20, 62.5%) and small sample size of our study. G2 grade was observed in 12 patients and there was no G3 tumor or neuroendocrine carcinoma. In the literature, the percentage of of G1 tumors ranges from 61% to 96.6% (17, 18).
We did not detect gender-based differences in OS or DFS accordingly to current literature (18).
Distant metastases are described to have a low prognostic impact on the DF (21). In our cohort, the analysis of DFS did not show any significant difference with or without synchronous metastases (p = 0.1) but can be explained by the small sample size. The 5-year OS was 58%, indicating to select patients who can take advantage of a radical surgical approach. A radical surgical approach should be attempted to gain R0 resection and avoid tumor recurrence. This should include a complete oncological resection of the primary with resection of all regional lymph nodes (17, 21, 22). The timing of surgery and surgical approach depends on clinical presentation. As seen in our cohort, a radical surgical approach is crucial for the oncological outcome, with only 38% resulting in tumor recurrence. In our cohort, most patients received a radical oncological resection, which included a hemicolectomy or segmental small-bowel resection. Concerning synchronous liver metastases, two patients were radically resected with either hemihepatectomy or atypical liver resection. In two cases radical local ablation with stereotactic radiofrequency (RFA) was performed. Only one patient with multiple liver lesion was treated in a palliative setting with platin-base chemotherapy.
In our cohort, a tumor diameter with more than 3cm is a negative prognostic factor as seen in Tables 2, 3. This could be explained due to higher frequencies of perineural invasion as well as local lymph node invasion in these cases. Curiously, not the nodal status not the presence of synchronous metastases and even the tumor grading showed any correlation with the patients’ prognosis. Of note, these results are not simply explained by the small sample size. It should be also considered that our series consists of only well-differentiated SBNET (G1 and G2) which show good response to multimodal strategies contemplating local and systemic treatment as well. Not only a multimodal strategy but also a multidisciplinary approach was shown to be clinically relevant in the management of NEN patients (19).
Tumor recurrence was detected during oncological follow-up, on the one hand due to elevated CgA-levels, on the other hand, via imaging (PET-CT, CT-scan). Treatment of tumor-recurrence was with either a radical surgical approach, RFA or systemic chemotherapy. Only one patient did not receive specific therapy for tumor recurrence due to advanced age and comorbidities. As Watzka et al. described in their work, patients benefit from resection of hepatic metastases with respect to survival rate (88.5% 5-year survival rate with R0/R1 resection vs. 69.1% with R2- or no resection of hepatic metastases) (19). Elevated CgA levels are reported to associate with shorter survival (17, 18, 23). As seen in our cohort, laboratory testing of CgA-levels during oncological follow-up is an excellent tool to early diagnose tumor recurrence, as elevated levels correlate with tumor burden (24). The measurement of CgA is cheap and straightforward to achieve and can be performed at the general practitioner's office.
Especially because SBNEN represent a rare entity with rising incidence (7, 8, 17), it is crucial to address the primary symptoms at diagnosis, the therapeutic management and the long-term oncological outcome of this special entity. Due to their relative seldom incidence, previous literature data are quite inhomogeneous (Table 4) and nowadays further studies, in particular randomized controlled trials, are needed to better uniform the clinical management of these entities (25–34).
This single-center retrospective analysis certainly has its limitations. Due to the small sample size, we could not describe significant differences in survival between various parameters in detail. As described above, tumor size was one of the most critical factors regarding the OS and DFS. Furthermore, for us an aggressive surgical approach seems crucial for patients’ disease-free and overall survival.
In addition to those findings, a strict and consequent follow-up, including laboratory testing of CgA before primary surgery and during each follow-up appointment and repeated imaging with at least a PET-CT scan once a year, helps to detect tumor recurrence early.
Conclusion
SBNEN are mostly asymptomatic; therefore, preoperative diagnostic work-up is complex. Diagnosis often happens by incident. According to our results, an aggressive surgical regimen and a strict follow-up period seem to be valuable therapeutic strategies to achieve a favorable oncological outcome.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethikkommission der Medizinischen Universität Innsbruck. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. The study was approved by the local ethics committee (Vote number 1435/2021).
