
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 25 August 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.990702
This article is part of the Research TopicArtificial Intelligence in Colorectal CancersView all 7 articles
Purpose: This study aims to identify the independent risk factors in the low anterior resection syndrome (LARS) after surgery for colorectal cancer (CRC).
Method: This was a retrospective, single-institution study in the Second Affiliation Hospital of Dalian Medical University, China. Patients underwent sphincter-preserving low anterior resection with total or partial mesorectal resection (with or without protective ileostomy) and completed a self-filled questionnaire over the phone to assess postoperative bowel dysfunction from January 2017 to December 2019. The predictors of LAR were evaluated using univariate and multivariate analyses.
Result: The study population was 566 patients, 264 (46.64%), 224 (39.58%), and 78 (13.78%) patients with no, minor, and major LARS, respectively. In the univariate analysis, independent factors such as tumor location and size, anastomotic height, protective ileostomy, post-operation chemoradiotherapy, tumor T stage, lymphatic nodal metastasis classification, surgery duration, and time interval for closure of stoma were significantly associated with LARS points while we found the tumor T stage and lymphatic nodal metastasis classification as the new independent risk factors compared with the last decade studies. In the multivariate analysis, factors such as low and middle tumor location and protective ileostomy, and post operation treatment, nodal metastasis classification were the independent risk factors for major LARS.
Conclusion: The new independence risk factors were tumor T stage and lymphatic nodal metastasis status in univariate analysis in our study, with anastomotic height, low and middle tumor location, protective ileostomy, post-operation chemoradiotherapy, nodal metastasis status increasing LARS point in multivariate analysis after surgery for CRC.
Low anterior resection syndrome (LARS) is a common functional bowel disorder that develops after anal-sparing rectal cancer surgery.
The LARS score was developed to allow an assessment of the syndrome.
A few studies have attempted to identify LARS risk factors, but have generally failed to comprehensively report the statistical significance of the factors identified.
This is important to raise awareness among clinicians and researchers to focus on this syndrome, to improve prevention and treatment of bowel disorders such as LARS, as well as to inform patients.
CRC is the third most common cancer in the world, accounting for more than one third of all cancers, with an age-standardized rate of 7.7 per 100,000, and among them the rectal cancer is accounting for 5% in all cases (1–4). With the advances in chemotherapy, radiotherapy, and surgical techniques, the long-term survival rate is increasing after CRC surgery regardless of the rising incidences of these diseases (5–7).
The major surgical procedure for rectal cancer involves abdominoperineal resection (APR or called as Mile's procedure) and low anterior resection (LAR) with preservation of sphincter muscles. In recent years, LAR with total mesorectal excision (TME) is the gold standard in rectal cancer surgery (8, 9). LAR and partial mesorectal resections are the most appropriate surgical procedures for upper rectal cancer (1, 5, 10, 11). Laparoscopic LAR is a technically difficult procedure that involves transection of the intraperitoneal rectum in a limited pelvic cavity, and the undesirable result of this surgery is low anterior resection syndrome (LARS). The prevalence is around 80%–90%, and patients experience LARS with varying degrees of severity after sphincter-preserving LAR surgery (1–3, 9, 12, 13).
The conception of LARS is hard to define and involves some altered evacuation status after LAR. It can be described as a “disordered bowel function after rectal resection, leading to a detriment in quality of life.” (14–17). The etiology of LARS is poorly understood, and it seems that the anatomical components and physiological functions of normal defecation, which may be damaged during surgery, are not well established (9, 18). The colorectal experts established LARS scoring system which had five-item validated questionnaire evaluating the bowel functions after CRC surgery in 2012, and this questionnaire has been used to evaluate LARS worldwide. (Table 1) (5, 19, 20). They also focused to find the risk factors influencing LARS happening, and many studies reported the several risk factors for predicting the severity of LARS. Unfortunately, they had some limitations of sample size and insufficient following up. Therefore, we think it is important to identify the risk factors of LARS using comprehensive understandable scoring system and prevent this undesirable result of CRC surgery.
