- 1Department of Orthorpedic Oncology, Changzheng Hospital, Naval Military Medical University, Shanghai, China
- 2Naval Medical Center, Naval Military Medical University, Shanghai, China
- 3Department of Radiology, Changzheng Hospital, Naval Military Medical University, Shanghai, China
Objective: This study aims to highlight the use of robots in surgery and that of tube-assisted minimally invasive surgery for spinal metastases, as well as elaborate on the concept of invasion-controlled surgery (ICS).
Summary of background: Many patients with spinal metastasis cancer cannot afford serious complications when undergoing traditional open surgery because of their poor physical condition. Robots and minimally invasive technology have been introduced into the field of spine surgery and they have shown significant advantages.
Methods: Six patients who underwent robot and working tube-assisted ICS for spinal metastases. Relevant demographic, medical, surgical, and postoperative data were collected from medical records and analyzed.
Results: Mean operative time was 3.8 h and the mean length of the surgical incision was 4.9 cm. The mean estimated blood loss was 400 ml. The mean bedtime and hospital length of stay were 3.2 days and 6.5 days, respectively. No obvious complications were reported during treatment. The mean accuracy of screw placement was 98%. The mean time for further system treatment after surgery was 5.8 days. All patients experienced significant pain relief. The mean preoperative visual analog scale (VAS) was 7.83 points. The mean VAS at 1 day, 1 week, and 1 month after surgery were 2.83, 1.83, and 1.17 points, respectively. Frankel grade was improved in five of six patients. One patient preoperatively with Frankel grade D was the same postoperatively.
Conclusion: The concept of ICS is suitable for patients with spinal metastases. Robot and working tube-assisted ICS for spinal metastases is one of the safest and most effective treatment methods.
Introduction
According to the GLOBOCAN 2020 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer, an estimated 19.3 million new cancer cases were reported worldwide (1). Approximately 70% of cancer patients have spinal metastases (2). In recent years, the incidence of spine metastases is increasing due to the aging population and improved targeted therapy (3). Roughly one-third of patients with spinal metastases develop symptoms, including intractable pain, neurological dysfunction, and/or spinal instability (4). Surgical treatment is an effective means to solve these issues (5, 6) and has been evolving towards approaches that are both precise and minimally invasive (7).
Nonetheless, numerous patients with spinal metastasis cancer cannot afford serious complications that might result from undergoing traditional open surgery due to their poor physical condition (8) or relatively short survival time. Moreover, a comprehensive review report by Gelalis et al. (9) stated that pedicle screw misplacement rates were 0.1% to 31% using the freehand technique (10). A misplaced pedicle screw can result in complications including dural tearing, neural damage, and vascular or visceral injury. The rate of complications in relation to pedicle screw misplacement increased to 54% (11–14). Traditional open surgery complications and pedicle screw misplacement will result in a slower recovery time or a further decline in life quality.
Robots and minimally invasive techniques have been introduced into the field of spine surgery and they have demonstrated outstanding advantages. Numerous studies suggested that robot-assisted spine surgery offers screw placement accuracy, efficiency, as well as superior safety, when compared to the freehand technique (15). Robot-assisted technology could significantly reduce unnecessary tissue separation exposure and cause lower blood loss (16). In the meantime, the intra-operative radiation dose would also be reduced (15). Furthermore, with the application of targeted drugs and immune checkpoint inhibitors, the systemic control of many patients with malignant tumors is optimistic. Due to the lengthy healing period and potential complications associated with open surgical incisions, it would be inadvisable to implement the aforementioned treatment methods immediately.
In this study, we proposed the concept of invasion-controlled surgery (ICS) for spinal metastasis. It is a minimally invasive surgery that aims to enhance, in a short time, the quality of life of patients who have spinal metastasis by performing a well-controlled operation. Based on this surgical concept, we implemented a new surgery on six patients: robot and working tube-assisted ICS of spinal metastases. This article describes the indications, characteristics, and patient benefits of this surgical procedure.