Author contributions
AP: design, analysis and writing. VK and RB: data acquisition, analysis, and writing. EG, KE, HE, RK-R, DÖ: execution and correction. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Scott AT, Howe JR. Management of small bowel neuroendocrine tumors. J Oncol Pract. (2018) 14(8):471–82. doi: 10.1200/JOP.18.00135
2. Larouche V, Akirov A, Alshehri S, Ezzat S. Management of small bowel neuroendocrine tumors. Cancers. (2019) 11(9):1395. doi: 10.3390/cancers11091395
3. Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pathology: diagnosis and prognostic stratification. Neuroendocrinology. (2017) 105(3):196–200. doi: 10.1159/000457956
4. Wang R, Zheng-Pywell R, Chen HA, Bibb JA, Chen H, Rose JB. Management of gastrointestinal neuroendocrine tumors. Clin Med Insights Endocrinol Diabetes. (2019) 12:1179551419884058. doi: 10.1177/1179551419884058
5. Xavier S, Rosa B, Cotter J. Small bowel neuroendocrine tumors: from pathophysiology to clinical approach. World J Gastrointest Pathophysiol. (2016) 7(1):117–24. doi: 10.4291/wjgp.v7.i1.117
6. Huang W, Nebiolo C, Esbona K, Hu R, Lloyd R. Ki67 index and mitotic count: correlation and variables affecting the accuracy of the quantification in endocrine/neuroendocrine tumors. Ann Diagn Pathol. (2020) 48:151586. doi: 10.1016/j.anndiagpath.2020.151586
7. Tran CG, Sherman SK, Howe JR. Small bowel neuroendocrine tumors. Curr Probl Surg. (2020) 57(12):100823. doi: 10.1016/j.cpsurg.2020.100823
8. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3(10):1335–42. doi: 10.1001/jamaoncol.2017.0589
9. Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. (2003) 348(12):1134–49. doi: 10.1056/NEJMra021405
10. Oberg KE. The management of neuroendocrine tumours: current and future medical therapy options. Clin Oncol R Coll Radiol G B. (2012) 24(4):282–93. doi: 10.1016/j.clon.2011.08.006
11. Rienke A, Wiedenmann B, Auernhammer C, Bartenstein P, Bartsch DK, Begum N, et al. Practice guideline neuroendocrine tumors - AWMF-reg. 021-27. Z Gastroenterol. (2018) 56(6):583–681. doi: 10.1055/a-0604-2924
12. Niederle B, Pape UF, Costa F, Gross D, Kelestimur F, Knigge U, et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the Jejunum and ileum. Neuroendocrinology. (2016) 103(2):125–38. doi: 10.1159/000443170
13. Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. (2016) 103(2):172–85. doi: 10.1159/000443167
14. Muttillo EM, Mazzarella G, Picardi B, Rossi S, Cinelli L, Diana M, et al. Treatment strategies for neuroendocrine liver metastases: a systematic review. HPB. (2022) 24(11):1832–43. doi: 10.1016/j.hpb.2022.06.009
15. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
16. Zhang S, Zheng C, Chen Y, Xu Q, Ma J, Yuan W, et al. Clinicopathologic features, surgical treatments, and outcomes of small bowel tumors: a retrospective study in China. Int J Surg Lond Engl. (2017) 43:145–54. doi: 10.1016/j.ijsu.2017.05.076
17. Pasquer A, Walter T, Hervieu V, Forestier J, Scoazec JY, Lombard-Bohas C, et al. Surgical management of small bowel neuroendocrine tumors: specific requirements and their impact on staging and prognosis. Ann Surg Oncol. (2015) 22(3):742–9. doi: 10.1245/s10434-015-4620-2
18. Ter-Minassian M, Chan JA, Hooshmand SM, Brais LK, Daskalova A, Heafield R, et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocr Relat Cancer. (2013) 20(2):187–96. doi: 10.1530/ERC-12-0340
19. Watzka FM, Fottner C, Miederer M, Weber MM, Schad A, Lang H, et al. Surgical treatment of NEN of small bowel: a retrospective analysis. World J Surg. (2016) 40(3):749–58. doi: 10.1007/s00268-016-3432-2
20. Landry CS, Lin HY, Phan A, Charnsangavej C, Abdalla EK, Aloia T, et al. Resection of at-risk mesenteric lymph nodes is associated with improved survival in patients with small bowel neuroendocrine tumors. World J Surg. (2013) 37(7):1695–700. doi: 10.1007/s00268-013-1918-8
