- 1Department of Breast Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Breast / Thyroid Surgery, Jinhua Municipal Central Hospital, Jinhua, China
Background: The association between tumor location and breast cancer prognosis has been controversial. We sought to explore the relationship between tumors located in central and nipple portion (TCNP) and Chinese breast cancer.
Patients and methods: A total of 1,427 breast cancer patients were recruited. There were 328 cases of TCNP and 1,099 cases of tumors in the breast peripheral quadrant (TBPQ). The chi-square test was used to compare different variables between TCNP and TBPQ groups. A one-to-one propensity score matching (PSM) was applied to construct a matched sample consisting of pairs of TCNP and TBPQ groups. Kaplan–Meier curves were used for survival analysis of disease-free survival (DFS), breast cancer-specific survival (BCSS) and overall survival (OS). The Cox proportional hazards regression model was applied to identify prognostic risk factors.
Results: The median follow-up time was 58 months. Compared to TBPQ, TCNP patients had significantly larger tumor size, more frequent metastasis to lymph nodes (LN) and more proportions of TNM stage II–III. DFS, OS and BCSS rates were markedly lower in the TCNP group as compared to the TBPQ group before and after PSM (all p < 0.05). Multivariate Cox analysis showed that TCNP was an independent prognostic factor for breast cancer. Subgroup analysis indicated that for breast molecular subtypes and TNM stage II-III breast cancer, TCNP were related to worse prognosis. Multivariate logistic regression revealed that TCNP was an independent contributing factor for LN metastasis.
Conclusion: In Chinese breast cancer, compared to TBPQ, TCNP is associated with more LN metastasis and poorer prognosis.
Introduction
Breast cancer is the most common malignant tumor in women, which threaten to women's health seriously. In recent years, the importance of active screening and early diagnosis have been well acknowledged in the field which significantly improved the prognosis of breast cancer (1). However, breast cancer is still one of the main causes of female death, especially in developing countries, even more than lung cancer (2). Therefore, it is critical to identify prognostic risk factors of breast cancer so proper treatment strategy can be timely adjusted.
Studies have shown that many clinicopathological factors affected the prognosis of breast cancer patients, such as age, tumor size, body mass index (BMI), TNM stage, histological grade, lymph node (LN) status, Ki67, hormone receptor and human epidermal growthfactor receptor 2 (HER2) expressions (3–6). In recent years, molecular typing and gene detection have gradually entered the clinical practice to guide the precise treatment of breast cancer and improve the prognosis of breast cancer patients (7–9). However, the prognosis of breast cancer patients with similar clinicopathological characteristics can be varied even though receiving the same treatment, indicating that there are still many other factors affecting the prognosis.
Breast cancer most commonly locates at the upper outer quadrant and rarely at the lower inner quadrant of the breast. Previous studies have confirmed that the location of the primary mass of breast cancer could affect the prognosis of patients (10–14). Typically, tumor located at the medial quadrant was associated with worse prognosis, and the inner lower quadrant location was considered as an independent risk factor for prognosis in breast cancer patients (15–17). It's well-recognized that axillary lymph node (ALN) metastasis is of predictive clinical significance for survival of breast cancer. And the possibility of ALN metastasis might be related to the primary location of tumor. Bevilacqua et al. suggested that the incidence of ALN metastasis is 32.3% when tumor is in central position, higher than tumor of upper-inner-quadrant (20.6%) (18). Japanese researchers investigated a cohort of 313 cases and found that T1 and T2 breast cancer patients with a tumor located closer to the nipple have a higher risk of sentinel lymph node (SLN) metastases (19). However, with regard to the effect of tumors located in central and nipple portion (TCNP) in Chinese breast cancer patients, few studies have been reported.
The purpose of this study was to investigate the clinicopathological characteristics of TCNP and to uncover its prognosis value in Chinese patients.
Patients and methods
Study population
The clinical and pathological information of 1,517 breast cancer patients who admitted to our department from December 2014 to December 2018 were collected. In this study, TCNP was defined as the tumor whose center was within 2 cm of the nipple, including the nipple-areola complex. We choose a 2 cm margin because it has been indicated that tumors within this zone involve the nipple-areolar complex in up to 50% of patients (20, 21). Tumors in the breast peripheral quadrant were defined as TBPQ (including upper outer quadrant, upper inner quadrant, lower outer quadrant, lower inner quadrant, but overlapping sites were excepted). The inclusion criteria were as follows: (1) females aged over 18 years old; (2) breast cancer as the first and only malignant primary tumor; (3) unilateral breast cancer; (4) defined tumour location (TCNP or TBPQ); (5) tumors must located in the same quadrant; (6) American Joint Committee on Cancer stages (the seventh AJCC System) TNM stage I–III; (7) breast molecular subtype (luminal A, luminal B, HER2 enriched, and triple-negative) (22). (8) known of estrogen receptor (ER); progesterone receptor (PR); HER2 expression; ki67; histological grade; tumor size and LN metastasis status; surgery type; radiation/chemotherapy information; (9) active follow-up. The exclusion criteria were: (1) male; (2) bilateral breast cancer; (3) occult breast cancer; (4) tumors were not clearly located and (or) involved two or more quadrants; (5) stage IV breast cancer at diagnosis; (6) patients who received neoadjuvant chemotherapy; (7) people with incomplete clinical or pathological information. According to the inclusion and exclusion criteria, 1,427 cases of breast cancer were finally enrolled, including 328 cases of TCNP and 1,099 cases of TBPQ. All patients have signed informed consent.
Statistical analysis
Disease-free survival (DFS) was the primary end point. Overall survival (OS) and breast cancer-specific survival (BCSS) were the secondary end points. DFS was defined as the time from date of diagnosis to local or regional recurrence, distant organ metastasis, contralateral breast cancer, death or last follow-up. OS was defined as the time from diagnosis to death or last follow-up. BCSS was defined as the time from diagnosis to the death caused by breast cancer. Chi-square test was used to compare the clinical and pathological factors between TCNP and TBPQ groups. A one-to-one propensity score matching (PSM) analysis was carried out to balance the differences in baseline covariates, and we set the match tolerance as 0.02. Kaplan–Meier curves were applied to measure the DFS, BCSS, and OS between TCNP and TBPQ, and the differences were determined by log-rank test. Cox proportional hazard models were used to estimate risk ratios for prognostic factors. Logistic regression was applied to present the relationship between tumor location and LN metastasis. Two-sided p < 0.05 was considered statistically significant. All statistical analysis was completed by SPSS 26.0.