In this study, we tried to identify the independent risk factors influencing LARS after rectal cancer resection based on the recent database for the advanced research.
This was a retrospective study with prospectively collected information from the Second Affiliation Hospital of Dalian Medical University in China. All the patients were diagnosed with CRC and underwent sphincter-preserving LAR with intensive treatments from January 2017 to December 2019.
Any patient diagnosed with CRC and underwent LAR was included in this study without any age or gender specifications, and tumor location ranging from 5–25 cm off the anal verge. All patients underwent colonoscopy, CT (or MRI test if necessary) and other tests, and were diagnosed as rectal cancer.
1. Patients with unresectable cancers.
2. Patients assessed as more than ASA grade 3.
3. Patients with poor-quality total mesorectal excision (TME) surgery or breached circumferential tumor margins in complete mesocolic excision (CME) surgery.
4. Patients who underwent abdominal perineal resection (APR, also called as Miles procedure) or proctosigmoidectomy (Hartmann procedure).
5. Patients who did not complete the LARS questionnaire or follow-up.
6. There was no pediatric patient in our study.
Every patient was followed up for more than one year after LAR surgery and filled a LARS score questionnaire. The endpoint was the completion of the analysis in January 2021.
All resections were performed by five of professionally-certified and fellowship-trained colorectal surgeons, who all shared a similar case volume over the study years.
LARS questionnaire was used for assessing the bowel function and included the following items: flatus incontinence, liquid stools status, frequency, clustering, and urgency. Every item has three options with a defined scoring system used for evaluating the severity. The patients were divided into the no (0–20), minor (21–29), and major (30–42) LARS groups depending on their total score (Table 2).
We used the Chinese version of the questionnaire. Patient demographics, pre-and-post operative data, surgery information, and pathological data were obtained from the hospital database, and the three groups were compared. We measured the tumor location using the specimen from the anal verge after surgery, and the tumor location was divided into four degrees, such as low (=<5 cm), middle (5–10 cm), high (10–15 cm), and sigmoid (>15 cm). The anastomotic height was measured based on the tumor location and operation procedure in the surgery. The cancer stage was defined using the 8th edition American Joint Commission on Cancer (AJCC) Tumor Node Metastasis (TMN) classification system. In this study, the pathological stage was defined as the cancer stage after surgery.
LARS scores were assessed for more than one year after an operation during follow-up. In this study, patients received phone calls and explained the questionnaire in detail, and they were asked to complete a validated Chinese version of the questionnaire designed to evaluate LARS score after CRC surgery. We rechecked the addresses and phone numbers for the patients who did not receive the calls, then reminded them or their family members to complete the questionnaire. The follow-up process was completed over three months.
We searched the PubMed (“Title/Abstract” add to the query box) and Web of Science Core Collection database (“TI = Title” and “AB = Abstract” add to the query box) from January 2011 to December 2021, using a combination of relevant Medical Subject Heading terms and keywords: (low anterior resection* OR LAR* OR low anterior resection syndrome* OR LARS*) AND (risk factor* OR independent factor* OR independent risk factor* OR quality of life* OR QoL*) AND (rectal cancer* OR colorectal cancer* OR colon cancer*) AND (surgery* OR operation* OR resection*). And we selected the most citied and suitable 21 papers, which researched about the risk factors of LARS among the 3,450 papers (642 papers from PubMed, 2,808 papers from Web of Science), and summarized their risk factors reported before.
The Ethics Committee of the Second Affiliation Hospital of Dalian Medical University approved this study. All patients were given information regarding the surgery and informed consent was obtained before surgery.
