- Department of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu, China
Background: The prognostic nutrition index (PNI), which has been evaluated in various kinds of cancers, offered a simple yet effective approach to predict the prognosis. The aim of this meta-analysis is to reveal the correlation between preoperative PNI and the prognosis of patients with pancreatic ductal adenocarcinoma (PDAC) who underwent curative resection.
Methods: We searched the PubMed, Embase, Web of Science and Cochrane Library databases, and extracted the hazard ratio (HR) with 95% confidential interval (CI) from eligible studies. The pooled HR with 95% CI was applied to evaluate the association between PNI and overall survival (OS), recurrence-free survival (RFS).
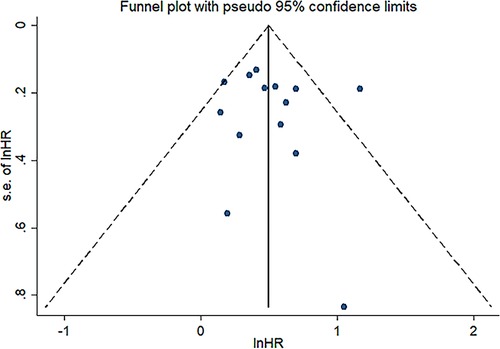
Results: A total of fourteen studies with 3,385 patients were included for meta-analysis. The results (the pooled HR: 1.664, 95% CI: 1.424–1.994, I² = 42.6%, p value = 0.046) indicated that low preoperative PNI was closely related to poor OS. In addition, the results suggested that PNI was negatively correlated with RFS (the pooled HR: 1.369, 95%CI: 1.080–1.734). The robustness of these pooled results was verified by our subgroup analysis and sensitivity analysis. Moreover, different cutoff values among studies are responsible for the heterogeneity of pooled HR of OS through meta-regression analysis (p value = 0.042). Funnel plots, Begg's test (p value = 0.228) and Egger’s test (p value = 0.702) indicated no significant publication bias in OS.
Conclusion: Preoperative PNI might be a promising marker to predict the prognosis of PDAC patients who underwent curative resection.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignant digestive system tumors with a 5-year survival rate of approximately 9% (1). Surgical resection is taken as the only curative therapy for PDAC, and the 5-year survival rate after radical resection is about 20% (2). Despite advancements in medical technology, the prognosis of PDAC is still very poor. Therefore, it is vital to identify a marker that can predict the prognosis for patients with PDAC.
An increasing number of studies have shown that inflammation and nutrition status play a significant role in oncogenesis, progression and metastasis (3–5). Inflammatory indices, such as neutrophil-to-lymphocyte ratio (NLR) (6), platelet-to-lymphocyte ratio (PLR) (7) and controlling nutritional status (CONUT) score (8), have been applied to predict the prognosis of patients with PDAC. Prognostic nutritional index (PNI) was initially reported by Buzby and colleagues in 1980, and it calculated as 158 – 16 (ALB) – 0.78 (TSF) – 0.20 (TFN) – 5.8 (DH). (ALB is serum albumin level (g/100 ml), TSF is triceps, skinfold (mm), TFN is serum transferrin level (mg/100 ml) and DH is delayed hypersensitivity reactivity to any of three recall antigens (mumps, streptokinase-streptodornase, candida) graded as 0, 1, 2) (9). Then in 1984, Onodera T. developed a relatively simple and convenient formula of PNI to assess the risk of postoperative complications and the prognosis of gastrointestinal cancer patients after surgery, which was 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (10). Subsequently, Onodera’s PNI was widely utilized to predict the prognosis of various cancers since 2010s, including gastric cancer (11), hepatocellular cancer (12), lung cancer (13), colorectal cancer (14–16), etc. A few studies have investigated the relationship between the PNI and the prognosis of PDAC (17–19). The results of two previous meta-analysis studies indicated that low PNI was related to poorer OS. Nevertheless, they analyzed mixed patients who treated with surgery alone, chemotherapy/chemoradiotherapy alone or preoperative chemotherapy/chemoradiotherapy followed by surgery, which could bring about bias, and the conclusion might not be very reliable. These preoperative treatment regimes, especially the chemotherapy, may decline the lymphocyte count and albumin concentration via myelosuppression and chemotherapy toxicity, which could impact the calculation of PNI subsequently. Hence, our aim was to perform a systematic review and meta-analysis of the current published studies to evaluate the clinical significance of PNI as a preoperative prognostic factor in patient with PDAC underwent curative resection.
Materials and methods
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (20).
