
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 30 August 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.997169
This article is part of the Research Topic Acute Care Surgery, Emergency Surgery, Surgical Outcomes View all 7 articles
Background: Anorectal melanoma is a rare tumor with a dismal prognosis. The only promising treatment for anorectal melanoma is surgery, either extensive resection (ER) or local excision (LE). However, the optimal extent of resection is still controversial. The purpose of this study was to investigate whether the survival outcomes of anorectal melanoma at different stages are influenced by the surgical approaches (LE or ER) using the National Institute of Health's Surveillance, Epidemiology, and End Results Program (SEER) database.
Methods: The Surveillance, Epidemiology and End Results (SEER) database was queried to identify patients treated for anorectal melanoma (2000–2018). Overall survival (OS) and disease-specific survival (DSS) outcomes were compared for the two surgical approaches (ER or LE) stratified by stage (localized, regional and distant).
Results: A total of 736 patients were included in the study. Details of previous surgical procedures were available for 548 of the study patients: 360 (65.7%) underwent LE, and 188 (34.3%) underwent ER. In localized cases, 199 underwent LE, and 48 underwent ER. The OS (median 45 vs. 29 months, 5-year rate 41.7% vs. 23.4%) and DSS (median 66 vs. 34 months, 5-year rate 51% vs. 30.7%) of patients undergoing ER were significantly better (p = 0.009 and 0.041, respectively) than those who received LE. Multivariate analysis showed that the type of surgery was an independent prognostic factor for both OS and DSS. Among the regional cases, 89 cases had LE, and 96 cases had ER. Patients with regional disease who underwent ER had no significant differences in OS (23 vs. 21 months; p = 0.866) or DSS (24 vs. 24 months; p = 0. 907) compared to patients who underwent LE. In distant cases, 72 cases had LE, and 44 cases had ER. Patients with metastatic disease who had ER also had similar OS (median 11 vs. 8 months; p = 0.36) and DSS (median 11 vs. 8 months; p = 0.593) to those who underwent LE.
Conclusion: Extensive resection can improve the long-term prognosis of localized anorectal melanoma compared to local excision, but the prognosis of the two surgical techniques is comparable in both regional patients and distant patients.
Anorectal melanoma is a rare disease with poor prognosis due to early metastasis, and its incidence has been continuously increasing over time (1, 2). Anorectal melanoma accounts for 0.4% to 1.6% of all malignant melanomas, 23.8% of all mucosal melanomas, and 1% of all anorectal malignant tumors, making it the third most prevalent site for melanoma after the skin and eyes (3–5). The symptoms of anorectal melanoma, on the other hand, are ambiguous and can be found in a variety of anorectal illnesses, such as hemorrhoidal disease, polyps, and squamous cell carcinoma, thereby delaying its diagnosis and treatment (6–8). The overall survival of anorectal melanoma remains dismal, with a 5-year overall survival (OS) rate ranging from 10% to 20% (1, 9).
Because of its low incidence, experience in the surgical treatment of anorectal melanoma is still limited. Surgical resection, either extensive resection (ER) or local excision (LE), is the most promising treatment for anorectal melanoma, providing the opportunity for long-term survival (10–12). However, the optimal extent of resection is still controversial. Several recent studies reported comparable survival outcomes between ER and LE for the treatment of locoregional anorectal melanoma (13, 14). In contrast, some earlier studies found that abdominoperineal resection provided a significantly longer survival than LE (15, 16).
The abovementioned studies did not distinguish between localized and regional patients; therefore, the conclusions might not be suitable for all anorectal melanoma patients. Due to the low overall incidence and difficulty of early detection, localized cases of anorectal melanoma are generally rare. Currently, only a handful of studies with small sample sizes have been conducted to verify the prognostic impact of various surgical types on anorectal melanoma at various stages, and contradictions exist in these studies (16, 17). The current study aims to investigate whether the survival outcomes of anorectal melanoma at different stages are influenced by the surgical type (LE or ER) using the National Institute of Health's Surveillance, Epidemiology, and End Results Program (SEER) database.
All patients diagnosed with melanomas of the anorectum were identified from 18 registries of the Surveillance, Epidemiology and End Results (SEER, http://seer.cancer.gov/) database between January 1, 2000, and December 31, 2018. The included patients satisfied the following criteria: anatomic sites of rectum or anus (site code ICD-O-3: “Rectum” and “Anus, Anal Canal and Anorectum”.); histologically diagnosed as malignant melanoma (Histologic type ICD-O-3 codes: 8720–8772, malignant Behavior code ICD-O-3 code: 3). The exclusion criteria were as follows: lost to follow-up, and unknown stage. Finally, a total of 736 patients with malignant melanoma were included in this study. The patients were divided into 3 groups by tumor stage according to the Clinical Combined Summary Stage developed for SEER: localized disease (stage I), regional disease (stage II, representing cases with regional lymph-nodal metastasis) and distant disease (stage III, referring to disease with distant metastasis). Each group was further divided into LE or ER subgroups according to the extent of surgery, and patients who did not undergo surgical resection or for whom the surgical method was unclear were excluded in this step (Figure 1).