Material and methods
Patients' selection
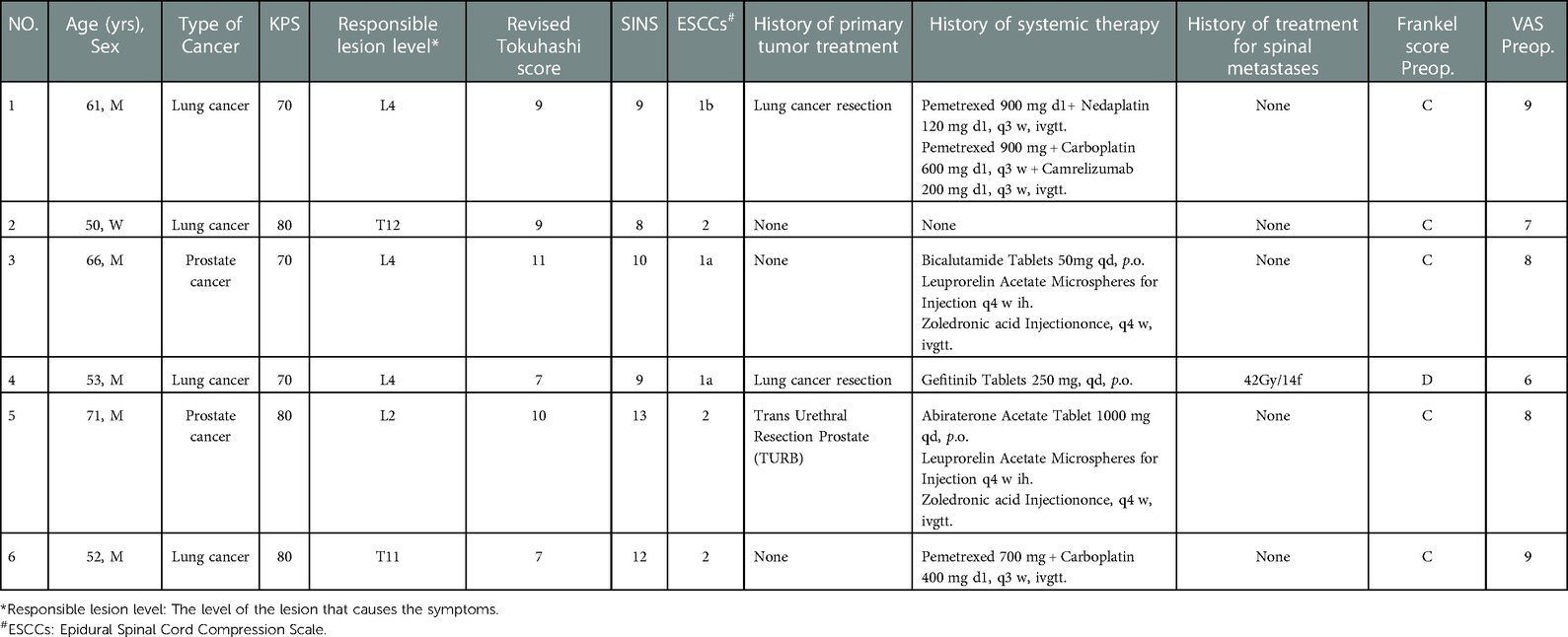
Six patients were diagnosed with malignant tumors and spinal metastases between May and August of 2021. There were five men and one woman with an average age of 59 years (range 50–71 years) and an average Karnofsky Performance Scale (KPS) score of 75 (range 70–80). All patients had symptoms of mechanical pain, nerve root, or/and spinal cord compression. Each patient signed informed consent and underwent robot and working tube-assisted ICS of spinal metastases. The robot was manufactured by Shanghai Jiao Information Technology Development Co., Ltd., and the catheter was manufactured by Medtronic, Inc. All patients had a limited life expectancy, with a mean revised Tokuhashi score of 8.8 (range 7–11). Table 1 provides a summary of the preceding key statistics. Before surgery, the patients underwent anteroposterior and lateral radiographs, computed tomography (CT), and magnetic resonance imaging (MRI) examination of the segment with the lesion and two to three adjacent segments. The average spine instability neoplastic score (SINS) was 10.2 points (range 8–13 points) according to the CT image. MRI demonstrated lesions were all located in the lower thoracic spine or lumbar spine. The lesions involved unilateral articular joints and led to unilateral nerve root or/and spinal cord compression. CT and MRI images are depicted in Figure 1. The responsible lesion segment involves a part of the side of the vertebral body and yet does not exceed the midline of the vertebral body. The patients with spinal metastasis from a malignant tumor required surgical intervention, but their physical condition was generally poor, and the minimally invasive surgical treatment plan was determined through multidisciplinary consultation.