Search strategies
PubMed, Embase, Web of Science, and Cochrane Library databases were searched for eligible articles up to March 1st, 2022. The search was conducted using medical subject headings (MeSH) in combination with free text words. The search strategy in PubMed database was the following: (“Pancreatic Neoplasms” [MeSH Terms] OR ((“Pancreatic” [Title/Abstract] OR “pancreas” [Title/Abstract]) AND (“adenocarcinoma” [Title/Abstract] OR “carcinoma” [Title/Abstract] OR “cancer” [Title/Abstract] OR “neoplasm*” [Title/Abstract] OR “tumor” [Title/Abstract]))) AND (“Prognostic Nutritional Index” “[Title/Abstract]” OR “Prognostic Nutritional Indices” “[Title/Abstract]” OR “PNI” [Title/Abstract]).
Inclusion and exclusion criteria
All studies included in the meta-analysis were selected according to the following inclusion criteria: (1) studies including patients who underwent curative surgical resection and confirmed as PDAC by histopathological or pathological analysis, (2) PNI was calculated using Onodera’s simplified formula, and measured before surgery, (3) studies investigating the relationship between preoperative PNI and the prognosis of PDAC, (4) hazard ratio (HR) with 95% confidence interval (CI) or other necessary data was available, and (5) studies written in English and published in full-text. The exclusion criteria were as follows: (1) patients received any preoperative neoadjuvant chemotherapy, chemoradiotherapy, or immunotherapy, (2) abstracts, case reports, editorials, letters, systematic reviews, and comments, (3) studies with incomplete data, (4) studies enrolled the overlapped or same population, and (5) duplicate studies.
Data extraction
Two investigators (PCZ and ZWW) independently extracted necessary data from included studies and any disagreements were resolved by discussion till reach consensus. The following data were extracted from each study: first author, publication year, country, study design, age of the study population, male/female, sample size, cutoff value of PNI, tumor stage, duration of follow-up, operation, outcome measures, type of analysis, and recurrence-free survival (RFS) and overall survival (OS) with HR and their 95% CI. Because of confounding factor adjustment, the multivariate analysis was preferred when the HRs for OS or RFS were obtained using both univariate and multivariate analyses. If HR with 95% CI was not provided in original studies, we extracted from the survival curve by using Engauge Digitizer software (https://markummitchell.github.io/engauge-digitizer/).
Quality assessment
The Newcastle-Ottawa quality assessment Scale (NOS) was used to evaluate the quality of included studies. The NOS consists of 3 aspects: selection (4 points maximum), comparability (2 points maximum) and outcomes (3 points maximum). Studies with a score of six or higher were considered as high-quality studies (21). This work was also performed independently by our two investigators (PCZ and ZWW). (Supplementary Table S1)
Statistical analysis
Meta-analysis was conducted using Stata 14.0 software (https://www.stata.com/stata14/). The pooled HR with 95% CI was used to evaluate the relationship between the preoperative PNI and the outcome in patients with PDAC. The heterogeneity of pooled HR was accessed using Cochran’s Q test and Higgins I² statistic. Q test p value < 0.1 or I² > 50% was considered significant heterogeneity and random-effect model was applied to estimate the pooled HR. While heterogeneity was not significant (Q test p value > 0.1 or I² < 50%), a fixed-effect model was used. To reduce and explain the heterogeneity of OS among studies, subgroup analyses, meta-regression analysis and sensitivity analysis were applied. Furthermore, publication bias was visually checked through funnel plot, and then quantitatively analyzed by Begg’s and Egger’s tests. All statistical tests were two-sided, and p value less than 0.05 were defined as statistically significant.