All data collection and statistical analyses were performed using EndNote 20.0, Excel 2019, and Social Science SPSS Advanced Statistics 26.0 (IBM Software Group). The mean, standard deviation, and median values (interquartile range) were used to describe the normal and non-normal distribution measurement data. Frequency (percentage) was used to describe the classification data. The one-way ANOVA and nonparametric tests were used to compare the measurement and classification data between the groups. Statistical significance was set at p < 0.05.
First, we used univariate analysis to find factors with significant associations with LARS. Then, we performed the multivariate analysis with the variables representing significant differences in the univariate analysis. We confirmed the risk factors associated with LARS using the ordered logistic regression analysis.
We collected 660 patient data from the hospital database, and 566 patients responded completely (85.76%). Among the 660 patients, 32 could not be contacted, 26 did not respond, 29 returned incomplete questionnaires, and seven died because of several causes, including the other diseases or accidents. We excluded these 94 patients from the analyses. Therefore, the study population was 566 patients with 354 men and 221 women (Figure 1).
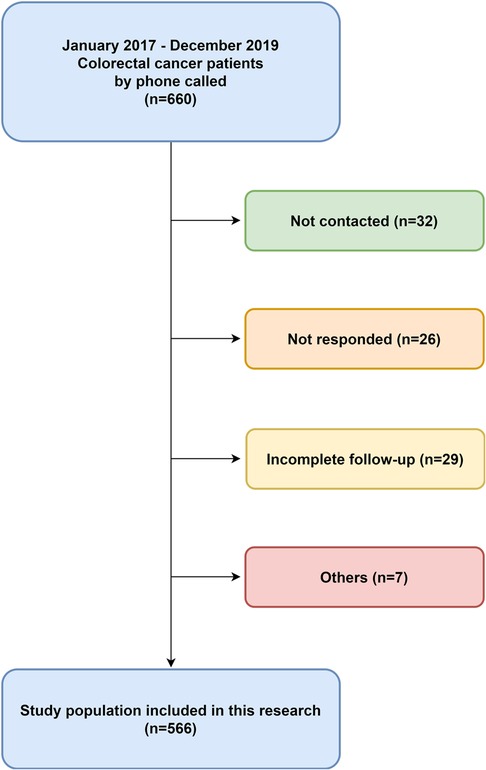
Figure 1. Patients collection diagram. This was a retrospective, single-institution study with colorectal cancer who had undergone low anterior resection from January 2017–December 2019.
The mean age was 63.44 y (64.60 y and 61.50 y for men and women, respectively). The median follow-up was 15.6 months (10–22 months) after surgery. Depending on the LARS score, we divided the patients into the no LARS, minor LARS, and major LARS groups with 264 (46.64%), 224 (39.58%), and 78 (13.78%) patients, respectively. Laparoscopic surgery and protective ileostomy were performed on 514 (90.81%) and 354 (41.34%) patients, respectively. There were 10 incidences of anastomosis leakage (1.8%) (Table 2).
The results of the univariate analysis are shown in Table 3. LARS was significantly more frequent in patients with factors, such as low tumor location and tumor size, protective ileostomy versus no ileostomy, post-operation chemoradiotherapy, tumor T stage, nodal, long surgery duration, and time interval between ileostomy closure. In contrast, gender, age, BMI, surgery type (laparoscopy or open procedure), pre-operation chemoradiotherapy, and tumor metastases were not associated with LARS development.
In the multivariate analysis, the independent risk factors related with LARS were anastomotic height, low and middle tumor locations, nodal classification and protective ileostomy (Table 4).
Sphincter-preserving low anterior resection (LAR) improves the quality of life (QoL) of patients with middle and low colorectal cancer, and several large randomized clinical trials have reported the safety and feasibility of this procedure (21, 22). Therefore, it has become a popular treatment method (23, 24). However, the undesirable result of this procedure is the bowel dysfunction called low anterior resection syndrome (25). About 80% of patients who undergo this procedure experience varying degrees of LARS (26, 27).