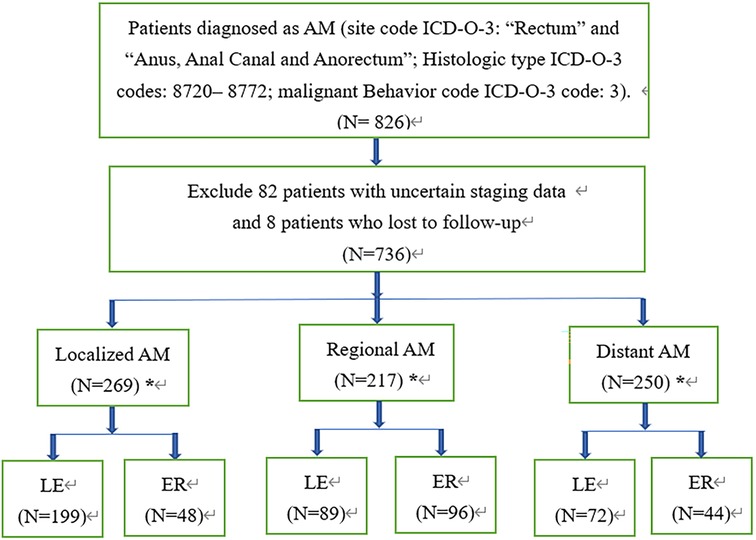
Figure 1. Flowchart for screening and statistical analysis of AM. *Stands for removing patients who have not undergone surgery, and the surgical method is unknown in the subsequent comparative analysis; AM, anorectal melanoma; LE, local excision; ER, extensive resection.
In this study, LE includes “local tumor excision”, “polypectomy” and “excisional biopsy”. ER refers to the more extensive surgical approaches with pararectal lymph node removal, including “abdominal perineal resection”, “total proctectomy”, “total proctocolectomy” and “proctectomy”. Specifically, in rectal melanoma, cases with RX Summ Surg Prim Site (1998+) codes of 10–28 were identified as LE; in contrast, cases with Summ–Surg Prim Site (1998+) codes of 30–70 were categorized as ER. LE (Summ–Surg Prim Site codes: 10–27) and ER (Summ Surg Prim Site codes: 60–63) in anal melanoma were extracted similarly.
The survival data were calculated by SEER*Stat software using the survival session tool. In overall survival (OS), any death was considered an event, whereas in disease-specific survival (DSS), only death from the specific cancer was considered.
SPSS version 25.0 (IBM Corp.) was used for statistical analysis and data management. Categorical variables are indicated as category names (number of cases, percentage), whereas continuous variables are indicated as the mean ± standard deviation or median. Chi-square analysis or Fisher's exact test was used to compare categorical data. Continuous variables were compared using the t test or Mann–Whitney test.
Survival curves were constructed using the Kaplan–Meier method, and the statistical significance of differences in survival was determined using the log-rank test. The Cox-hazard model was used for the univariate analysis and multivariate analysis. Variables with p < 0.1 in univariate analysis were included in multivariate analysis. The Wald backward method was used for multivariate analysis. A two-sided P value less than 0.05 indicated a significant difference. The SEER database is publicly available, and all patient information is deidentified; therefore, this study was granted an exemption from institutional review board approval.
Among all 736 patients, 292 had rectal melanoma (39.7%), and 444 had anal melanoma (60.3%). The mean age was 67.9 ± 14.3 years, and the female-to-male ratio was 1.45:1. The number and proportion of cases with localized, regional and distant diseases were 269 (36.5%), 217 (29.5%) and 250 (34%), respectively. There were significant differences among the 3 groups in age, tumor location (rectum or anus), date of diagnosis, radiation, chemotherapy and surgery (Table 1).
Overall, patients with anorectal melanoma had a median OS of 17 months and a 5-year survival rate of 17%. Patients with localized disease had a median OS of 29 months and a 5-year survival rate of 25.6%, which were significantly superior to those of the regional (median OS 21 months, 5-year rate 16.1%; p = 0.013) and distant groups (median OS 7 months, 5-year rate 8.9%; p < 0.001).
Not surprisingly, patients who underwent surgical resection showed significantly longer OS (median 21 vs. 7 months; p < 0.001) and DSS (median 24 vs. 8 months; p < 0.001) than those who failed to undergo resection, irrespective of the stage classification. Furthermore, the stratified analyses based on tumor staging demonstrated that patients with both localized disease (median OS 31 vs. 12 months, p < 0.001; DSS 38 vs. 12 months, p < 0.001) and regional disease (median OS 22 vs. 12 months; p = 0.02; DSS 24 vs. 13 months; p = 0.024) could benefit from surgical treatment. However, for patients with distant metastases, surgical resection failed to show any statistically significant survival advantages (median OS 8 vs. 6 months, p = 0.051; DSS 8 vs. 6 months, p = 0.086), as shown in Table 2.