Results
Study selection
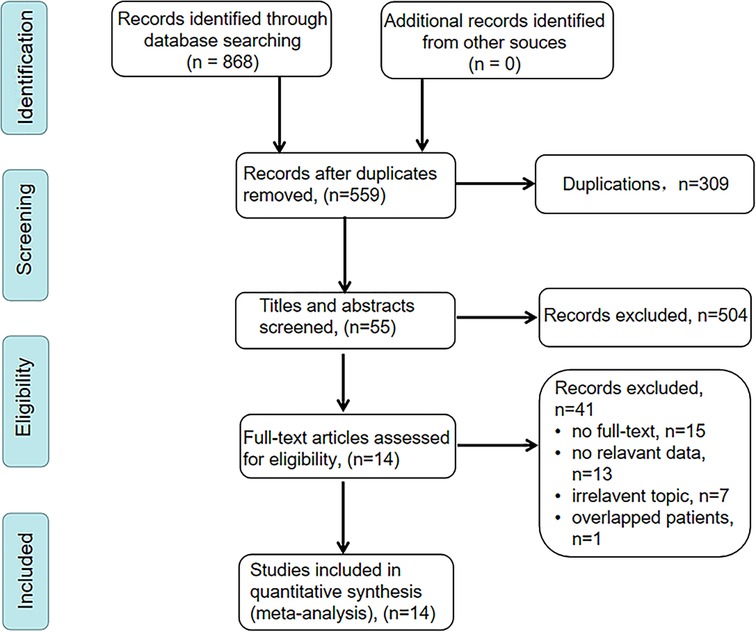
We searched PubMed, Embase, Web of Science, and Cochrane Library databases, and a total of 868 articles were initially retrieved. After removing 309 duplicates, 559 articles remained. After screening the titles and abstracts, 455 articles were excluded for being irrelevant topics, reviews or meta-analysis, conference abstracts, or meeting. Among the remained 104 articles, only 55 articles were performed among patients who underwent curative resection. Finally, 14 articles met our inclusion criteria and 3,385 patients were included in this meta-analysis (17–19, 22–31). The detailed selection process was illustrated in Figure 1.
Clinical characteristic of enrolled studies
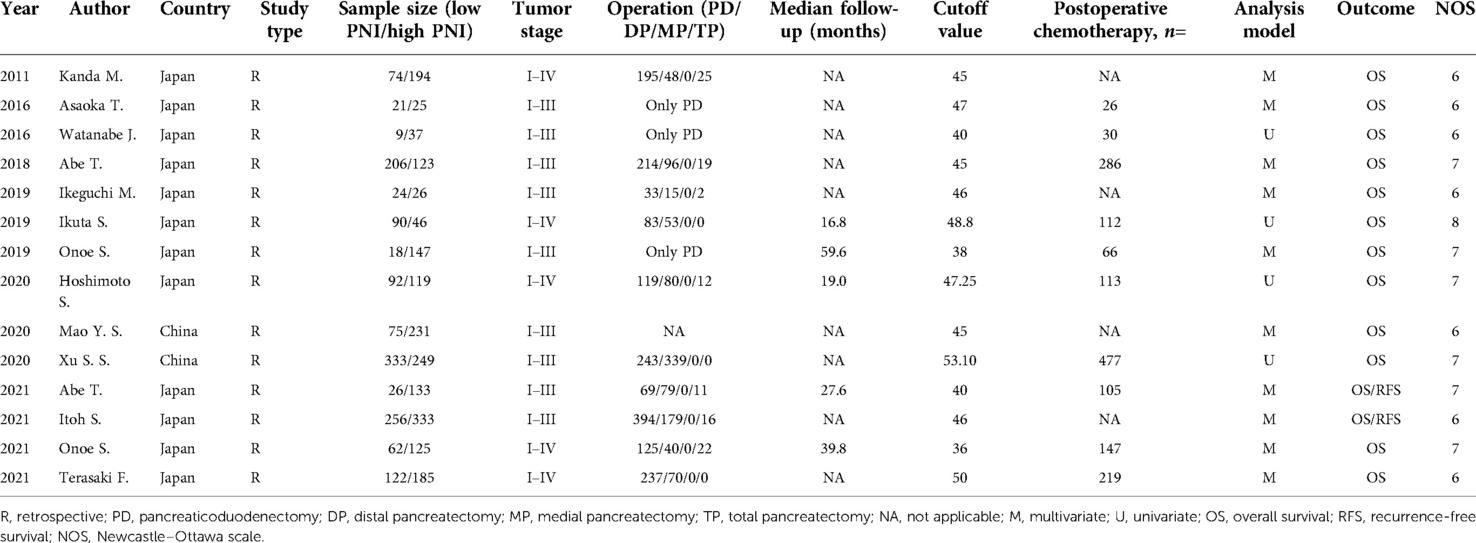
The main characteristics of included studies were presented in Table 1. These included studies were retrospective studies, and mainly published in the past ten years. All included studies used the Onodera’s PNI. Twelve of fourteen studies were from Japan, and two studies were from China. The sample size of enrolled studies varied from 46 to 589. In all selected articles, the correlation between PNI and OS was presented, while RFS was additionally analyzed in two studies. The preoperative PNI cut off value were not consistent ranged from 36 to 53.10. Multivariate analyses were conducted in ten of fourteenth studies. The scores of study quality assessed by NOS ranged from 6 to 8.