LARS generally consists of fecal incontinence, urgency, and incomplete evacuation or evacuation difficulties. Several articles reported the leakage of gas and stool, stool clustering, frequent bowel movements, evacuation, and urgency as the main complaints (1, 7, 14, 17, 28, 29). LARS can have two types of symptoms, the first type appears within 6–12 months after surgery, which is called short-term symptoms. They are usually caused by short-lived neorectal irritabilities during the postoperative period, and includes fecal urgency, incontinence, and increased frequency. The second type extends for more than one year after surgery. They are called long-term symptoms and are most likely caused by constant changes, and includes constipation, feelings of incomplete excretion, and bowel-emptying difficulties (7, 10, 28). Some patients show characteristics of both types. They alternate between the two patterns or experience both at the same time (30–33). These symptoms are caused because of damage to several factors, such as nerves and muscles of defecation (18, 24, 34).
LAR surgery can injure components of the anal canal, such as the internal anal sphincter, longitudinal conjunctive muscle, or hiatus ligament, or can cause mechanical or nerve damage through injury to these organs. The resection of the rectum, division of the coccygeus muscle, and/or damage to the nerve supply can impair rectal function. The remaining rectum is small and does not function properly, and the hypermotility of the remnant colon can affect the manifestation of urge fecal incontinence (7, 16, 17).
The first idea for LARS scoring system came up in 1998, and the Memorial Sloan Kettering Cancer Center Bowel Function Instrument (MSKCC-BFI) created the 18 items validated scoring system in 2004 that can be used to assess the bowel function after LAR (35). This scoring system surveys several factors, including diet number, form, quality and timing of bowel movements, sensation of flatus, anti-diarrheal medication usage, and fecal incontinence. This scoring system ranged from 18–90, higher scores indicate better levels of bowel function. However, this scoring system was not universally applicable and could not be widely used (1, 14, 36). The second idea of LARS scoring system which had five-item validated questionnaire evaluating the bowel functions after CRC surgery in 2012, and this questionnaire has been used to evaluate LARS worldwide.
The risk factors of severe LARS are related to the anastomotic height, pre and postoperative chemoradiotherapy, anastomotic leakage, and protective ileostomy etc. (8, 9, 32, 37, 38).
In our study, we firstly identified the independent risk factors associated with LARS in univariate analysis, including tumor location and tumor size, anastomotic height, protective ileostomy versus no ileostomy, post-operation chemoradiotherapy, tumor T stage, nodal classification, long surgery duration, and time interval between ileostomy closure, while the tumor T stage and nodal classification were clarified as the new independent risk factors while the last decade studies have not reported.
When having low anterior resection procedure for CRCs, it takes time for the bowel to adapt after the operation, which helps in intestinal function recovery. And protective ileostomy was performed, the patients have difficulties controlling their defecation. The loss of bowel functions leads to stool defecation without consciousness, and this phenomenon adversely affects LARS recovery.
Tumor location, size, T stage and lymphatic nodal characteristics are directly related to surgical range and procedures; therefore, LARS is directly influenced by these three factors (39–41). But this theory is suggested in our study and the other studies have no mentioned the tumor T stage and nodal classification as the risk factors in their researches.
The side effect of neoadjuvant radiotherapy and chemotherapy is intestinal dysfunction, which is caused by nerve and muscle damage in the colon (38, 42–44). In 2017, L.M. Jimenez-Gomez et al., (8) reported risk factors, such as TME and neoadjuvant and adjuvant radiotherapy can increase the risk of major LARS. In 2020, Theresa H. Nguyen et al., (1) proved that neoadjuvant and adjuvant radiotherapy were risk factors for LARS, especially major LARS, even in patients with large rectal residuals. And several studies have shown that LARS is divided into incontinence-dominant and frequency-dominant modes. Each mode is associated with different risk factors. The incontinence-dominant mode is related to preoperative radiotherapy and postoperative complications. The frequency-dominant mode is related to the low tumor location from the anal margin; however, the overall main LARS is related to poor quality of life. The frequency-dominant type of LARS has a more profound impact on postoperative quality of life (10, 20, 45). In 2019, Keiji Koda et al., (18) showed that removing most of the rectum can damage the internal sphincter muscle and/or rectal wall, and deconstruct structures around the levator hiatus, are factors involved in the development of LARS symptoms.