Among the 269 localized cases, 22 cases were excluded due to no surgery or unclear surgical method, and finally, 199 localized cases with LE and 48 cases undergoing ER were included in the analysis. Patients undergoing ER were younger (median 62.6 vs. 70.3 years, p = 0.001), while more patients in the LE subgroup received radiotherapy (21.6% vs. 6.3%, p = 0.014). The sex, tumor location, race, date of diagnosis and chemotherapy were comparable between the 2 subgroups, as shown in Supplementary Table S1. The survival analysis showed that ER was associated with significantly better OS (median 45 vs. 29 months, 5-year rate 41.7% vs. 23.4%; p = 0.009) and DSS (median 66 vs. 34 months, 5-year rate 51% vs. 30.7%; p = 0.041) than LE (Figure 2).
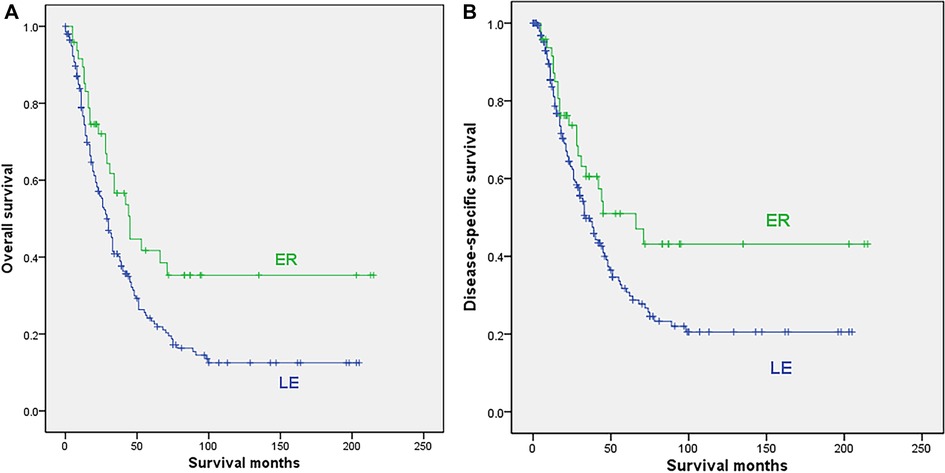
Figure 2. Survival curves for patients with localized disease who underwent local excision (LE) or extensive resection (ER); (A) overall survival; (B) disease-specific survival.
Only 137 of 199 cases in the LE subgroup and 35 of 48 patients in the ER subgroup had tumor size information, and the mean sizes were 31.3 mm and 31.7 mm, respectively, with no significant difference (p = 0.959). Seventeen patients undergoing LE and 32 patients in the ER subgroup had postoperative lymph node biopsy data, with average numbers of lymph node biopsies of 4.2 and 11.8, respectively. All lymph node biopsies were negative.
Univariable Cox regression analysis was conducted to determine the survival significance of these variables (sex, age, race, date of diagnosis, location, type of surgery, radiation, and chemotherapy), and the results indicated that RE was associated with better OS (HR = 0.579, p = 0.01) and DSS (HR = 0.626, p = 0.043) than LE. Multivariate analysis showed that the type of surgery, age and race were independent prognostic factors for OS, and the type of surgery and race were independent prognostic factors for DSS of localized anorectal melanoma patients (Table 3, Supplementary Table S4).
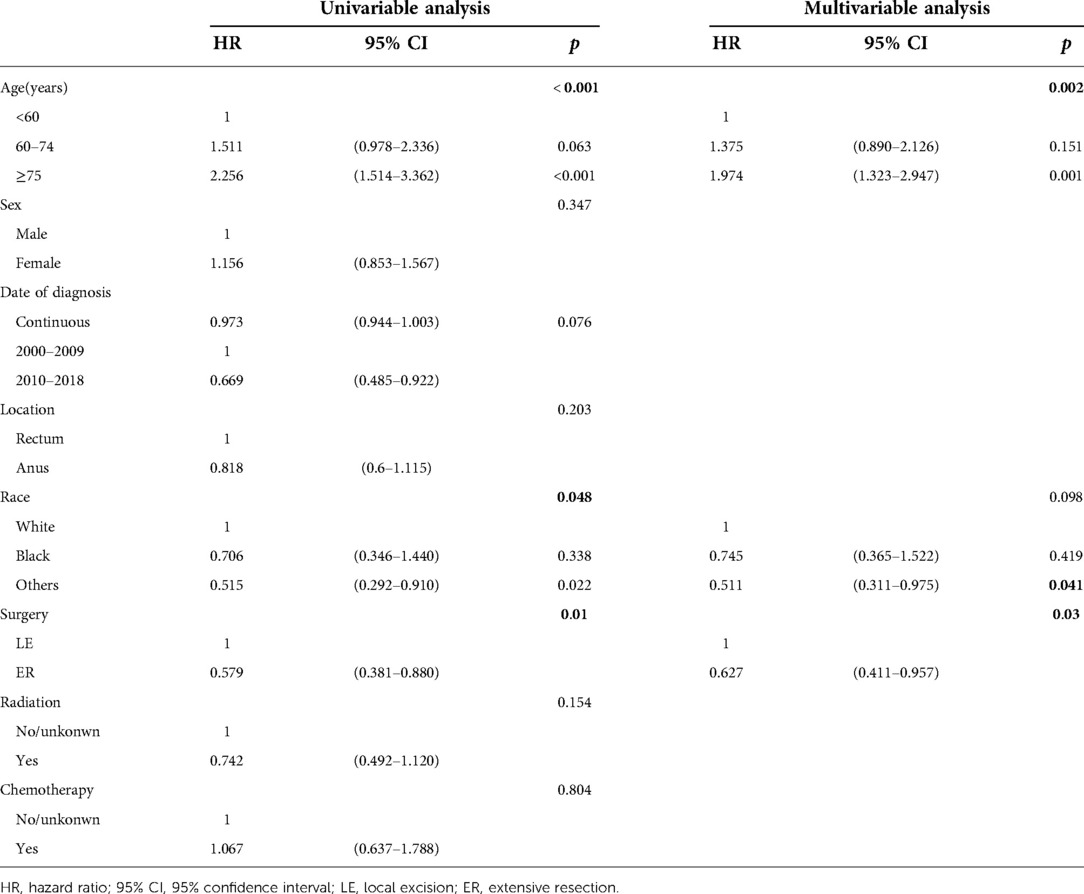
Table 3. Cox regression analysis of prognostic factors influencing OS for patients with localized disease.