Figure 1. MRI image (A) and CT image (B) show metastatic tumor involve unilateral articular joints and cause nerve root involvement and mild epidural compression. The red arrow points to the location of the lesion.
Surgical techniques and tools
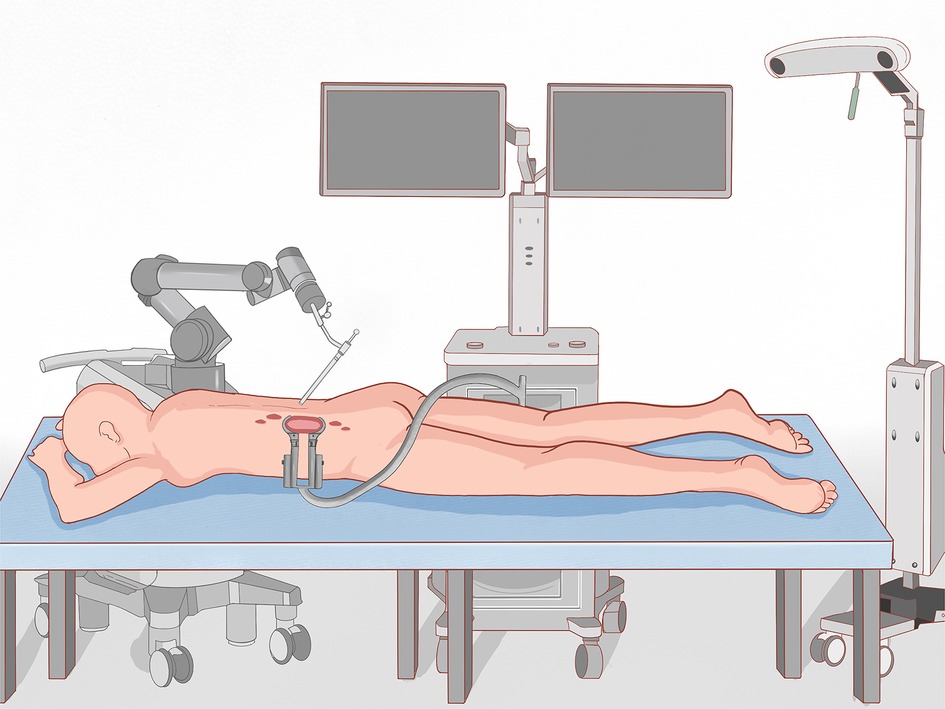
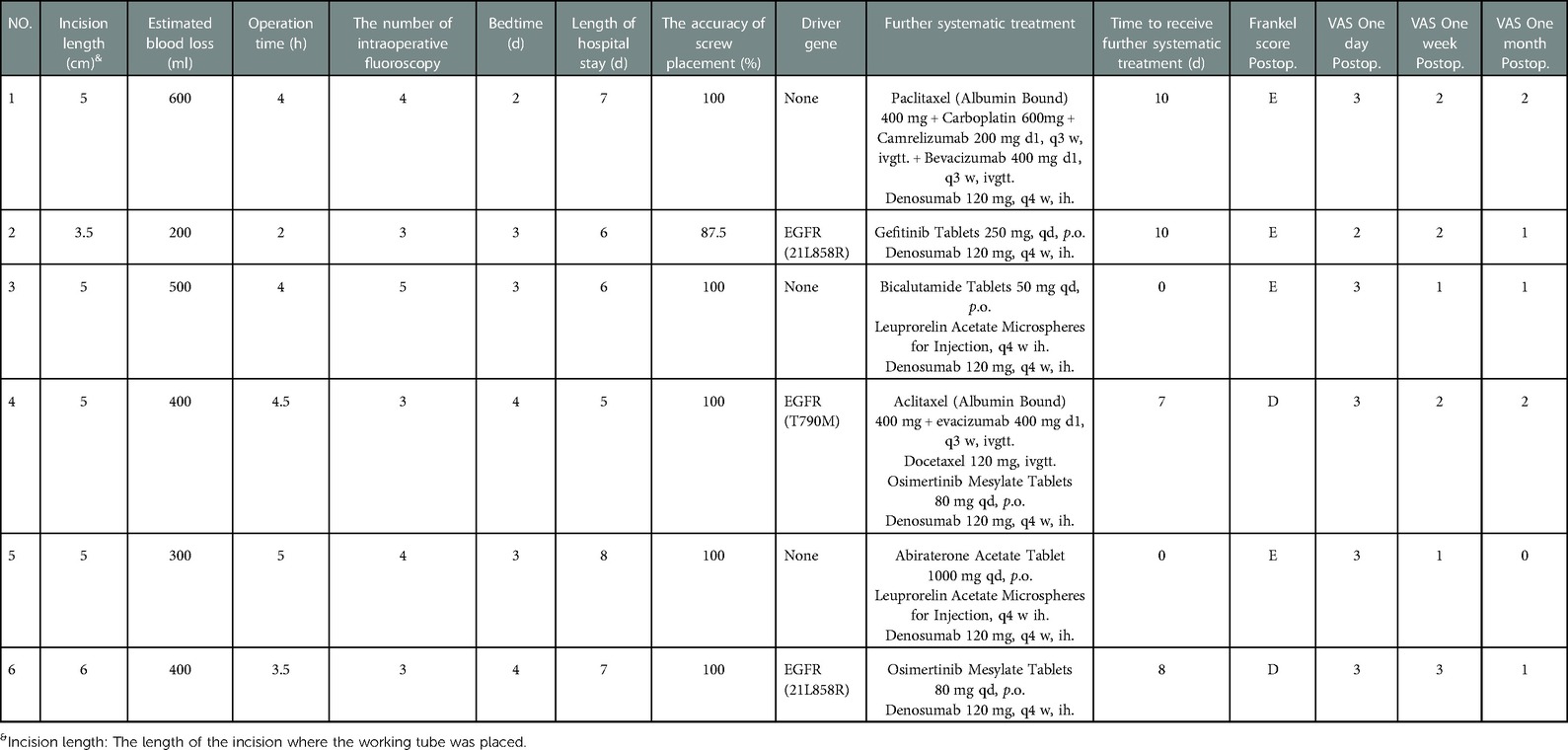
Before surgery, valuable spine imaging was obtained, including anteroposterior and lateral radiographs, and a thin-slice (1-mm) CT scan of the responsible lesion segment and the upper and lower two or three adjacent vertebral spine levels. Based on these images, we built a virtual three-dimensional (3D) model of the spine on the robot host software and determined the appropriate length, diameter, direction, and insertion angle of the pedicle screw on this model. During surgery, the patient was positioned prone on a radiolucent operating table, allowing for anteroposterior and lateral fluoroscopic control with x-rays (Figure 2). Furthermore, the robot positioned the skin projection of the pedicle screw entry point one by one and created a small incision of approximately 1 cm on the skin (Figure 3A). With the assistance of the prepared robot, the placement of pedicle screws along the predetermined trajectory was performed individually. The screws we employed were bone cement injectable canulated pedicle screws (CICPS, from Shanghai Sanyou Medical Co., Ltd). After the x-ray fluoroscopy displayed that the position and direction of the screw were appropriate, we inserted the bone cement into the CICPS. The number of x-ray fluoroscopy during surgery was counted (Table 2). We also employed the robot to accurately locate the skin projection of the responsible lesion segment and make a 3 cm-5 cm incision at the location. The thoracolumbar fascia was exposed, and