In recent years, significant incidences of postoperative intestinal dysfunction and the prospects of a good prognosis have made radical resection plus neoadjuvant radiotherapy the standard treatment. However, there are some practical difficulties to perform the complete radical resection. In this theory, full-dose neoadjuvant chemotherapy can reduce tumor size similar to radiotherapy plus chemotherapy, reducing the possibilities of local recurrence in patients undergoing surgical resection. It also reduces the incidences of distant metastases. These studies have shown that neoadjuvant chemotherapy is usually an effective method for the treatment of locally advanced rectal cancer, and the effects are satisfactory (46, 47). Considering that neoadjuvant chemotherapy has no significant effect on bowel function, it may be a reasonable treatment option for major LARS patients (38).
In our study, only post-operative chemoradiotherapy was identified as a risk factor for severe LARS development in terms of neoadjuvant and adjuvant treatment of CRCs. We thought that this result came from the differences in treatments and conditions according to every country and national race.
In 2021, Suzuki, N et al., (48) also reported anastomotic complications, such as leakage, which was confirmed to be associated with a 3.5-fold increase in the incidences of major LARS. However, we could not find anastomotic complications increasing the incidences of major LARS in our study, and we thought this was due to the development of operation skills and reliable management of patients after operation in recent years.
Several studies have suggested an algorithm for the treatment of LARS, including conservative therapies, biofeedback, and sacral nerve stimulation. In 2019, Chirs George et al., (42) reported that conservative treatment (internal medicine, physical therapy, and trans-anal irrigation), invasive surgery (neuromodulation), and multimodal therapy were the main methods for treating LARS in patients. If these treatments were not working wonderfully, it's recommended to perform stoma surgery. The definitive stoma surgery was considered if major LARS persisted for more than 2 years (7, 24, 29). In 2021, K. Neumann et al., (49) found that transanal endoscopic microsurgery (TEM) for rectal tumors was associated with significantly reduced hospitalization costs, which far exceeded the cost of acquiring and maintaining the technology, and reduced the incidence of LARS, so recommended that if possible use TEM to treat rectal cancer.
When we are focusing on the number of articles published each year for the last ten years, the publications and citations trend to increase obviously (Figure 2: downloaded from Web of Science Core Collection). This shows that research for LARS and improving QoL is recently one of the major focuses in the colorectal fields as patient requests. And, the independent factors are similar to the others, including pre- and post-surgery chemoradiotherapy, poor TME procedure, tumor height from the anal verge, anastomosis height and leakage, temporary protective ileostomy, and complications after surgery (Table 5). We thought it would give a well-updated knowledge for future studies. We thought there are some limitations in our study such as not enough numbers of database, single institution study design and no mentions on LARS treatment. These can affect the undesirable effects on the study results and general ideas. We hope an updated and advanced study is needed for a better understanding to provide more information on LARS treatment strategies improving the quality of life.
The new independence risk factors were tumor T stage and lymphatic nodal metastasis status in univariate analysis, while anastomotic height, low and middle tumor location, protective ileostomy, post-operation chemoradiotherapy, nodal metastasis status was increasing LARS points after CRC surgery in multivariate analysis in our study.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Second Affiliation Hospital of Dalian Medical University. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: HR, XC and HK, investigation: ZX, ZG, data curation: KK, YR, HK, writing-original draft preparation: HR, HK, writing-review and editing: HK, XC, YR, supervision and project administration: HR, ZX and XC. All authors contributed to the article and approved the submitted version.