After excluding cases with no surgery or unclear surgical methods, 89 cases with LE and 96 cases undergoing ER were finally included in the analysis. Patients undergoing ER were also younger (median 65 vs. 69.6 years, p = 0.02). The sex, race, date of diagnosis, radiation and chemotherapy were all comparable between the 2 subgroups (Supplementary Table S2). The survival analysis indicated that the type of surgery showed no significant influence on either OS (median 23 months for ER vs. 21 months for LE, 5-year rate of 18% for ER vs. 17.8% for LE; p = 0.866) or DSS (median 24 months for both ER and LE, 5-year rate of 19.6% for ER vs. 20.2% for LE; p = 0. 907), as shown in Figure 3.
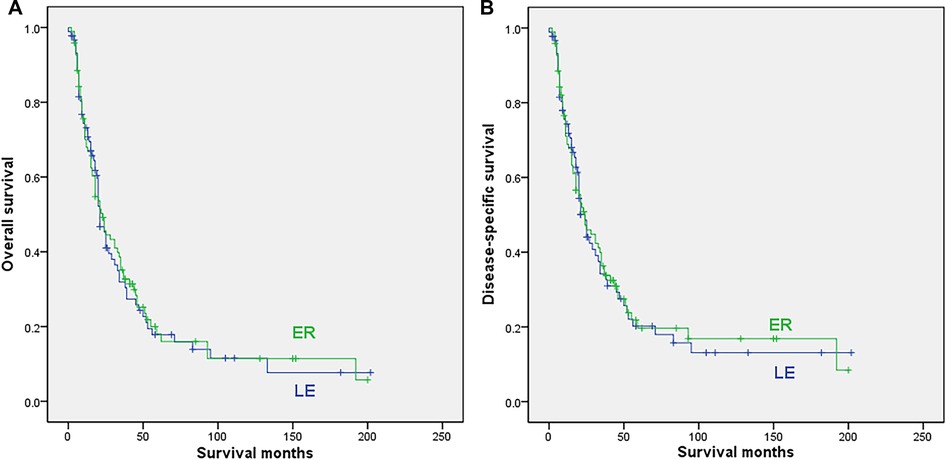
Figure 3. Survival curves for patients with regional disease who underwent local excision (LE) or extensive resection (ER); (A) overall survival; (B) disease-specific survival.
The univariable Cox regression analysis showed that patients receiving chemotherapy had a worse prognosis for OS (p = 0.02) and DSS (p = 0.014). Multivariate analysis showed that age and chemotherapy were independent prognostic factors for both the OS and DSS of regional anorectal melanoma patients (Table 4, Supplementary Table S5).