its incision allowed for a Wiltse muscle-splitting technique-compliant gentle dissection between the muscles (17). Moreover, the Wiltse approach allowed direct access to the unilateral articular joints. We consequently maintained and expanded the space between the muscles by introducing an expandable working tube (Figure 3B,C). The diameter was 22 mm when not expanded. When expanded, the maximum width of the upper part was 50–55 mm, and the maximum width of the lower part was 80–100 mm. The working tube was widened and moved moderately as required to visualize the altered anatomy. Unilateral lamina, facet joints, and pedicle could be removed to complete dorsal decompression as well as nerve root release under the expandable working tube (Figure 3C). The part of the vertebral body involved by the lesion (not more than the midline of the vertebral body), was removed to complete the ventral side stress reliever. After the resection, the stability of the anterior column of the vertebral body could be compromised. In this case, namely case 3, we placed a proper amount of bone cement in the missing vertebral body position via the expandable working tube. Accordingly, the scope of the lesion that could be excised under the expandable working tube was 1–6 or 7–12 areas of the Weinstein-Boriani-Biagini spine surgical staging (WBB staging), and WBB staging areas 2–3 or 10–11 are the most convenient for operations. After the procedure, the longitudinal connecting rod was inserted, the nut was tightened, and a drainage tube was placed at the excision site. All patients underwent further systematic treatment, such as targeted therapy, immunotherapy, or chemotherapy, under conditions permitted by their bodies following surgery, and the time spent receiving treatment was tallied (Table 2).