Results
Clinicopathological features of TCNP and TBPQ
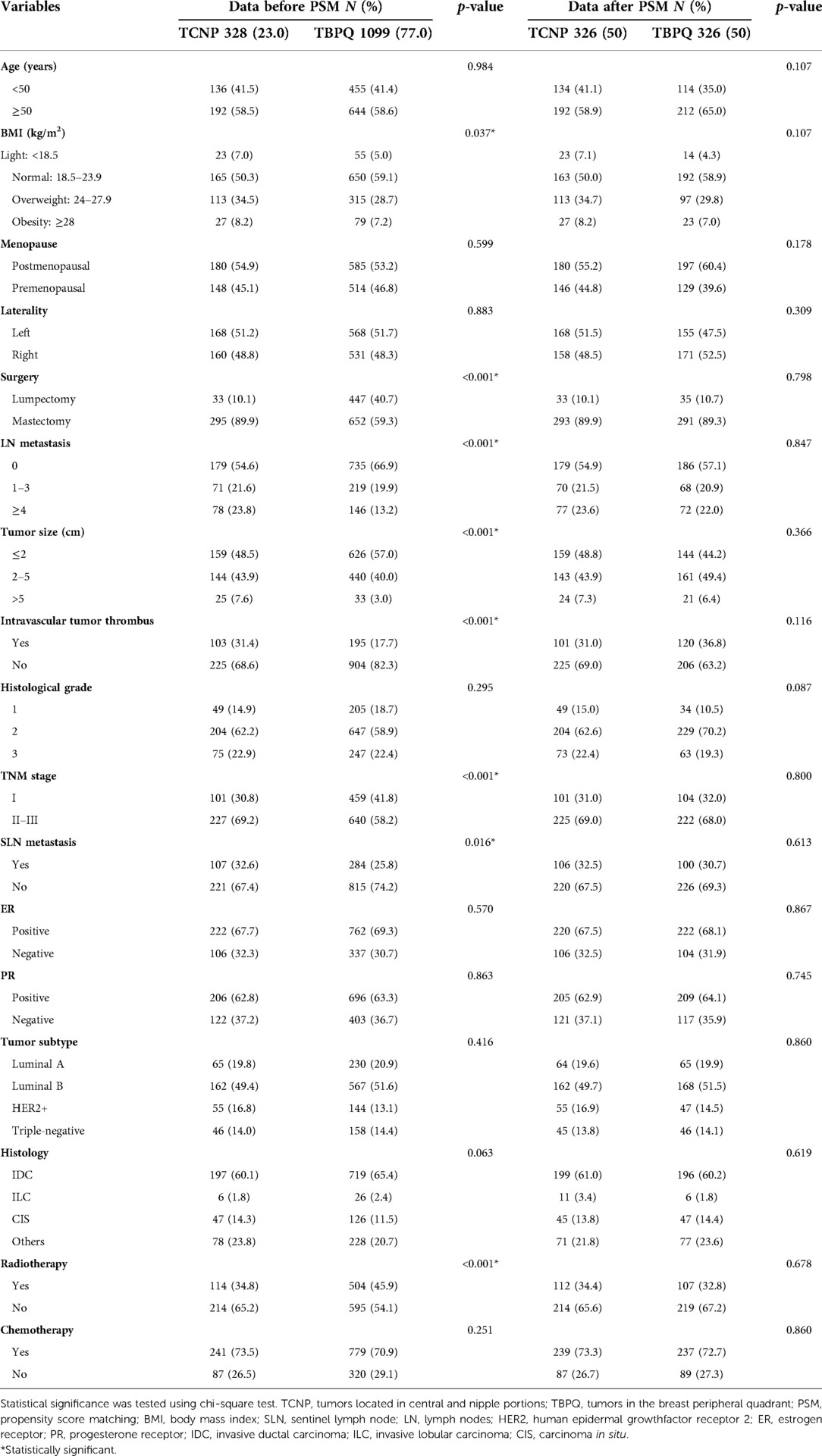
As mentioned in above, a total of 1,427 breast cancer patients were eventually enrolled in this study with a median follow-up time of 58 months. The results before PSM indicated that compared to the TBPQ group, patients in the TCNP group had significantly larger tumor size (43.9% vs. 40.0% for tumor size of 2–5 cm; 7.6% vs. 3.0% for tumor size of >5 cm, p < 0.001), higher BMI (overweight and obese group: 42.7% vs. 35.9%, p = 0.037), higher rate of SLN metastasis (32.6% vs. 25.8%, p = 0.016), more frequent LN metastasis (1–3: 21.6% vs. 19.9%; ≥4: 23.8% vs. 13.2%, p < 0.001) more intravascular tumor thrombus (31.4% vs. 17.7%, p < 0.001) and more patients with advanced stages (TNM stage II–III) (69.2% vs. 58.2%, p < 0.001). The results also showed that more TCNP patients underwent mastectomy (89.9% vs. 59.3%, p < 0.001) but less of them received radiotherapy (34.8% vs. 45.9%, p < 0.001) (Table 1). These results reminded us that TCNP has unique clinical features which differ from TBPQ.
After 1:1 matching, 326 patients in the TCNP group were matched as compared with 326 patients in the TBPQ group. There were no notable differences between the two groups after PSM (Table 1).