21. Landerholm K, Zar N, Andersson RE, Falkmer SE, Järhult J. Survival and prognostic factors in patients with small bowel carcinoid tumour. Br J Surg. (2011) 98(11):1617–24. doi: 10.1002/bjs.7649
22. Rajaretnam NS, Meyer-Rochow GY, Hallett J, Law C, Pasieka J, Koea J, et al. Surgical management of primary small bowel NET presenting acutely with obstruction or perforation. World J Surg. (2021) 45(1):203–7. doi: 10.1007/s00268-020-05689-7
23. Chatani P, Aversa J, McDonald J, Khan T, Keutgen X, Nilubol N. Preoperative serum chromogranin-A is predictive of survival in locoregional jejuno-ileal small bowel neuroendocrine tumors. Surgery. (2021) 170(1):106–13. doi: 10.1016/j.surg.2021.01.048
24. Baudin E, Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin JC, et al. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. (1998) 78(8):1102–7. doi: 10.1038/bjc.1998.635
25. Søreide O, Berstad T, Bakka A, Schrumpf E, Hanssen LE, Engh V, et al. Surgical treatment as a principle in patients with advanced abdominal carcinoid tumors. Surgery. (1992) 111(1):48–54. PMID: 1728075
26. Schindl M, Kaczirek K, Passler C, Kaserer K, Prager G, Scheuba C, et al. Treatment of small intestinal neuroendocrine tumors: is an extended multimodal approach justified? World J Surg. (2002) 26(8):976–84. doi: 10.1007/s00268-002-6628-6
27. Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. (2006) 140(6):891–7; discussion 897–8. doi: 10.1016/j.surg.2006.07.033
28. Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. (2009) 89(4):471–6. doi: 10.1159/000197899
29. Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. (2009) 16(3):885–94. doi: 10.1677/ERC-09-0042
30. Norlén O, Stålberg P, Öberg K, Eriksson J, Hedberg J, Hessman O, et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg. (2012) 36(6):1419–31. doi: 10.1007/s00268-011-1296-z
31. Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of vater:a population-based study. J Gastrointest Surg. (2014) 18(2):354–62. doi: 10.1007/s11605-013-2365-4
32. Mosquera C, Koutlas NJ, Fitzgerald TL. Localized high-grade gastroenteropancreatic neuroendocrine tumors: defining prognostic and therapeutic factors for a disease of increasing clinical significance. Eur J Surg Oncol. (2016) 42(10):1471–7. doi: 10.1016/j.ejso.2016.07.137
33. Noujaim MG, Green J, Min M, Schlieve CR, Patel K, Cahan M, et al. Carcinoids and capsules: a case series highlighting the utility of capsule endoscopy in patients with small bowel carcinoids. Gastroenterol Res. (2017) 10(6):347–51. doi: 10.14740/gr937w
Keywords: neuroendocrine tumors, small-bowel neuroendocrine tumors, diagnostic workup, surgical treatment, oncological outcome
Citation: Kroepfl V, Bellotti R, Gasser E, Esswein K, Esser H, Kafka-Ritsch R, Öfner D and Perathoner A (2023) Small bowel neuroendocrine tumors: An analysis of clinical presentation, diagnostic workup and surgical approach—A single center retrospective study. Front. Surg. 10:1072435. doi: 10.3389/fsurg.2023.1072435
Received: 17 October 2022; Accepted: 13 March 2023;
Published: 3 April 2023.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Jose M. Ramia, Hospital General Universitario de Alicante, SpainPaolo Mercantini, Sapienza University of Rome, Italy
© 2023 Kroepfl, Bellotti, Gasser, Esswein, Esser, Kafka-Ritsch, Öfner and Perathoner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Perathoner YWxleGFuZGVyLnBlcmF0aG9uZXJAaS1tZWQuYWMuYXQ=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Veronika Kroepfl
Veronika Kroepfl Ruben Bellotti†
Ruben Bellotti† Elisabeth Gasser
Elisabeth Gasser Hannah Esser
Hannah Esser Reinhold Kafka-Ritsch
Reinhold Kafka-Ritsch Dietmar Öfner
Dietmar Öfner Alexander Perathoner
Alexander Perathoner