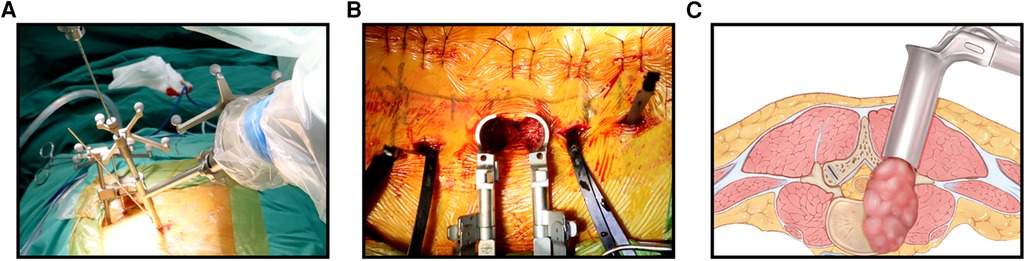
Figure 3.. (A): Robot-assisted positioned the skin projection of the pedicle screw entry point. (B): Complete decompression and resection under the working tube. (C): Schematic diagram of the working tube and the extent of the lesion.
Results
During the patient's hospitalization, data on operation time, incision length, operation time, estimated blood loss during the operation, bedtime, hospital stay, and complications were collected. Visual analog scales (VAS) and the Frankel grade classification were employed to, respectively, measure pain and neurological deficit before surgery and at discharge. The accuracy of screw placement was evaluated by CT imaging according to a Gertzbein and Robbins classification system (18): Grade A, in the pedicle; Grade B, perforation <2 mm; Grade C, perforation >2 mm but < 4 mm; Grade D, perforations > 4 mm but <6 mm; and Grade E, perforations >6 mm. Grade A and Grade B were regarded as accurate nail placement (19). This data is displayed in Table 2.
Operative data
The mean operative time was 3.8 h (range 2–5 h) and the mean length of the surgical incision of the decompression tube was 4.9 cm (range 3.5 cm–6 cm). The mean estimated blood loss was 400 ml (range 200–600 ml), and no patient needed a blood transfusion during or after the operation. The mean bedtime and length of hospital stay were 3.2 days (range 2–4 days) and 6.5 days (range 5–8 days), respectively.
Complications
There were no reported complications during treatment. All patients underwent x-ray and CT scans after surgery and the mean accuracy of the robot-assisted screw placement was 98% (range 87.5%–100%). During the duration of the study, no instrument failures were reported. The mean time to receive further systematic treatment was 7.67 days (range 0–21 days). The mean follow-up time was 5 months (range 4–7 months), and no patients were lost during follow-up. During the follow-up, it was determined that no patients had wounds that did not heal properly or other adverse surgical complications.
Neurological course
Four of the six patients (66.66%) improved by two Frankel grades (from C to E), and these four patients were able to walk independently without pain during hospitalization. One patient (16.66%) improved by one Frankel grade (from C to D grade). One patient (16.66%, Case 4), who had a Frankel grade D before the operation was still grade D after the operation, and yet the patient claimed the pain was significantly reduced. No patients experienced pain or spinal cord compression at the treatment level again during the follow-up period (Figure 4).