Survival analysis between TCNP and TBPQ
The Kaplan–Meier curves were used to assess the differences of DFS, BCSS and OS between two groups. Before PSM, the 5-year DFS rates of patients with TCNP were significantly lower than those of TBPQ population (78.3% vs. 90.7%, p < 0.001) (Figure 1A). Next, we discovered that the 5-years OS rates of patients in the TCNP group were significantly worse than those of patients in the TBPQ group (82.4% vs. 94.5%, p < 0.001) (Figure 1B). Such difference was also observed in BCSS between TCNP and TBPQ (83.3% vs. 95.4%, p < 0.001) (Figure 1C). Similarly, we still found the notable difference between the two groups after PSM. The survival analysis of the matched groups showed that TCNP exhibited worse outcomes for DFS (5-year DFS: 78.5% vs. 86.3%, p = 0.032) (Figure 2A), OS (5-year OS: 85.4% vs. 92.1%, p = 0.0215) (Figure 2B) and BCSS (5-year BCSS: 86.2% vs. 92.8%, p = 0.018) (Figure 2C).

Figure 1. Kaplan–Meier survival curves of DFS (A), OS (B) and BCSS (C) between TCNP and TBPQ before PSM. DFS, disease-free survival; BCSS, breast cancer specific survival; OS, overall survival; PSM, propensity score matching.

Figure 2. Kaplan–Meier survival curves of DFS (A), OS (B) and BCSS (C) between TCNP and TBPQ after PSM. DFS, disease-free survival; BCSS, breast cancer specific survival; OS, overall survival; PSM, propensity score matching.
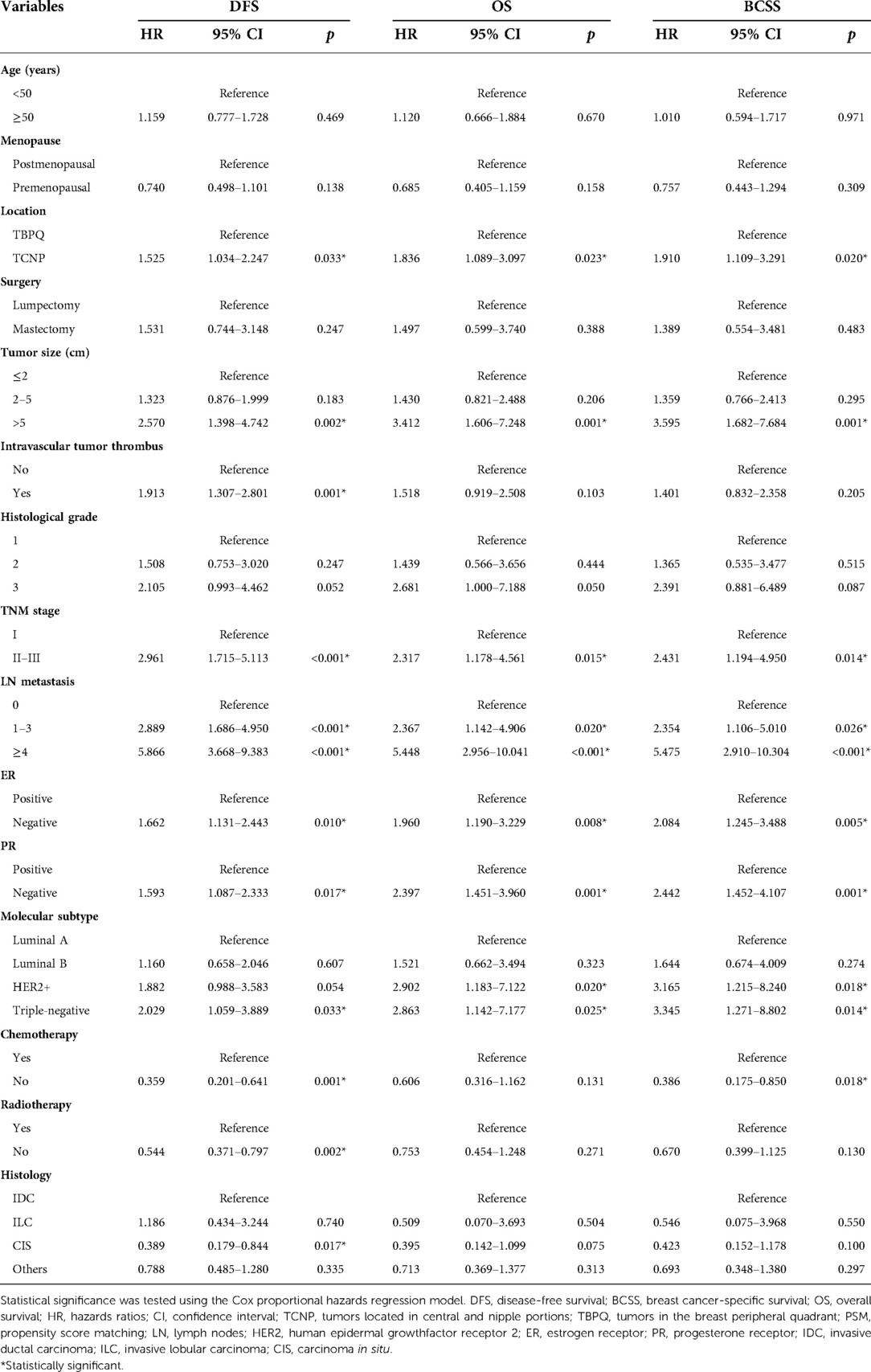
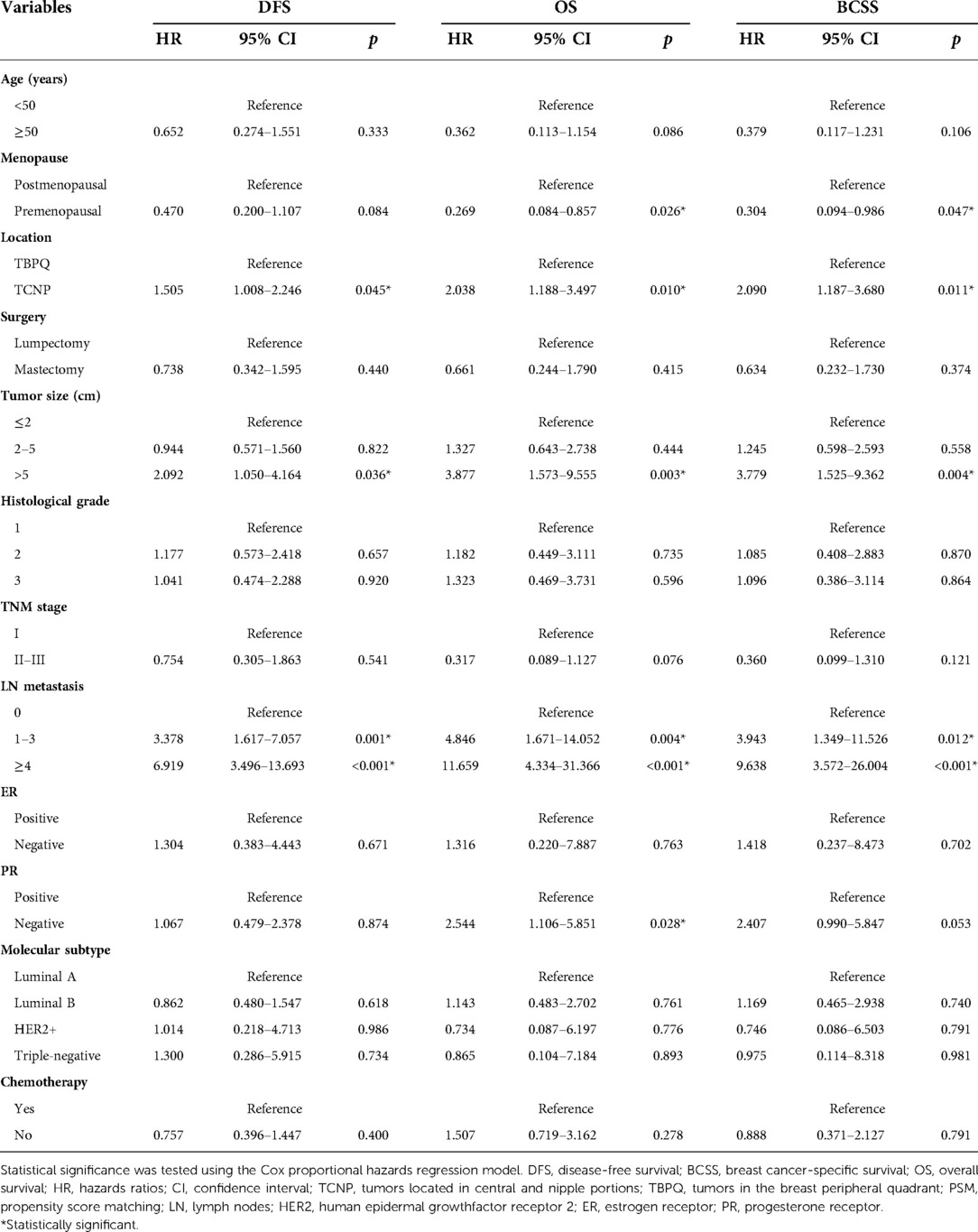
Furthermore, the Cox proportional hazards regression model were used to identify risk factors of DFS, OS and BCSS. Univariable Cox regression analysis presented that TCNP or TBPQ location, tumor size, intravascular tumor thrombus, TNM stage, LN metastasis, ER, PR, molecular subtype, chemotherapy, radiotherapy and histology were all associated with DFS. The significant predictors of OS were TCNP or TBPQ location, tumor size, TNM stage, LN metastasis, ER, PR and molecular subtype. Similarly, TCNP or TBPQ location, tumor size, TNM stage, LN metastasis, ER, PR, molecular subtype and chemotherapy were responsible for BCSS (Table 2). To balance the effect of factors, we further included the covariates that were clinically worth exploring or had p < 0.05 in the univariate Cox analysis into the multivariate Cox analysis. The results suggested that TCNP or TBPQ location, tumor size and LN metastasis were independent predictors