Relationship between PNI and OS
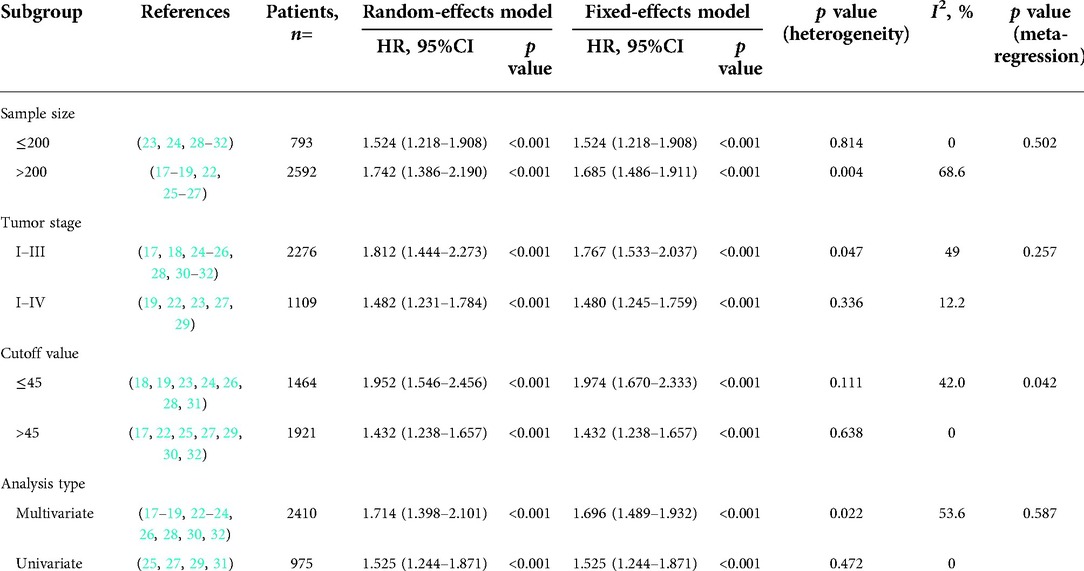
As illustrated in Figure 2, a total of 14 studies were enrolled in this meta-analysis, and the results indicated that patients with low PNI had significantly worse OS (HR = 1.664, 95%CI: 1.424–1.944, I2 = 42.6%, p value = 0.046). Subgroup analyses were conducted based on the sample size, tumor stage, cutoff value, and analysis model, and the results revealed that low PNI was still associated with inferior OS in all subgroups. Meta-regression analysis was performed to explore the heterogeneity, and the p value of cutoff value subgroup was 0.042, which indicated that using different cutoff value among studies might be the source of heterogeneity. Meanwhile, PNI was confirmed as an independent preoperative prognostic factor of OS in 6 studies (17–19, 23, 26, 28). All results of subgroup analyses and meta-regression analyses were shown in Table 2.
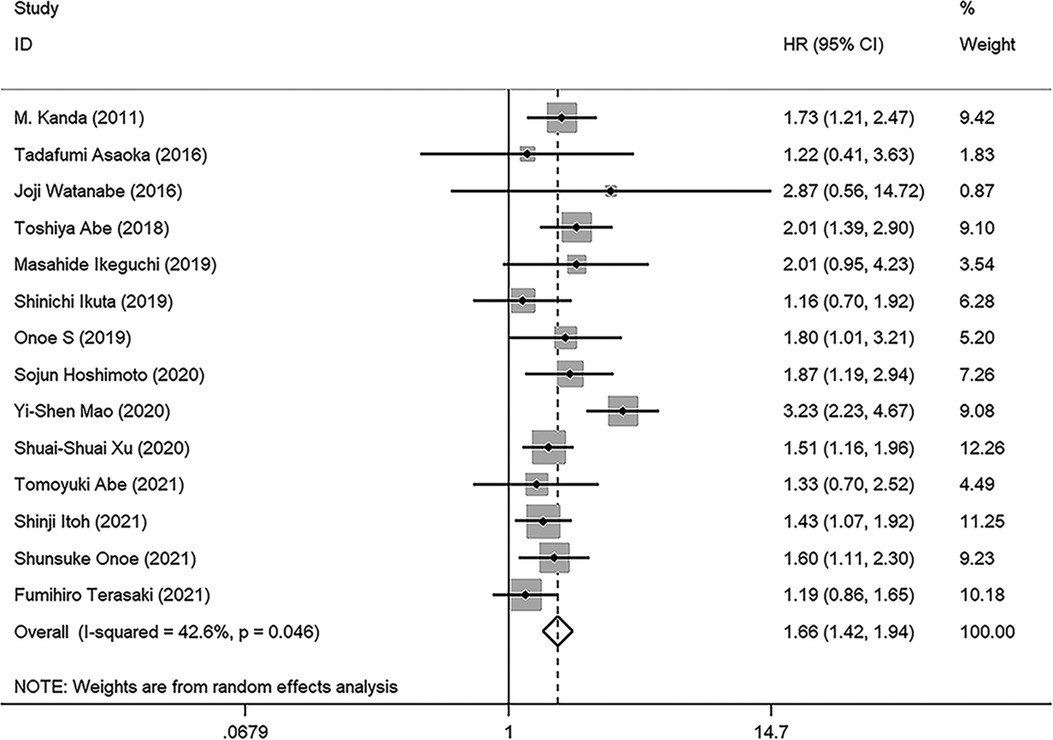
Figure 2. Forest plot of the association between PNI and OS. (OS, overall survival; HR, hazard ratio; CI, confidence interval).