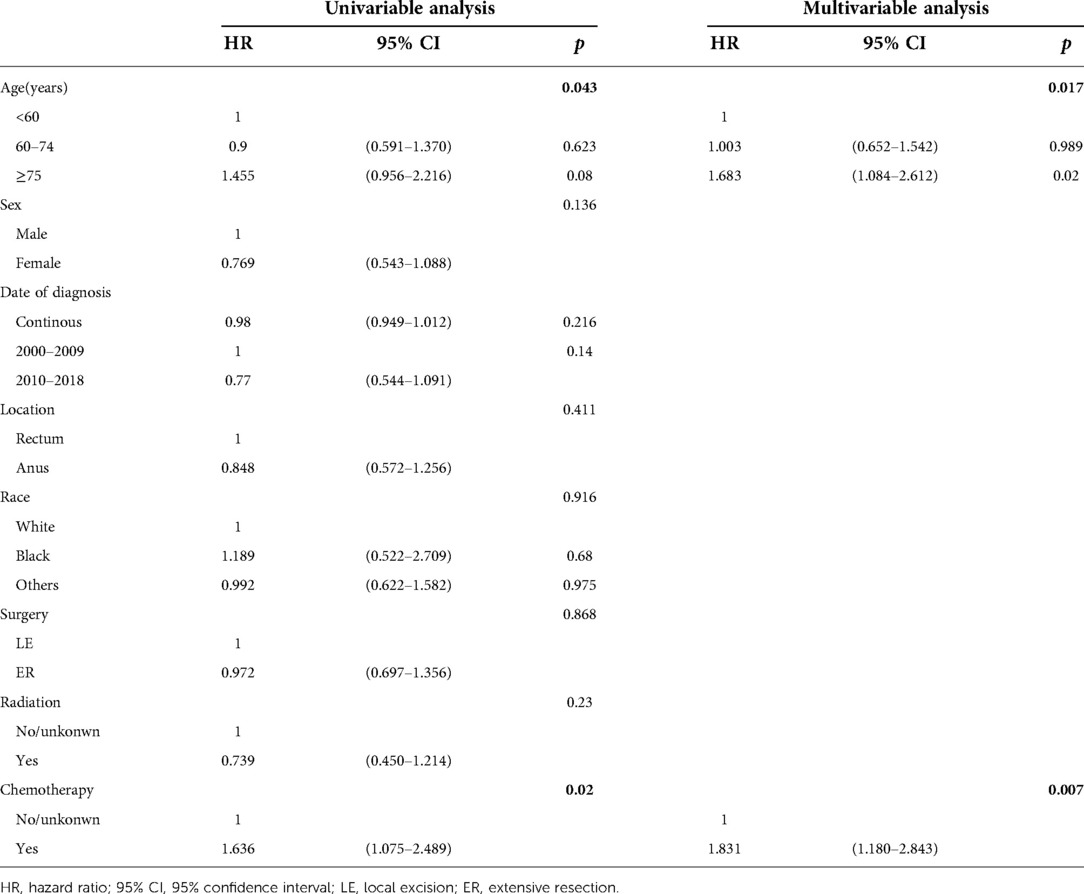
Table 4. Cox regression analysis of prognostic factors influencing OS for patients with regional disease.
In this group, 72 cases with LE and 44 cases undergoing ER were finally included in the analysis. The mean age, location, race, date of diagnosis, radiation and chemotherapy were comparable between the 2 subgroups, while the proportion of females was significantly higher (p = 0.017) in the ER subgroup, as shown in Supplementary Table S3. The survival analysis showed that ER was not associated with either OS (median 11 vs. 8 months; 5-year rate not reached for ER vs. 7.9% for LE; p = 0.36) or DSS (median 11 vs. 8 months, 5-year rate not reached for ER vs. 9.4% for LE; p = 0.593) for patients with distant disease (Figure 4).
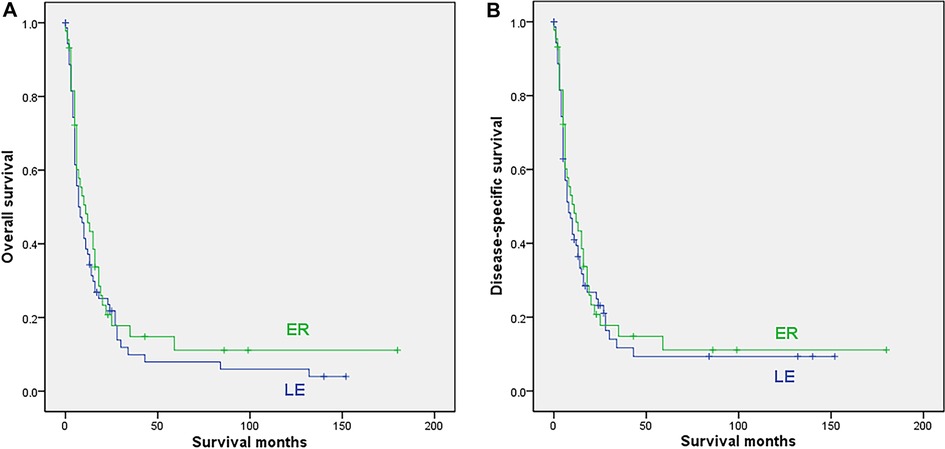
Figure 4. Survival curves for patients with distant disease who underwent local excision (LE) or extensive resection (ER); (A) overall survival; (B) disease-specific survival.
The univariable Cox regression analysis showed that older age and race, except white and black, were related to both worse OS (p = 0.002; p = 0.05) and DSS (p = 0.007; p = 0.04). Multivariate analysis showed that age was an independent prognostic factors for OS, and age, tumor location were independent prognostic factors for DSS in distant anorectal melanoma patients (Table 5; Supplementary Table S6).