of DFS (all p < 0.05), while menopause, TCNP or TBPQ location, tumor size, LN metastasis and PR were significant independent predictors of OS (all p < 0.05). Also, we found menopause, TCNP or TBPQ location, tumor size and LN metastasis were visibly associated with BCSS (all p < 0.05). TCNP was finally validated to be an independent risk factor for breast cancer prognosis (DFS: hazard ratios = 1.505, 95% CI: 1.008–2.246, p = 0.045; OS: hazard ratios = 2.038, 95% CI: 1.188–3.497, p = 0.010; BCSS: hazard ratios = 2.090, 95% CI: 1.187–3.680, p = 0.011) (Table 3).
Subgroup analysis
Now that TCNP was verified to be significantly negatively correlated with DFS, OS and BCSS of breast cancer, we were interested to further evaluated the potential prognostic value of TCNP in subgroups. Patients in TCNP or TBPQ were further stratified based on important clinical features. For patient with TNM stage II–III, we found that TCNP was a worse prognostic indicator for DFS (p = 0.001), OS (p < 0.001) and BCSS (p < 0.001) (Figure 3). Noticeable difference could also be seen in subgroups of breast molecular subtypes. For luminal B, HER2 enriched and triple-negative breast cancer, TCNP patients had decreased DFS, OS and BCSS as compared to TBPQ patients (all p < 0.05) (Figures 4D–L). Although prognostic value of TCNP for OS in luminal A patients had no significance (p = 0.166) (Figure 4B), TCNP still gave visible prognostic value for predicting poorer DFS (p < 0.001) and BCSS (p = 0.022) in luminal A subgroup (Figures 4A,C). Furthermore, we performed a detailed analysis on prognosis of lateral and medial breast cancers. There were 681 patients with tumors in the lateral quadrant and 418 patients with tumors in the medial quadrant. Compared to the medial group, the group of lateral location were older 62.1% vs. 52.9% for age ≥50 years, p = 0.003) and had more proportion of postmenopausal patients (57.0% vs. 47.1%, p = 0.001), larger tumor size (45.7% vs. 38.7% for tumor size of >2 cm, p = 0.048), more SLN (28.0% vs. 22.2%, p = 0.033) and LN metastases (≥4: 15.4% vs. 9.6%, p = 0.019). Besides, TNM stage was more advanced in lateral group (61.1% vs. 53.6% for TNM stage II–III, p = 0.014). Survival analysis demonstrated that no significant differences were seen for DFS, OS and BCSS between the two groups before and after PSM (all p > 0.05) (see Supplementary Material for details).