Relationship between PNI and RFS
Two studies reported the prognostic value of PNI for RFS (17, 24), the pooled results were: HR: 1.369, 95%CI: 1.080–1.734, I2 = 0, p value = 0.689, which suggested patients with lower PNI had shorter RFS than those with high PNI (Figure 3).
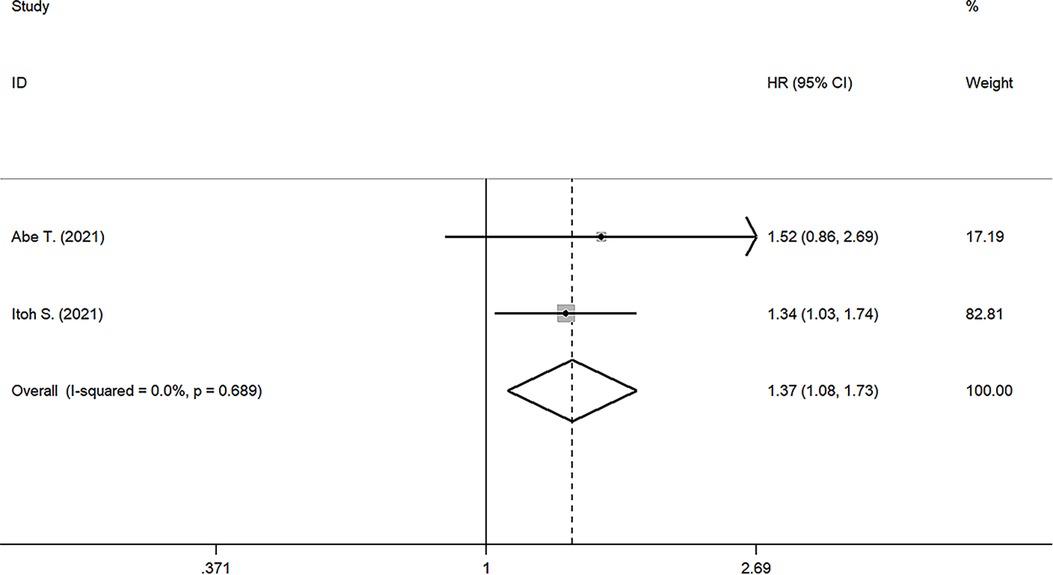
Figure 3. Forest plot of the association between PNI and RFS. (RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval).
Sensitivity analysis and publication bias
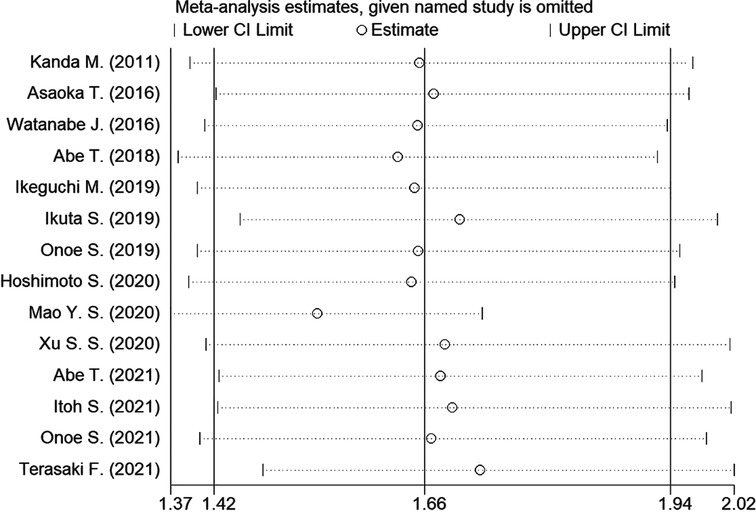
Sensitivity analysis was conducted to assess the effect of individual studies on the pooled HR of OS, and the result revealed that omitting any individual studies had no significant effect on the pooled HR (Figure 4). Furthermore, publication bias was investigated, and there was no obvious asymmetry in the funnel plot upon visual inspection (Figure 5), then Begg's and Egger's tests yielded p values of 0.228 and 0.702, respectively, which indicated that there was no distinct publication bias among included studies.