The authors are thankful to the Second Affiliation Hospital of Dalian Medical University for providing the sufficient database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nguyen TH, Chokshi RV. Low anterior resection syndrome. Curr Gastroenterol Rep. (2020) 22(10):48. doi: 10.1007/s11894-020-00785-z
2. Vollebregt PF, Wiklendt L, Ang D, Venn ML, Mekhael M, Christensen P, et al. Altered anal slow-wave pressure activity in low anterior resection syndrome: short case series in two independent specialist centres provide new mechanistic insights. Colorectal Dis. (2021) 23(2):444–50. doi: 10.1111/codi.15502
3. McDermott FD. Altered colonic motility is associated with low anterior resection syndrome, by Keane et al. Colorectal Dis. (2021) 23(2):338. doi: 10.1111/codi.15547
4. Pieniowski EHA, Nordenvall C, Palmer G, Johar A, Tumlin Ekelund S, Lagergren P, et al. Prevalence of low anterior resection syndrome and impact on quality of life after rectal cancer surgery: population-based study. BJS Open. (2020) 4(5):935–42. doi: 10.1002/bjs5.50312
5. Buchli C, Martling A, Sjovall A. Low anterior resection syndrome after right- and left-sided resections for colonic cancer. BJS Open. (2019) 3(3):387–94. doi: 10.1002/bjs5.50128
6. Stelzner S, Kupsch J, Mees ST. Low anterior resection syndrome-causes and treatment approaches. Chirurg. (2021) 92(7):612–20. doi: 10.1007/s00104-021-01398-6
7. Christensen P, Im Baeten C, Espin-Basany E, Martellucci J, Nugent KP, Zerbib F, et al. Management guidelines for low anterior resection syndrome - the MANUEL project. Colorectal Dis. (2021) 23(2):461–75. doi: 10.1111/codi.15517
8. Jimenez-Gomez LM, Espin-Basany E, Trenti L, Marti-Gallostra M, Sanchez-Garcia JL, Vallribera-Valls F, et al. Factors associated with low anterior resection syndrome after surgical treatment of rectal cancer. Colorectal Dis. (2017) doi: 10.1111/codi.13901.28963744
9. Sapci I, Velazco JS, Xhaja X, Aiello A, Gorgun E, Stocchi L, et al. Factors associated with noncomplete mesorectal excision following surgery for rectal adenocarcinoma. Am J Surg. (2019) 217(3):465–8. doi: 10.1016/j.amjsurg.2018.10.051
10. Kim MJ, Park JW, Lee MA, Lim HK, Kwon YH, Ryoo SB, et al. Two dominant patterns of low anterior resection syndrome and their effects on patients’ quality of life. Sci Rep. (2021) 11(1):3538. doi: 10.1038/s41598-021-82149-9
11. Zhou X, Wang B, Li F, Wang J, Fu W. Risk factors associated with nonclosure of defunctioning stomas after sphincter-preserving low anterior resection of rectal cancer: a meta-analysis. Dis Colon Rectum. (2017) 60(5):544–54. doi: 10.1097/DCR.0000000000000819
12. Keane C, Paskaranandavadivel N, Vather R, Rowbotham D, Arkwright J, Dinning P, et al. Altered colonic motility is associated with low anterior resection syndrome. Colorectal Dis. (2021) 23(2):415–23. doi: 10.1111/codi.15465
13. Rodrigues B, Rodrigues F, Buzatti K, Campanati RG, da Luz MMP, da Silva RG, et al. Feasibility study of transanal irrigation using a colostomy irrigation system in patients with low anterior resection syndrome. Dis Colon Rectum. (2022) 65(3):413–20. doi: 10.1097/dcr.0000000000002005
14. Hu J, Sun J, Wang Y, Sun X, Tong W, Hu H. Knowledge, attitudes, practices, and related factors of low anterior resection syndrome management among colorectal surgery nurses: a multicenter cross-sectional study. Support Care Cancer. (2021) 29(7):4129–36. doi: 10.1007/s00520-020-05922-y