Figure 3. Kaplan–Meier survival curves of DFS (A), OS (B) and BCSS (C) between TCNP and TBPQ in TNM stage II–III subgroup. DFS, disease-free survival; BCSS, breast cancer specific survival; OS, overall survival.

Figure 4. Kaplan–Meier survival curves of DFS, OS and BCSS between TCNP and TBPQ in different breast cancer molecular subtypes. DFS (A), OS (B) or BCSS (C) between TCNP and TBPQ in luminal A breast cancer. DFS (D), OS (E) or BCSS (F) between TCNP and TBPQ in luminal B breast cancer. DFS (G), OS (H) or BCSS (I) between TCNP and TBPQ in HER2 enriched breast cancer. DFS (J), OS (K) or BCSS (L) between TCNP and TBPQ in triple-negative breast cancer. DFS, disease-free survival; BCSS, breast cancer specific survival; OS, overall survival.
TCNP and LN metastasis
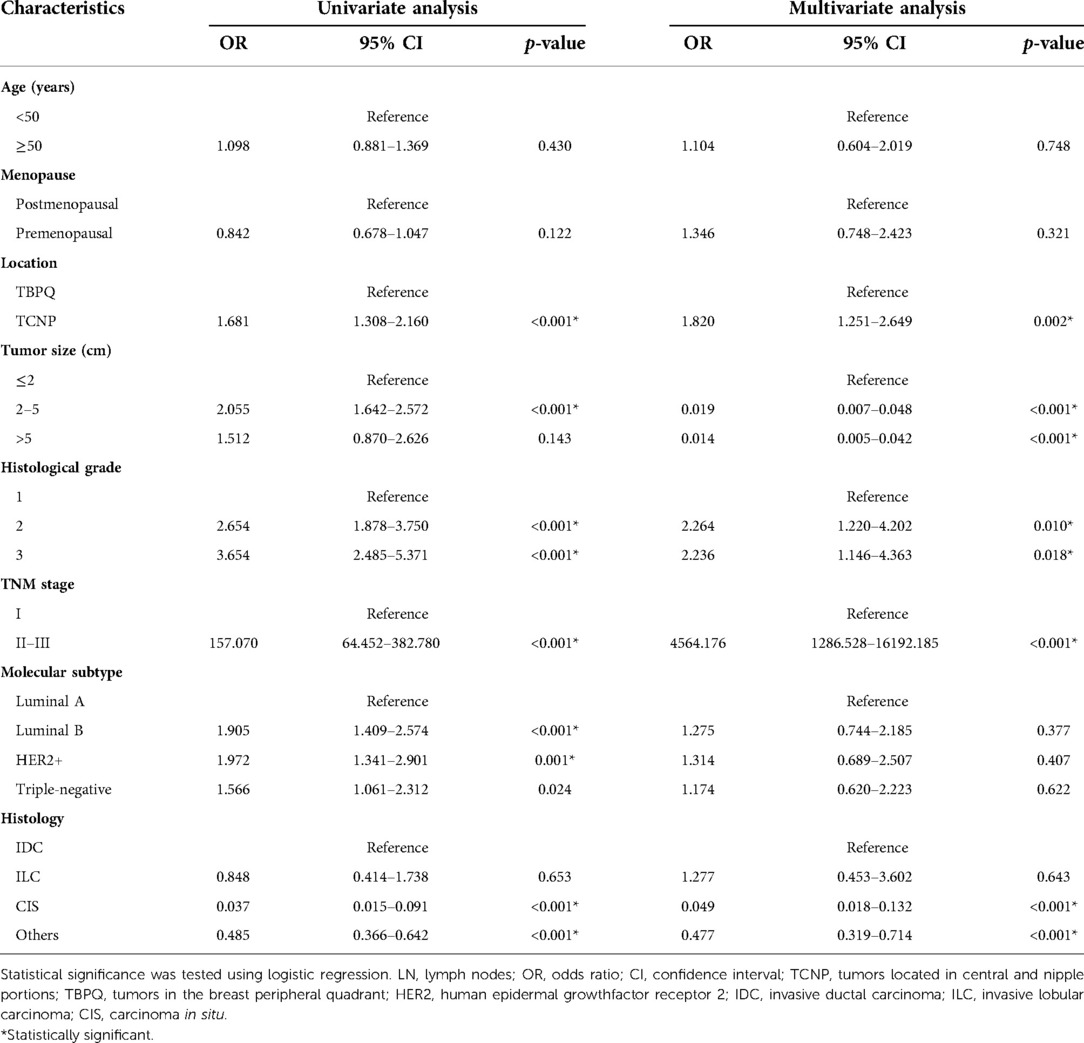
In addition, we analyzed the correlation between unfavorable prognosis of TCNP patients and LN metastasis. Univariate logistic analysis was performed and significant (p < 0.05) variables (TCNP or TBPQ location, tumor size, histological grade, TNM stage, molecular subtype and histology) were further incorporated into the multivariate logistic regression analysis. TCNP was finally proved to be more susceptible to LN metastasis than TBPQ (OR = 1.820, 95% CI: 1.251–2.649, p = 0.002) (Table 4).
Discussion
Although several studies have shown that the location of the primary tumor has an important effect on prognosis, it has not been well adopted in clinic as a prognostic risk factor (23–28). Therefore it is critical to better understand the association between location of the primary tumor and its influence on the disease outcome of breast cancer in order to develop specific treatment in the future. TCNP is a relatively unique site, and has been rarely studied in the field, especially in Asian populations. In this retrospective study of 1,427 cases, we uncovered that among Chinese population, TCNP presents distinct clinicopathological features and worse prognosis than TBPQ.