Discussion
Prognostic nutritional index (PNI) was previously known as a nutritional evaluation index, it has recently been reported to be useful to estimate postoperative morbidity and predict the prognosis in PDAC patients. In our meta-analysis, fourteen studies with a total of 3,385 patients were included (17–19, 22–32). The pooled results showed that lower preoperative PNI was association with poorer OS and RFS. Moreover, results from subgroup analyses and sensitivity analysis further validated the robustness of pooled results.
According to the meta-regression analysis, the diversity of cutoff value might be the source of heterogeneity. There were several methods to determine cutoff value. Among these studies, five studies were defined with a receiver operating characteristic curve (ROC) (17, 24, 27, 29, 31), three with the minimum p value approach (22, 25, 28), and one set the worst tertile of PNI as cutoff value (23). In other studies, mean or median value was used in two studies (30, 32), and three had no clear explanation (18, 19, 26). As a consequence, the cutoff value for PNI ranged from 36 to 53.10. The ROC curve approach maybe the most common way to identify cutoff value. The ROC could reflect the 1-specificity values (false positive rate, X-axis) and the sensitivity values (true positive rate, Y-axis) for each potential threshold, and we were able to determine the cutoff value with high accuracy. The minimum p value approach, also called maximal Chi square statistics approach, was another common method. Each value was assessed as potential threshold, and Chi squared tests were utilized. The maximal Chi square value corresponding threshold was recognized as the optimal cutoff value, however, the type I error rate might be higher due to multiple testing of this method (33, 34). The best way to determine cutoff value is still up for debate, and we have not been able to come to a consistent conclusion. Hence, more multi-institutional data analyses were required to reach a definitive conclusion about cutoff values.
PNI was calculated by albumin and lymphocyte. Albumin, mainly synthesized by hepatocytes, was closely related to nutritional status. Hypoalbuminemia showed the level of malnutrition and cachexia of cancers patients. Some cytokines in tumor microenvironment, such as TNF-α, played an optimal role in the pathogenesis of malnutrition in pancreatic cancer (35). TNF-α could selectively inhibit the gene expression of albumin, causing hypoalbuminemia. Some research indicates that nutrition is a crucial determinant of immune response, which may be impaired by hypoalbuminemia (36). Thus, low levels of serum albumin can be recognized as a marker of poor prognosis in PDAC (37, 38). It was widely acknowledged that lymphocytes were indispensable components of immune system and tumor microenvironment (4). Immune surveillance was considered as the vital part of anti-tumor immunity, however, tumor cells might escape the surveillance by reducing CD4+ and CD8+ lymphocytes causing lymphocytopenia (39). Low lymphocyte counts lead to insufficient immunological responses in tumor microenvironment and result in cancer progression. In addition, the impairment of lymphocyte subsets may be reversed when resecting primary tumor (40). What is more, malnutrition and weak immune could increase the risk of postoperative complications, such as bleeding, pancreatic fistula and infection (26, 41). Therefore, PNI might be a promising predictor of prognosis in patients with PDAC.
There were several limitations in our meta-analysis. Firstly, all studies we selected are retrospective in design, so the potential bias was not inevitable. Secondly, the ethnicity of all included patients is Asian, and we expect the more similar studies can be conducted in Caucasians and Africans. Thirdly, HR and 95% CI of one study was estimated according to the survival curve (31), which might not be very accurate. It would affect the pooled HR. Fourthly, patients with tumor located in pancreas head usually underwent pancreaticoduodenectomy, while distal pancreatectomy or medial pancreatectomy were always performed when tumor locates in pancreas body or tail. Different surgical procedures were associated with different prognosis, and it may result in bias. Finally, all include studies were published in English, and potential publication bias cannot be ignored.