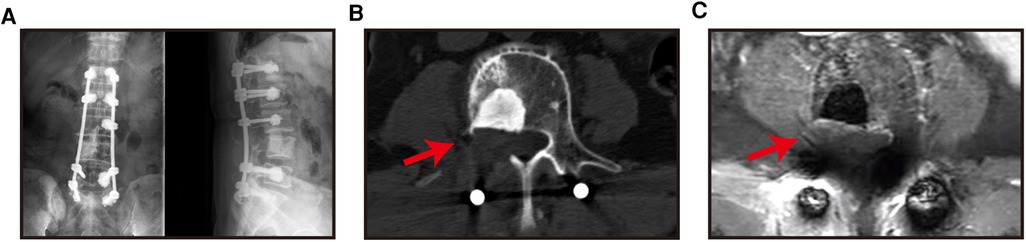
Figure 4.. X-ray (A), CT images (B) and MRI images (C) of patients were reviewed 6 months after surgery.
Pain alleviation
All patients' pain were significantly reduced. (Figure 5) as they all experienced massive pain relief. The mean preoperative VAS was 7.83 points. The mean VAS at 1 day, 1 week, and 1 month after surgery were 2.83, 1.83, and 1.17 points, respectively, meaning a decrease of more than 5 points. During the period of follow-up, none of the patients experienced a recurrence of pain.

Figure 5.. The X-ray (A), CT image (b) and MRI image (C) of the patient at 6 months after the operation showed spine no local progression (the red arrow is the decompression area, and item with high density at the vertebral body in B is intraoperative bone cement).
Discussion
In patients with malignant tumors, spinal metastasis is more prevalent. It may cause severe symptoms and serious damage to the quality of life. Once neurological signs are present, there is level I evidence that direct decompressive surgery is far superior to radiotherapy alone in patients with spinal metastasis (20). The revised Tokuhashi score (21) asserts that the majority of patients with lung cancer have a survival period of fewer than 6 months. Nonetheless, during the last decades, clinicians have witnessed remarkable developments in molecular targeted therapy, particularly the second development of immune checkpoint inhibitors that have significantly enhanced the prognosis of lung cancer patients (22). Consequently, we can perform palliative surgery on lung cancer patients with neurological signs. The surgical treatment is aimed at palliative care (23)—to relieve pain, repair and protect nerve function, and correct spinal instability, as well as strengthen the quality of life of patients. Notwithstanding, conventional open surgery may cause significant bleeding during the operation and there are numerous risks involved, including excessive muscle trauma, and nerve and blood vessel damage, which may cause major complications after surgery. Additionally, the risks of conventional open surgery will be more prominent for patients who are elderly, with underlying diseases, poor nutritional status as well as for those who have previously received chemotherapy, radiotherapy, or glucocorticoid therapy. Hence, the proposed concept of ICS for spinal metastases benefits patients in several ways.
The six patients with spinal metastases who were included in this study all had nerve root pain, spinal instability, or neurological dysfunction. Vertebroplasty could not effectively solve these problems. ICS was the most reliable method of surgery to rebuild spine stability and remove the responsible lesions, and thus decompress the spinal cord and nerve roots.
The robot-assisted technique is a superior method to place pedicle screws in the spine, due to its higher accuracy, safety, and the feasibility rates of the procedure. It could benefit from the following characteristics. Firstly, the data of preoperative robot planning has the advantages of intuitiveness, accuracy, and repeatability. Secondly, the intraoperative fluoroscopy image is synchronized with the preoperative CT image during the operation, which is one of the reasons why high accuracy can be achieved. Additionally, the robot can also avoid most human distinctions and fatigue-related errors, such as a surgeon's trembling hands or poor coordination. These features allow the robot to have an active role in screw placement precision, operation time, and radiation exposure.
On the other hand, we analyzed the characteristics of the patients' lesions, which predominantly affected unilateral facet joints, resulting in unilateral nerve root or spinal cord compression. The operation under the small incision and expandable working tube was able to achieve the purpose of resectioning the lesion and relieving the compression with minimal invasion, which also incorporated the ICS concept for spinal metastasis. Consequently, the mean surgical incision was 4.9 cm, the mean intraoperative blood loss was 400 ml, the postoperative neurological function of the patient improved, and the pain was relieved. These and the absence of obvious complications confirm the efficacy of this minimally invasive technique.