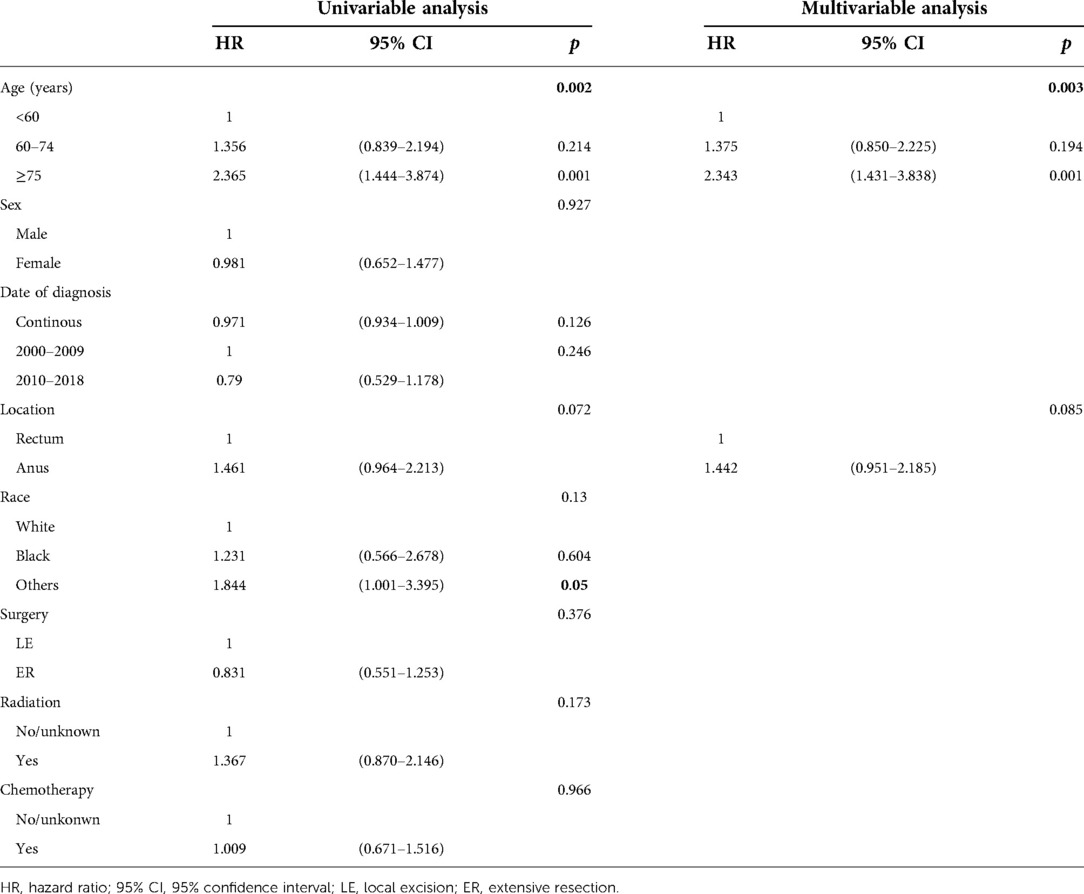
Table 5. Cox regression analysis of prognostic factors influencing OS for patients with distant disease.
The data of this SEER-based study indicated the dismal prognosis of anorectal melanoma, with a 5-year OS rate of only 17%. However, the 5-year survival rate was 25.6% in patients with localized disease, which was significantly better than those with regional or distant disease. Surgical resection was associated with a significant survival advantage for anorectal melanoma patients with either localized or regional disease, while this benefit was not seen for patients with distant metastases. Regarding different surgical types (LE or ER), no significant prognostic difference was found between the ER and LE subgroups in patients with either regional or distant disease. However, our results demonstrated that ER could significantly increase both OS and DSS in patients with localized anorectal melanoma when compared to LE.
In 1995, a retrospective study of 74 cases from a single institution found that abdominoperineal resection was associated with a higher chance of long-term survival for patients with localized anorectal melanoma, so the authors concluded that abdominoperineal resection should be considered in localized anorectal melanoma patients, especially those with smaller tumors and no evidence of nodal metastases (16). Some recent studies have drawn different conclusions. In 2021, Jutten et al. (17) reported their results of 71 localized anorectal melanoma patients and found no significant differences in survival between LE and ER procedures (25 vs. 21 months, p = 0.228). In the study, 10 of 48 patients (21%) who received LE originally finally underwent ER after a median of 4 months. In a recent meta-analysis, a total of 278 localized cases from 8 studies were analyzed, and the results showed no significant difference (OR = 1.30, 95% CI. 0.62 to 2.72, p = 0.49) in OS between the LE and ER groups (18). However, this study employed the odds ratio as a summary statistic for time-to-event outcomes, which has been questioned because not all patients had events, and the follow-up durations and individual patients in those studies were nonhomogeneous (19). Our study is by far the largest stratified study, and our results from 247 localized anorectal melanoma cases demonstrated that ER could significantly increase both OS and DSS (p = 0.009 and p = 0.041, respectively) compared to LE. Multivariate analysis showed that ER was an independent prognostic factor for OS (HR = 0.627; p = 0.03) and DSS (HR = 0.624; p = 0.044).