Conclusion
To sum up, our meta-analysis revealed that PDAC patients with lower preoperative PNI level had a worse prognosis. The limitation of this study also cannot be overlooked, and more well-designed studies with large sample size and different ethnicity are required to overcome these limitations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
PCZ, ZWW, CW and BLT conceived and designed the study. PCZ, ZWW, XH and ZHW were responsible for the collection and assembly of data, data analysis, and interpretation. PCZ, ZWW, and CW were involved in writing the manuscript. PCZ, XH, ZHW and BLT revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Sichuan Science and Technology Program (No. 2021YFS0234) and Sichuan University West China hospital (No. 20HXFH055)
Acknowledgments
The authors thank all the medical staff who contributed to the maintenance of the medical record database
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.992641/full#supplementary-material.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. (2019) 16:11–26. doi: 10.1038/s41571-018-0112-1
3. Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. (2012) 143:550–63. doi: 10.1053/j.gastro.2012.07.009
4. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
5. Hamaker ME, Oosterlaan F, van Huis LH, Thielen N, Vondeling A, van den Bos F. Nutritional status and interventions for patients with cancer - a systematic review. J Geriatr Oncol. (2021) 12:6–21. doi: 10.1016/j.jgo.2020.06.020
6. Asaoka T, Miyamoto A, Yamamoto K, Haraguchi N, Miyake M, Nishikawa K, et al. Preoperative neutrophil-to-lymphocyte ratio (NLR) and serum CA19-9 as significant predictors for pancreatic head cancer. HPB. (2014) 16:28.
7. Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. (2012) 29:3092–100. doi: 10.1007/s12032-012-0226-8
8. Dang C, Wang M, Zhu F, Qin T, Qin R. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with pancreatic cancer undergoing radical surgery. Asian J Surg. (2021) 45(6):1237–45. doi: 10.1016/j.asjsur.2021.08.011
9. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) 139:160–7. doi: 10.1016/0002-9610(80)90246-9
10. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5. PMID: 6438478
11. Wang SH, Zhai ST, Lin H. Role of Prognostic Nutritional Index in patients with gastric cancer: a meta-analysis. Minerva Med. (2016) 107:322–7. PMID: 27285120
12. Wang Z, Wang J, Wang P. The prognostic value of prognostic nutritional index in hepatocellular carcinoma patients: a meta-analysis of observational studies. PLoS One. (2018) 13:e0202987. doi: 10.1371/journal.pone.0202987
13. Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: review and meta-analysis. Clin Chim Acta. (2018) 486:303–10. doi: 10.1016/j.cca.2018.08.030
14. Hu Z, Li Y, Mao W, Chen B, Yang L, Meng X. Impact of nutritional indices on the survival outcomes of patients with colorectal cancer. Cancer Manag Res. (2020) 12:2279–89. doi: 10.2147/CMAR.S243172
15. Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. (2020) 20:208. doi: 10.1186/s12885-020-6698-6
16. Lee SJ, Kim K, Park HJ. Meta-analysis on the neutrophil-lymphocyte ratio in rectal cancer treated with preoperative chemoradiotherapy: prognostic value of pre- and post-chemoradiotherapy neutrophil-lymphocyte ratio. Front Oncol. (2022) 12:778607. doi: 10.3389/fonc.2022.778607
17. Itoh S, Tsujita E, Fukuzawa K, Sugimachi K, Iguchi T, Ninomiya M, et al. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study. Pancreatology. (2021) 21(7):1356–63. doi: 10.1016/j.pan.2021.08.003
18. Abe T, Nakata K, Kibe S, Mori Y, Miyasaka Y, Ohuchida K, et al. Prognostic value of preoperative nutritional and immunological factors in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. (2018) 25:3996–4003. doi: 10.1245/s10434-018-6761-6
19. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. (2011) 98:268–74. doi: 10.1002/bjs.7305
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
22. Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, et al. The preoperative controlling nutritional status (CONUT) score is an independent prognostic marker for pancreatic ductal adenocarcinoma. Updates Surg. (2021) 73:251–9. doi: 10.1007/s13304-020-00792-9
23. Onoe S, Yokoyama Y, Kokuryo T, Igami T, Mizuno T, Yamaguchi J, et al. A presurgical prognostic stratification based on nutritional assessment and carbohydrate antigen 19-9 in pancreatic carcinoma: an approach with nonanatomic biomarkers. Surgery. (2021) 169:1463–70. doi: 10.1016/j.surg.2020.11.035