15. Pape E, Pattyn P, Van Hecke A, Somers N, Van de Putte D, Ceelen W, et al. Impact of low anterior resection syndrome (LARS) on the quality of life and treatment options of LARS - A cross sectional study. Eur J Oncol Nurs. (2021) 50:101878. doi: 10.1016/j.ejon.2020.101878
16. Moon J, Monton O, Smith A, Garfinkle R, Zhao K, Zelkowitz P, et al. Interactive online informational and peer support application for patients with low anterior resection syndrome: patient survey and protocol for a multicentre randomized controlled trial. Colorectal Dis. (2021) 23(5):1248–57. doi: 10.1111/codi.15602
17. Keane C, Fearnhead NS, Bordeianou L, Christensen P, Espin Basany E, Laurberg S, et al. International consensus definition of low anterior resection syndrome. Colorectal Dis. (2020) 22(3):331–41. doi: 10.1111/codi.14957
18. Koda K, Yamazaki M, Shuto K, Kosugi C, Mori M, Narushima K, et al. Etiology and management of low anterior resection syndrome based on the normal defecation mechanism. Surg Today. (2019) 49(10):803–8. doi: 10.1007/s00595-019-01795-9
19. Dalsgaard P, Emmertsen KJ, Mekhael M, Laurberg S, Christensen P. Nurse-led standardized intervention for low anterior resection syndrome. A population-based pilot study. Colorectal Dis. (2021) 23(2):434–43. doi: 10.1111/codi.15497
20. Mekhael M, Kristensen HO, Larsen HM, Juul T, Emmanuel A, Krogh K, et al. Transanal irrigation for neurogenic bowel disease, low anterior resection syndrome, faecal incontinence and chronic constipation: a systematic review. J Clin Med. (2021) 10(4). doi: 10.3390/jcm10040753
21. Farag A, Mashhour AN, Elbarmelgi MY. Taeniectomy versus transverse coloplasty as neorectum after low rectal resection. World J Surg. (2019) 43(4):1137–45. doi: 10.1007/s00268-018-04890-z
22. Shimizu H, Yamaguchi S, Ishii T, Kondo H, Hara K, Takemoto K, et al. Who needs diverting ileostomy following laparoscopic low anterior resection in rectal cancer patients? Analysis of 417 patients in a single institute. Surg Endosc. (2020) 34(2):839–46. doi: 10.1007/s00464-019-06837-4
23. Sakr A, Sauri F, Alessa M, Zakarnah E, Alawfi H, Torky R, et al. Assessment and management of low anterior resection syndrome after sphincter preserving surgery for rectal cancer. Chin Med J (Engl). (2020) 133(15):1824–33. doi: 10.1097/CM9.0000000000000852
24. Keane C, Wells C, O'Grady G, Bissett IP. Defining low anterior resection syndrome: a systematic review of the literature. Colorectal Dis. (2017) 19(8):713–22. doi: 10.1111/codi.13767
25. van der Heijden JAG, Qaderi SM, Verhoeven R, Custers JAE, Klarenbeek BR, Maaskant-Braat AJG, et al. Transanal total mesorectal excision and low anterior resection syndrome. Br J Surg. (2021) 108(8):991–7. doi: 10.1093/bjs/znab056
26. Marti WR, Curti G, Wehrli H, Grieder F, Graf M, Gloor B, et al. Clinical outcome after rectal replacement with side-to-end, colon-J-pouch, or straight colorectal anastomosis following total mesorectal excision: a Swiss prospective, randomized, multicenter trial (SAKK 40/04). Ann Surg. (2019) 269(5):827–35. doi: 10.1097/SLA.0000000000003057
27. Bazzell A, Madsen LT, Dains J. Clinical management of bowel dysfunction after low anterior resection for rectal cancer. J Adv Pract Oncol. (2016) 7(6):618–29. PMCID: PMC586612829588867