Patients with spinal metastases should receive additional systematic treatment as soon as possible after surgery, that is, they should transition from short-term palliative surgery to long-term tumor control. Nonetheless, the prerequisites are that a patient has no obvious postoperative complications, the surgical wound heals and the systemic condition recovers as soon as possible. These are the exact objectives pursued by ICS.
Nevertheless, the implementation of such surgery also has some constraints. For instance, the popularity of spinal robots is not significant, the range of decompression under the working tube cannot be overly large, and the operation of decompression under the working tube requires an experienced spinal surgeon to perform the operation.
Conclusions
The concept of invasion-controlled surgery is extremely suitable on the condition that patients with malignant spinal metastases and limited life expectancy require surgical treatment. Moreover, the six cases of surgery in this article were specific cases of ICS for spinal metastasis, which further indicates that it is a safe and effective method of treatment that can lessen complications, promote rapid recovery of patients after surgery, and enable patients to enter more quickly the next step of treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SZ, BL, and PW have contributed equally to this study. Correspondence to: WX and JX. Conception and design: WX and JX. Acquisition of data: SZ, MX, and JZ. Analysis and interpretation of data: SZ, BL, and PW. Prepared tables and figures: SZ, BL, and PW. Drafting of the article: SZ. Critically revising the article: all authors. Reviewed submitted version of the manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: WX. Study supervision: JX. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant No.81902733) and the Science and Technology Commission of Shanghai Municipality (Grant No. 17411950300, 17411950301, 19YF1448100 and 18411964400).
Acknowledgments
I am very grateful to my teachers, brothers, classmates, my wife, son, and myself. I will continue to contribute to medical research.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Gasbarrini A, Beisse R, Fisher C. Rhines L: spine metastasis. Int J Surg Oncol. (2011) 2011:375097. doi: 10.1155/2011/375097
3. Yaari LS, Novack L, Shemesh S, Sidon E, Haviv B, Sheinis D, et al. Patient outcomes and survival following surgery for spinal metastases. J Spinal Cord Med. (2021) 44:204–11. doi: 10.1080/10790268.2019.1610602
4. Park J-S, Park S-J, Lee C-S. Incidence and prognosis of patients with spinal metastasis as the initial manifestation of malignancy: analysis of 338 patients undergoing surgical treatment. Bone Joint J. (2019) 101-B:1379–84. doi: 10.1302/0301-620X.101B11.BJJ-2018-1600.R2
5. Park H-Y, Lee S-H, Park S-J, Kim E-S, Lee C-S, Eoh W. Minimally invasive option using percutaneous pedicle screw for instability of metastasis involving thoracolumbar and lumbar spine: a case series in a single center. J Korean Neurosurg Soc. (2015) 57:100–7. doi: 10.3340/jkns.2015.57.2.100
6. Rao PJ, Thayaparan GK, Fairhall JM, Mobbs RJ. Minimally invasive percutaneous fixation techniques for metastatic spinal disease. Orthop Surg. (2014) 6:187–95. doi: 10.1111/os.12114
7. Zhang Q, Han X-G, Xu Y-F, Fan M-X, Zhao J-W, Liu Y-J, et al. Robotic navigation during spine surgery. Expert Rev Med Devices. (2020) 17:27–32. doi: 10.1080/17434440.2020.1699405
8. Weber BR, Grob D, Dvorák J, Müntener M. Posterior surgical approach to the lumbar spine and its effect on the multifidus muscle. Spine. (1997) 22:1765–72. doi: 10.1097/00007632-199708010-00017
9. Gelalis ID, Paschos NK, Pakos EE, Politis AN, Arnaoutoglou CM, Karageorgos AC, et al. Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J. (2012) 21:247–55. doi: 10.1007/s00586-011-2011-3