First of all, our results showed that compared to patients in TBPQ group, patients from TCNP group were accompanied with larger tumor sizes (>2 cm), higher BMI, higher rates of LN metastasis, more advanced TNM stages (II-III) and more intravascular tumor thrombus. These unfavorable clinical characteristics of TCNP might contribute to its larger tumor burden and worse survival outcomes than TBPQ. On the one hand, TCNP patients had higher BMI and more adipose tissue especially in the breast might increase the difficulty for early detection of the mass when undergo the B-ultrasonic examination; on the other hand, during mammography, excessive x-ray penetration in the nipple-areola complex reduced the accuracy of the examination (29).
The prognostic value of primary tumor site is currently highly debated and remains unclear. Siotos et al. suggested that the site of the primary tumor might be an important feature affecting the prognosis of breast cancer in a whole cohort of 5,295 patients (10). Rummel et al. reported that although tumours in the central region were associated with less favourable outcome, these associations were not independent of location but rather driven by larger tumour size (30). Wu et al. found that in Chinese women with T1-2N0M0 breast cancer, the inner and lower quadrant was an independent risk factor for DFS and OS, while tumor in the central region had no prognostic value (31). Another study showed that in invasive ductal breast carcinoma, patients with tumors in the central and medial quadrants had significantly increased risk of recurrence, metastasis, and death compared to patients with tumors in other quadrants (32). However, in our study, the prognosis for lateral breast cancer was similar to that for medial breast cancer, which was consistent with the previous study by Jayasinghe et al. (33). Many factors, for example, LN metastasis, TCNP or TBPQ location, tumor size, TNM stage, ER, PR and molecular subtype were associated with DFS, OS and BCSS in univariate analysis. After multivariate analysis, TCNP was substantiated as an independent risk predictor over TBPQ for both DFS, OS and BCSS. The survival analysis revealed that among Chinese female patients, the DFS, BCSS and OS rates of TCNP were significantly lower than those of TBPQ. The findings of our research support another recent investigation which suggested that central breast cancer has poorer BCSS and OS than non-central breast cancer based on population from SEER database (34).
Further subgroup analysis demonstrated that TCNP was a poor prognostic indicator of DFS, OS and BCSS in Chinese patients with luminal B, HER2 enriched, triple-negative and TNM stage II–III breast cancer. As for luminal A subtype, TCNP still had power of predicting worse DFS and BCSS. These findings strongly supported that TCNP served as a robust indicator of poor prognosis among Chinese breast cancer patients.
The mechanism underlying poor prognosis for TCNP may be as follows. Firstly, tumors of TCNP are easily missed and delayed in diagnosis as mentioned above, which can affect treatment. Secondly, higher rate of ALN metastasis in TCNP is another leading cause for decreased survival. The number of ALN that metastases of breast cancer is of predictive clinical significance (35) and Involvement of ALN has been believed to be accountable for increasing breast cancer recurrence and mortality (36, 37). Those who initially presented with ALN metastases usually received worse survival after recurrence (38, 39). To date, a clear association between LN metastasis and lumps located near the nipple and areola has not been establish, yet many scholars hold a supportive attitude. A landmark study by Ansari et al. demonstrated that for every 1 cm decrease in the distance between the tumor and the nipple, the likelihood of LN positivity increases by 23% (40). A recent study by Yang et al. revealed that tumour-nipple distance was an independent predictor of ALN involvement. In LN-positive patients, the tumour-nipple distance was smaller (41). In this study, TCNP was found to have a higher proportion of LN metastasis than TBPQ. Through further univariate and multivariate logistic regression analysis, we finally elucidated that TCNP was an independent indicator for LN metastasis. Our result is in corroboration with the previous findings. Last but not least, the role of internal mammary lymph node (IMLN) in TCNP cannot be overlooked. IMLN is the second largest lymphatic drainage of breast cancer, after the ALN (42). It is generally believed that medial and central tumors more often drain to the IMLN than other quadrants and IMLN metastasis is always found concomitantly with ALN metastasis. A large sample retrospective study by Huang et al. indicated that the incidence of IMLN metastasis was 4.4%, 18.8%, 28.1%, and 41.5% for patients with negative ALN, 1–3 positive ALN, 4–6 positive ALN, seven or more positive ALN, respectively (43). The status of IMLN is also an important factor for determining the clinical stage, treatment strategy and prognosis of breast cancer patients. Veronesi et al. retrospectively analyzed 1,085 patients and showed that patients with ALN metastases only or with IMLN metastases only had similar prognosis, while patients with both axillary and internal mammary positive nodes had the worst prognosis (44). Although our study did not evaluate IMLN due to the deep anatomical location, small size of IMLN, low accuracy and sensitivity of the current used tracer and no consensus on the indications for IMLN biopsy and dissection, we successfully proved TCNP more prone to ALN metastases (even in subgroup of metastatic LN number ≥4). Therefore, we could logically assume that IMLN increases the adverse impact of TCNP on prognosis.
Our study has several important strengths. Firstly, this is a unique study that focused on the potential clinical value of TCNP for Chinese population. In China, the incidence rate of breast cancer has soared obviously in recent decades and our results represent real-world data that may be generalisable to routine clinical settings. Secondly, the relatively large sample size and long duration of follow up are other strengths of this analysis. Our results indicated that for TCNP patients, clinicians need to pay more attention to assessing the status of LN and improve preoperative evaluation comprehensively. Also, we suggest that it's necessary to consider whether the primary tumor site should be included in breast cancer staging guidelines.
This study has some limitations that should be noted. First, although the data is real and effective, this is a retrospective study from a single center and a selection bias cannot be entirely excluded. Second, evaluation of IMLN are not performed, therefore, we are unable to determine whether IMLN metastasize. More studies involving prospective and multicenter data collection are needed to confirm the clinical predicting value of tumor location in breast cancer patients of Asian/Chinese origin.