24. Abe T, Amano H, Kobayashi T, Hattori M, Hanada K, Nakahara M, et al. Efficacy of the physiobiological parameter-based grading system for predicting the long-term prognosis after curative surgery for resectable pancreatic cancer. Eur J Surg Oncol. (2021) 47:613–9. doi: 10.1016/j.ejso.2020.09.008
25. Xu S-S, Li S, Xu H-X, Li H, Wu C-T, Wang W-Q, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. (2020) 26:828–38. doi: 10.3748/wjg.v26.i8.828
26. Mao YS, Hao SJ, Zou CF, Xie ZB, Fu DL. Controlling nutritional status score is superior to prognostic nutritional Index score in predicting survival and complications in pancreatic ductal adenocarcinoma: a Chinese propensity score matching study. Br J Nutr. (2020) 124:1190–7. doi: 10.1017/S0007114520002299
27. Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Validation and clinical usefulness of pre- and postoperative systemic inflammatory parameters as prognostic markers in patients with potentially resectable pancreatic cancer. Pancreatology. (2020) 20:239–46. doi: 10.1016/j.pan.2019.12.004
28. Onoe S, Maeda A, Takayama Y, Fukami Y, Takahashi T, Uji M, et al. The prognostic impact of the lymphocyte-to-monocyte ratio in resected pancreatic head adenocarcinoma. Med Princ Pract. (2019) 28:517–25. doi: 10.1159/000501017
29. Ikuta S, Aihara T, Yamanaka N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac J Clin Oncol. (2019) 15:e109–14. doi: 10.1111/ajco.13123
30. Ikeguchi M, Goto K, Watanabe J, Urushibara S, Osaki T, Endo K, et al. Clinical importance of preoperative and postoperative prognostic nutritional index in patients with pancreatic ductal adenocarcinoma. Ann Hepatobiliary Pancreat Surg. (2019) 23:372–6. doi: 10.14701/ahbps.2019.23.4.372
31. Watanabe J, Otani S, Sakamoto T, Arai Y, Hanaki T, Amisaki M, et al. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg Today. (2016) 46:1258–67. doi: 10.1007/s00595-016-1308-6
32. Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, Yamamoto K, et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. (2016) 16:434–40. doi: 10.1016/j.pan.2015.10.006
33. Heinzl H, Tempfer C. A cautionary note on segmenting a cyclical covariate by minimum P-value search. Comput Stat Data Anal. (2001) 35:451–61. doi: 10.1016/S0167-9473(00)00023-2
34. Lausen B, Schumacher M. Evaluating the effect of optimized cutoff values in the assessment of prognostic factors. Comput Stat Data Anal. (1996) 21:307–26. doi: 10.1016/0167-9473(95)00016-X
35. Karayiannakis AJ, Syrigos KN, Polychronidis A, Pitiakoudis M, Bounovas A, Simopoulos K. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. (2001) 21:1355–8. doi: 10.2217/fon.09.136
36. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. (2010) 6:149–63. doi: 10.2217/fon.09.136
37. Agustsson T, Rydén M, Hoffstedt J, van Harmelen V, Dicker A, Laurencikiene J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. (2007) 67:5531–7. doi: 10.1158/0008-5472.CAN-06-4585
38. McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. (2008) 67:257–62. doi: 10.1017/S0029665108007131
39. Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. (2006) 32:22–8. doi: 10.1097/01.mpa.0000188305.90290.50
40. Xu YF, Lu Y, Cheng H, Shi S, Xu J, Long J, et al. Abnormal distribution of peripheral lymphocyte subsets induced by PDAC modulates overall survival. Pancreatology. (2014) 14:295–301. doi: 10.1016/j.pan.2014.05.797
Keywords: pancreatic cancer, meta-analysis, prognosis, surgery, prognostic nutritional index
Citation: Zhao P, Wu Z, Wang Z, Wu C, Huang X and Tian B (2022) Prognostic role of the prognostic nutritional index in patients with pancreatic cancer who underwent curative resection without preoperative neoadjuvant treatment: A systematic review and meta-analysis. Front. Surg. 9:992641. doi: 10.3389/fsurg.2022.992641
Received: 12 July 2022; Accepted: 22 August 2022;
Published: 9 September 2022.
Edited by:
Domenico Tamburrino, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Marcio F. Chedid, Division of General Surgery, Clinical Hospital of Porto Alegre, BrazilWeixing Wang, Renmin Hospital of Wuhan University, China
Joris Jaekers, University Hospitals Leuven, Belgium
Giampaolo Perri, University of Verona, Italy
© 2022 Zhao, Wu, Wang, Wu, Huang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bole Tian aHh0YmwwMzM4QDE2My5jb20=
†These authors have contributed equally to this work.
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Pengcheng Zhao†
Pengcheng Zhao† Zuowei Wu
Zuowei Wu Zihe Wang
Zihe Wang Bole Tian
Bole Tian