For patients with regional disease, although no survival difference was shown between the two surgery approaches, surgical treatment significantly improved both OS and DSS when compared to nonsurgical patients. A previous study found that metastasis to locoregional lymph nodes was a major prognostic factor (11). Theoretically, lymphadenectomy with extensive excision (ER) can help improve local control. However, consistent with all currently available studies, our results indicated that ER could not bring survival benefit for patients with regional anorectal melanoma (20–25). We believe this is totally due to the lack of effective systemic therapies for this disease. In our study, radiotherapy and chemotherapy both failed to improve the prognosis of patients. In recent years, the prognosis of many solid tumors has been significantly improved, which is mainly attributed to the progress of systemic therapy, including chemotherapy, targeted therapy and immunotherapy. With the help of effective systemic therapy, the role of surgery is to improve local cancer control. For anorectal melanoma, without the help of effective systemic therapy to ensure the overall prognosis, ER and lymphadenectomy have a very limited impact on survival, leading to a poor prognosis similar to that of patients undergoing LE.
For the same reason, patients with localized anorectal melanoma might benefit from ER. Currently, extensive surgery may be the only effective treatment that helps control occult regional metastasis, which could possibly have occurred beyond imaging and histological detection. Moreover, for patients with advanced (metastatic) disease, any extent of surgery could not improve the prognosis. Hopefully, in the near future, effective systematic treatments can be developed, so patients could be treated with a smaller extent of surgery to achieve a better prognosis. However, at present, it is still essential to individualize the surgical strategy for anorectal melanoma patients according to accurate preoperative staging.
Our study has several limitations. First, the SEER database lacks information on the resection margin status, recurrence, and postoperative complications, which have been reported to be linked to prognosis in previous studies (26–28). Second, this is a retrospective analysis based on the SEER database; hence, there will be some selection bias. In the localized and regional groups, for example, we discovered that patients who choose ER were younger; despite using multifactor analysis to compensate for the influence of age, this bias still persisted. Third, the results of this study lack external data validation. Therefore, a randomized controlled clinical study based on accurate preoperative staging is mandatory in the future.
In conclusion, anorectal melanoma is a rare tumor with a dismal prognosis. Currently, ER can benefit the prognosis of patients with localized disease but not for patients with regional disease. If effective systemic therapies could be developed in the future, the prognostic value of ER for patients with localized and regional metastases should be reassessed.
The data analyzed in this study was obtained from the National Institutes of Health (NIH) National Cancer institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program, the following licenses/restrictions apply: To request access to the SEER Incidence Databases, users must register through either the Institutional or Non-Institutional Account option. Requests to access these datasets should be directed to SEER, https://seerdataaccess.cancer.gov/seer-data-access.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Contributions: (1) CL: Conception and design, Administrative support, Collection and assembly of data, Collection and assembly of data, Manuscript writing, Final approval of manuscript; (2) JBZ: Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript; (3) CPT: Provision of study materials or patients, Manuscript writing, Final approval of manuscript; (4) PZ: Manuscript writing, Final approval of manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.997169/full#supplementary-material.
1. Chen H, Cai Y, Liu Y, He J, Hu Y, Xiao Q, et al. Incidence, surgical treatment, and prognosis of anorectal melanoma from 1973 to 2011: a population-based SEER analysis. Medicine (Baltimore). (2016) 95(7):e2770. doi: 10.1097/MD.0000000000002770
2. Trzcinski R, Kujawski R, Mik M, Sygut A, Dziki L, Dziki A. Malignant melanoma of the anorectum–a rare entity. Langenbecks Arch Surg. (2010) 395(6):757–60. doi: 10.1007/s00423-009-0586-5
3. Pessaux P, Pocard M, Elias D, Duvillard P, Avril MF, Zimmerman P, et al. Surgical management of primary anorectal melanoma. Br J Surg. (2004) 91(9):1183–7. doi: 10.1002/bjs.4592
4. Belli F, Gallino GF, Lo VS, Mariani L, Poiasina E, Leo E. Melanoma of the anorectal region: the experience of the National Cancer Institute of Milano. Eur J Surg Oncol. (2009) 35(7):757–62. doi: 10.1016/j.ejso.2008.05.001
5. Paolino G, Didona D, Macri G, Calvieri S, Mercuri SR. Anorectal melanoma. In: Scott JF, Gerstenblith MR, editors. Noncutaneous Melanoma [Internet]. Brisbane (AU): Codon Publications; (2018) Chapter 6. doi: 10.15586/codon.noncutaneousmelanoma.2018.ch6