Summarily, the current study indicated that TCNP is an independent prognostic factor for Chinese breast cancer, which is correlated with impaired survival and more likely to have LN metastasis. Our findings fill the important gap in the literature by discovering TCNP's role in Chinese breast cancer population. We suggest that prompt diagnosis and effective treatment are needed for TCNP patients in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by The First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Q-DH and G-LG: contributed to the study design and revised the article. X-HW, J-YZ and Y-QL: collected the data. W-DF and K-KL: analyzed and interpreted the data. W-DF and X-HW: contributed to draft the article. W-DF: wrote the article. All authors contributed to the article and approved the submitted version
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province of China (LY19H160026) and Zhejiang Provincial Health Department (2019KY454).
Acknowledgments
We would like to thank the Natural Science Foundation of Zhejiang Province of China (LY19H160026) and Zhejiang Provincial Health Department (2019KY454), whose financial support has made this study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.993263/full#supplementary-material.
Abbreviations
TCNP, tumors located in central and nipple portions; TBPQ, tumors in the breast peripheral quadrant; PSM, propensity score matching; DFS, disease-free survival; BCSS, breast cancer-specific survival; OS, overall survival; BMI, body mass index; LN, lymph nodes; SLN, sentinel lymph node; ALN, axillary lymph node; HR, hazards ratios; AJCC, American Joint Committee on Cancer; HER2, human epidermal growthfactor receptor 2; ER, estrogen receptor; PR, progesterone receptor; OR, odds ratio; CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; CIS, carcinoma in situ
References
1. Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. (2019) 1152:1–7. doi: 10.1007/978-3-030-20301-6_1
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Liu Z, Sahli Z, Wang Y, Wolff AC, Cope LM, Umbricht CB. Young age at diagnosis is associated with worse prognosis in the luminal A breast cancer subtype: a retrospective institutional cohort study. Breast Cancer Res Treat. (2018) 172:689–702. doi: 10.1007/s10549-018-4950-4
4. Ayoub NM, Yaghan RJ, Abdo NM, Matalka II, Akhu-Zaheya LM, Al-Mohtaseb AH. Impact of obesity on clinicopathologic characteristics and disease prognosis in pre- and postmenopausal breast cancer patients: a retrospective institutional study. J Obes. (2019) 2019:3820759. doi: 10.1155/2019/3820759
5. Vila J, Teshome M, Tucker SL, Woodward WA, Chavez-MacGregor M, Hunt KK, et al. Combining clinical and pathologic staging variables has prognostic value in predicting local-regional recurrence following neoadjuvant chemotherapy for breast cancer. Ann Surg. (2017) 265:574–80. doi: 10.1097/SLA.0000000000001492
6. Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, Hall PF, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. (2011) 29:4014–21. doi: 10.1200/JCO.2010.32.6462
7. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. (2018) 379:111–21. doi: 10.1056/NEJMoa1804710
8. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. (2018) 379:2108–21. doi: 10.1056/NEJMoa1809615
9. Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. (2016) 17:791–800. doi: 10.1016/S1470-2045(16)00163-7
10. Siotos C, McColl M, Psoter K, Gilmore RC, Sebai ME, Broderick KP, et al. Tumor site and breast cancer prognosis. Clin Breast Cancer. (2018) 18:e1045–52. doi: 10.1016/j.clbc.2018.05.007
11. Kroman N, Wohlfahrt J, Mouridsen HT, Melbye M. Influence of tumor location on breast cancer prognosis. Int J Cancer. (2003) 105:542–5. doi: 10.1002/ijc.11116
12. Gill PG, Birrell SN, Luke CG, Roder DM. Tumour location and prognostic characteristics as determinants of survival of women with invasive breast cancer: South Australian hospital-based cancer registries, 1987–1998. Breast. (2002) 11:221–7. doi: 10.1054/brst.2001.0400
13. Levi F, Randimbison L, Te VC, La Vecchia C. Influence of tumor location on breast cancer prognosis. Int J Cancer. (2003) 107:683–4. doi: 10.1002/ijc.11418
14. Brandon L, Youl P, Baade P, Whiteside E, Kimlin M. Location of residence and breast cancer stage: a SEER population-based analysis. Breast J. (2015) 21:693–5. doi: 10.1111/tbj.12499
15. Hwang KT, Kim J, Kim EK, Jung SH, Sohn G, Kim SI, et al. Poor prognosis of lower inner quadrant in lymph node-negative breast cancer patients who received no chemotherapy: a study based on nationwide Korean breast cancer registry database. Clin Breast Cancer. (2017) 17:e169–84. doi: 10.1016/j.clbc.2016.12.011
16. Sarp S, Fioretta G, Verkooijen HM, Vlastos G, Rapiti E, Schubert H, et al. Tumor location of the lower-inner quadrant is associated with an impaired survival for women with early-stage breast cancer. Ann Surg Oncol. (2007) 14:1031–9. doi: 10.1245/s10434-006-9231-5
17. Yang J, Tang S, Zhou Y, Qiu J, Zhang J, Zhu S, et al. Prognostic implication of the primary tumor location in early-stage breast cancer: focus on lower inner zone. Breast Cancer. (2018) 25:100–7. doi: 10.1007/s12282-017-0797-5
18. Bevilacqua J, Cody H 3rd, MacDonald KA, Tan LK, Borgen PI, Van Zee KJ. A prospective validated model for predicting axillary node metastases based on 2,000 sentinel node procedures: the role of tumour location [corrected]. Eur J Surg Oncol. (2002) 28:490–500. doi: 10.1053/ejso.2002.1268