10. Vaccaro AR, Harris JA, Hussain MM, Wadhwa R, Chang VW, Schroerlucke SR, et al. Assessment of surgical procedural time, pedicle screw accuracy, and clinician radiation exposure of a novel robotic navigation system compared with conventional open and percutaneous freehand techniques: a cadaveric investigation. Global Spine J. (2020) 10:814–25. doi: 10.1177/2192568219879083
11. Tjardes T, Shafizadeh S, Rixen D, Paffrath T, Bouillon B, Steinhausen ES, et al. Image-guided spine surgery: state of the art and future directions. Eur Spine J. (2010) 19:25–45. doi: 10.1007/s00586-009-1091-9
12. Gautschi OP, Schatlo B, Schaller K, Tessitore E. Clinically relevant complications related to pedicle screw placement in thoracolumbar surgery and their management: a literature review of 35,630 pedicle screws. Neurosurg Focus. (2011) 31:E8. doi: 10.3171/2011.7.FOCUS11168
13. Wang H, Zhou Y, Liu J, Han J, Xiang L. Robot assisted navigated drilling for percutaneous pedicle screw placement: a preliminary animal study. Indian J Orthop. (2015) 49:452–7. doi: 10.4103/0019-5413.159670
14. Zhang Q, Han X-G, Xu Y-F, Liu Y-J, Liu B, He D, et al. Robot-Assisted versus fluoroscopy-guided pedicle screw placement in transforaminal lumbar interbody fusion for lumbar degenerative disease. World Neurosurg. (2019) 125:e429–34. doi: 10.1016/j.wneu.2019.01.097
15. D'Souza M, Gendreau J, Feng A, Kim LH, Ho AL, Veeravagu A. Robotic-Assisted spine surgery: history, efficacy, cost, and future trends. Robotic Surgery (Auckland). (2019) 6:9–23. doi: 10.1155/2011/375097
16. Zhang J-N, Fan Y, He X, Liu T-J, Hao D-J. Comparison of robot-assisted and freehand pedicle screw placement for lumbar revision surgery. Int Orthop. (2021) 45:1531–8. doi: 10.1007/s00264-020-04825-1
17. Wiltse LL, Bateman JG, Hutchinson RH, Nelson WE. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg Am. (1968) 50:919–26. doi: 10.2106/00004623-196850050-00004
18. Solomiichuk V, Fleischhammer J, Molliqaj G, Warda J, Alaid A, von Eckardstein K, et al. Robotic versus fluoroscopy-guided pedicle screw insertion for metastatic spinal disease: a matched-cohort comparison. Neurosurg Focus. (2017) 42:E13. doi: 10.3171/2017.3.FOCUS1710
19. Ringel F, Stüer C, Reinke A, Preuss A, Behr M, Auer F, et al. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine. (2012) 37:E496–501. doi: 10.1097/BRS.0b013e31824b7767
20. Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet (London, England). (2005) 366:643–8. doi: 10.1016/S0140-6736(05)66954-1
21. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. (2005) 30:2186–91. doi: 10.1097/01.brs.0000180401.06919.a5
22. Yu W, Tang L, Lin F, Yao Y, Shen Z. Accuracy of tokuhashi score system in predicting survival of lung cancer patients with vertebral metastasis. J Neuro-Oncol. (2015) 125:427–33. doi: 10.1007/s11060-015-1934-7
Keywords: spinal metastases, invasion-controlled surgery, minimally invasive, robot, working tube
Citation: Zhou S, Li B, Wang P, Xu M, Zhao J, Duan S, Zhu Z, Xu W and Xiao J (2023) Robot and working tube-assisted invasion-controlled surgery for spinal metastases. Front. Surg. 10:1041562. doi: 10.3389/fsurg.2023.1041562
Received: 11 September 2022; Accepted: 25 January 2023;
Published: 24 February 2023.
Edited by:
Yu Zhu, Zhejiang University, ChinaReviewed by:
Luca Ambrosio, Campus Bio-Medico University, ItalyJianping Zeng, The First Affiliated Hospital of Nanchang University, China
© 2023 Zhou, Li, Wang, Xu, Zhao, Duan, Zhu, Xu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Xu eHV3ZWlfc3BpbmVAMTYzLmNvbQ== Jianru Xiao amlhbnJ1eGlhbzgzQDE2My5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Shangbin Zhou1,2,†
Shangbin Zhou1,2,† Pengru Wang
Pengru Wang Jian Zhao
Jian Zhao Wei Xu
Wei Xu Jianru Xiao
Jianru Xiao