6. de Meira JJ, Sobrado LF, Guzela VM, Nahas SC, Sobrado CW. Anorectal mucosal melanoma: a case report and literature review. Am J Case Rep. (2021) 22:e933032. doi: 10.12659/AJCR.933032
7. Meguerditchian AN, Meterissian SH, Dunn KB. Anorectal melanoma: diagnosis and treatment. Dis Colon Rectum. (2011) 54(5):638–44. doi: 10.1007/DCR.0b013e31820c9b1b
8. Biyikoglu I, Ozturk ZA, Koklu S, Babali A, Akay H, Filik L, et al. Primary anorectal malignant melanoma: two case reports and review of the literature. Clin Colorectal Cancer. (2007) 6(7):532–5. doi: 10.3816/CCC.2007.n.020
9. Callahan A, Anderson WF, Patel S, Barnholtz-Sloan JS, Bordeaux JS, Tucker MA, et al. Epidemiology of Anorectal Melanoma in the United States: 1992 to 2011. Dermatol Surg. (2016) 42(1):94–9. doi: 10.1097/DSS.0000000000000579
10. Yap LB, Neary P. A comparison of wide local excision with abdominoperineal resection in anorectal melanoma. Melanoma Res. (2004) 14(2):147–50. doi: 10.1097/00008390-200404000-00012
11. Ciarrocchi A, Pietroletti R, Carlei F, Amicucci G. Extensive surgery and lymphadenectomy do not improve survival in primary melanoma of the anorectum: results from analysis of a large database (SEER). Colorectal Dis. (2017) 19(2):158–64. doi: 10.1111/codi.13412
12. Falch C, Mueller S, Kirschniak A, Braun M, Koenigsrainer A, Klumpp B. Anorectal malignant melanoma: curative abdominoperineal resection: patient selection with 18F-FDG-PET/CT. World J Surg Oncol. (2016) 14(1):185. doi: 10.1186/s12957-016-0938-x
13. Ford MM, Kauffmann RM, Geiger TM, Hopkins MB, Muldoon RL, Hawkins AT. Resection for anal melanoma: is there an optimal approach? Surgery. (2018) 164(3):466–72. doi: 10.1016/j.surg.2018.05.026
14. Kaya S, Kement M, Altuntas YE, Altin O, Seker A, Mazmanoglu S, et al. Anal melanoma: outcomes of current surgical approaches. Niger J Clin Pract. (2018) 21(12):1622–6. doi: 10.4103/njcp.njcp_254_18
15. Abbas JS, Karakousis CP, Holyoke ED. Anorectal melanoma: clinical features, recurrence and patient survival. Int Surg. (1980) 65(5):423–6.7451062
16. Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum. (1995) 38(2):146–51. doi: 10.1007/BF02052442
17. Jutten E, Kruijff S, Francken AB, van Westreenen HL, Wevers KP. Survival following surgical treatment for anorectal melanoma seems similar for local excision and extensive resection regardless of nodal involvement. Surg Oncol. (2021) 37:101558. doi: 10.1016/j.suronc.2021.101558
18. Jutten E, Kruijff S, Francken AB, Lutke HM, van Leeuwen BL, van Westreenen HL, et al. Surgical treatment of anorectal melanoma: a systematic review and meta-analysis. BJS Open. (2021) 5(6):zrab107. doi: 10.1093/bjsopen/zrab107
19. Kazi M, Chatterjee A, Saklani A. Comment on: surgical treatment of anorectal melanoma: a systematic review and meta-analysis. BJS Open. (2022) 6(1):zrac015. doi: 10.1093/bjsopen/zrac015
20. Matsuda A, Miyashita M, Matsumoto S, Takahashi G, Matsutani T, Yamada T, et al. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma: a systematic review. Ann Surg. (2015) 261(4):670–7. doi: 10.1097/SLA.0000000000000862
21. Smith HG, Glen J, Turnbull N, Peach H, Board R, Payne M, et al. Less is more: a systematic review and meta-analysis of the outcomes of radical versus conservative primary resection in anorectal melanoma. Eur J Cancer. (2020) 135:113–20. doi: 10.1016/j.ejca.2020.04.041
22. Yeh JJ, Shia J, Hwu WJ, Busam KJ, Paty PB, Guillem JG, et al. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann Surg. (2006) 244(6):1012–7. doi: 10.1097/01.sla.0000225114.56565.f9
23. Menon H, Patel RR, Cushman TR, Amini A, Seyedin SN, Adams AC, et al. Management and outcomes of primary anorectal melanoma in the United States. Future Oncol. (2020) 16(8):329–38. doi: 10.2217/fon-2019-0715
24. Pei W, Zhou H, Chen J, Liu Q. Treatment and prognosis analysis of 64 cases with anorectal malignant melanoma. Zhonghua Wei Chang Wai Ke Za Zhi. (2016) 19(11):1305–8. doi: 10.3760/cma.j.issn.1671-0274.2016.11.021
25. Iddings DM, Fleisig AJ, Chen SL, Faries MB, Morton DL. Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patients? Ann Surg Oncol. (2010) 17(1):40–4. doi: 10.1245/s10434-009-0705-0
26. Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. (2007) 56(5):828–34. doi: 10.1016/j.jaad.2006.06.017
27. Henderson MA, Burmeister BH, Ainslie J, Fisher R, Di Iulio J, Smithers BM, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. (2015) 16(9):1049–60. doi: 10.1016/S1470-2045(15)00187-4
Keywords: anorectal melanoma, local resection, extensive resection, localized cases, survival
Citation: Liu C, Tang C, Zhang J and Zhu P (2022) Extensive resection improves overall and disease-specific survival in localized anorectal melanoma: A SEER-based study. Front. Surg. 9:997169. doi: 10.3389/fsurg.2022.997169
Received: 18 July 2022; Accepted: 10 August 2022;
Published: 30 August 2022.
Edited by:
Pietro Fransvea, Fondazione Policlinico Universitario A. Gemelli, IRCCS, ItalyReviewed by:
Giuseppe Tropeano, Agostino Gemelli University Polyclinic (IRCCS), Italy© 2022 Liu, Tang, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhu enVwZW5nQGNxbXUuZWR1LmNu
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.