19. Minami S, Sakimura C, Irie J, Tokai Y, Okubo H, Ohno T. Predictive factors among clinicopathological characteristics for sentinel lymph node metastasis in T1-T2 breast cancer. Cancer Manag Res. (2021) 13:215–23. doi: 10.2147/CMAR.S284922
20. Fowble B, Solin LJ, Schultz DJ, Weiss MC. Breast recurrence and survival related to primary tumor location in patients undergoing conservative surgery and radiation for early-stage breast cancer. Int J Radiat Oncol Biol Phys. (1992) 23:933–9. doi: 10.1016/0360-3016(92)90897-q
21. Morimoto T, Komaki K, Inui K, Umemoto A, Yamamoto H, Harada K, et al. Involvement of nipple and areola in early breast cancer. Cancer. (1985) 55:2459–63. doi: 10.1002/1097-0142(19850515)55:10<2459::aidcncr2820551025>3.0.co;2-l
22. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the st. gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. (2011) 22:1736–47. doi: 10.1093/annonc/mdr304
23. Colleoni M, Zahrieh D, Gelber RD, Holmberg SB, Mattsson JE, Rudenstam CM, et al. Site of primary tumor has a prognostic role in operable breast cancer: the international breast cancer study group experience. J Clin Oncol. (2005) 23:1390–400. doi: 10.1200/JCO.2005.06.052
24. Zucali R, Mariani L, Marubini E, Kenda R, Lozza L, Rilke F, et al. Early breast cancer: evaluation of the prognostic role of the site of the primary tumor. J Clin Oncol. (1998) 16:1363–6. doi: 10.1200/JCO.1998.16.4.1363
25. Gaffney DK, Tsodikov A, Wiggins CL. Diminished survival in patients with inner versus outer quadrant breast cancers. J Clin Oncol. (2003) 21:467–72. doi: 10.1200/JCO.2003.12.047
26. Sohn VY, Arthurs ZM, Sebesta JA, Brown TA. Primary tumor location impacts breast cancer survival. Am J Surg. (2008) 195:641–4. doi: 10.1016/j.amjsurg.2007.12.039
27. Lohrisch C, Jackson J, Jones A, Mates D, Olivotto IA. Relationship between tumor location and relapse in 6,781 women with early invasive breast cancer. J Clin Oncol. (2000) 18:2828–35. doi: 10.1200/JCO.2000.18.15.2828
28. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:290–303. doi: 10.3322/caac.21393
29. Da Costa D, Taddese A, Cure ML, Gerson D, Jr PR, Esserman LE. Common and unusual diseases of the nipple-areolar complex. Radiographics. (2007) 27(Suppl 1):S65–77. doi: 10.1148/rg.27si075512
30. Rummel S, Hueman MT, Costantino N, Shriver CD, Ellsworth RE. Tumour location within the breast: does tumour site have prognostic ability? Ecancermedicalscience. (2015) 9:552. doi: 10.3332/ecancer.2015.552
31. Wu S, Zhou J, Ren Y, Sun J, Li F, Lin Q, et al. Tumor location is a prognostic factor for survival of Chinese women with T1-2N0M0 breast cancer. Int J Surg. (2014) 12:394–8. doi: 10.1016/j.ijsu.2014.03.011
32. Wang YT, Chen ZJ, Zhang D, Zhou H, Cao XC, Wang X. Effect of the primary tumor location on the prognosis of breast invasive ductal carcinoma patients treated with radical mastectomy. Zhonghua Zhong Liu Za Zhi (Chin J Oncol). (2019) 41:686–92. doi: 10.3760/cma.j.issn.0253-3766.2019.09.008
33. Jayasinghe UW, Boyages J. Tumour location is not an independent prognostic factor for survival following a diagnosis of breast cancer. Breast. (2009) 18:41–6. doi: 10.1016/j.breast.2008.10.004
34. Ji F, Xiao WK, Yang CQ, Yang M, Zhang LL, Gao HF, et al. Tumor location of the central and nipple portion is associated with impaired survival for women with breast cancer. Cancer Manag Res. (2019) 11:2915–25. doi: 10.2147/CMAR.S186205
35. Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest. (2011) 121:2000–12. doi: 10.1172/JCI44751
36. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
37. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. (2014) 383:2127–35. doi: 10.1016/S0140-6736(14)60488-8
38. Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. (1999) 17:2334–40. doi: 10.1200/JCO.1999.17.8.2334
39. Rose BS, Jiang W, Punglia RS. Effect of lymph node metastasis size on breast cancer-specific and overall survival in women with node-positive breast cancer. Breast Cancer Res Treat. (2015) 152:209–16. doi: 10.1007/s10549-015-3451-y
40. Ansari B, Morton MJ, Adamczyk DL, Jones KN, Brodt JK, Degnim AC, et al. Distance of breast cancer from the skin and nipple impacts axillary nodal metastases. Ann Surg Oncol. (2011) 18(11):3174–80. doi: 10.1245/s10434-011-1957-z
41. Yang J, Yang Q, Mukherjee A, Lv Q. Distance between the tumour and nipple as a predictor of axillary lymph node involvement in breast cancer. Cancer Manag Res. (2021) 13:193–9. doi: 10.2147/CMAR.S262413
42. Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer. (1983) 51:1941–3. doi: 10.1002/1097-0142(19830515)51:10<1941::aidcncr2820511032>3.0.co;2-t
43. Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. (2008) 107:379–87. doi: 10.1007/s10549-007-9561-4
Keywords: breast cancer, tumors located in central and nipple portions, tumors in the breast peripheral quadrant, lymph nodes metastasis, prognosis
Citation: Fu W-D, Wang X-H, Lu K-K, Lu Y-Q, Zhou J-Y, Huang Q-D and Guo G-L (2022) Real-world outcomes for Chinese breast cancer patients with tumor location of central and nipple portion. Front. Surg. 9:993263. doi: 10.3389/fsurg.2022.993263
Received: 13 July 2022; Accepted: 20 September 2022;
Published: 3 October 2022.
Edited by:
Zhijun Dai, Zhejiang University, ChinaReviewed by:
Jinhong Zhu, Harbin Medical University Cancer Hospital, ChinaMa Xiaobin, Second Affiliated Hospital of Xi'an Jiaotong University, China
© 2022 Fu, Wang, Lu, Lu, Zhou, Huang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-Di Huang Njk3NzgyODlAcXEuY29t Gui-Long Guo Z3VvZ3VpbG9uZ0BzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Wei-Da Fu1,†
Wei-Da Fu1,† Gui-Long Guo
Gui-Long Guo