28. Martellucci J. Low anterior resection syndrome: a treatment algorithm. Dis Colon Rectum. (2016) 59(1):79–82. doi: 10.1097/DCR.0000000000000495
29. Rasulov AO, Baichorov AB, Merzlykova AM, Ovchinnikova AI, Semyanikhina AV. Surgical treatment of low anterior resection syndrome. Khirurgiia (Mosk). (2020) 11:53–60. doi: 10.17116/hirurgia202011153
30. Dulskas A, Smolskas E, Kildusiene I, Samalavicius NE. Treatment possibilities for low anterior resection syndrome: a review of the literature. Int J Colorectal Dis. (2018) 33(3):251–60. doi: 10.1007/s00384-017-2954-x
31. Farella M, Tuech JJ, Bridoux V, Coget J, Chati R, Resch B, et al. Surgical management by disk excision or rectal resection of low rectal endometriosis and risk of low anterior resection syndrome: a retrospective comparative study. J Minim Invasive Gynecol. (2021) 28(12):2013–24. doi: 10.1016/j.jmig.2021.05.007
32. Nocera F, Angehrn F, von Flue M, Steinemann DC. Optimising functional outcomes in rectal cancer surgery. Langenbecks Arch Surg. (2021) 406(2):233–50. doi: 10.1007/s00423-020-01937-5
33. Keane C, Park J, Oberg S, Wedin A, Bock D, O'Grady G, et al. Functional outcomes from a randomized trial of early closure of temporary ileostomy after rectal excision for cancer. Br J Surg. (2019) 106(5):645–52. doi: 10.1002/bjs.11092
34. Theodoropoulos GE, Liapi A, Spyropoulos BG, Kourkouni E, Frountzas M, Zografos G. Temporal changes of low anterior resection syndrome score after sphincter preservation: a prospective cohort study on repetitive assessment of rectal cancer patients. J Invest Surg. (2021):1–9. doi: 10.1080/08941939.2020.1864684
35. Essangri H, Majbar MA, Benkabbou A, Amrani L, Mohsine R, Souadka A. Transcultural adaptation and validation of the Moroccan Arabic dialect version of the Wexner incontinence score in patients with low anterior resection syndrome after rectal surgery. Surgery. (2021). doi: 10.1016/j.surg.2021.01.029
36. Burke JP. Low anterior resection syndrome - “braking” our focus with the pelvis. Colorectal Dis. (2021) 23(2):339–40. doi: 10.1111/codi.15548
37. Ekkarat P, Boonpipattanapong T, Tantiphlachiva K, Sangkhathat S. Factors determining low anterior resection syndrome after rectal cancer resection: a study in Thai patients. Asian J Surg. (2016) 39(4):225–31. doi: 10.1016/j.asjsur.2015.07.003
38. Zhang Q, An L, Yu R, Peng J, Yu K, Huang M, et al. The impact of neoadjuvant chemotherapy on low anterior resection syndrome after rectal cancer resection: a 6 months longitudinal follow-up. Asian J Surg. (2021). doi: 10.1016/j.asjsur.2021.02.010
39. Greenwood A, Keating J, Kenwright D, Shekouh A, Dalzell A, Dennett E, et al. Brief report: lymph node morphology in stage II colorectal cancer. PLoS One. (2021) 16(3):e0249197. doi: 10.1371/journal.pone.0249197
40. Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science. (2017) 357(6346):55–60. doi: 10.1126/science.aai8515
41. Jin M, Frankel WL. Lymph node metastasis in colorectal cancer. Surg Oncol Clin N Am. (2018) 27(2):401–12. doi: 10.1016/j.soc.2017.11.011
42. Cura Pales CG, An S, Cruz JP, Kim K, Kim Y. Postoperative bowel function after anal sphincter-preserving rectal cancer surgery: risks factors, diagnostic modalities, and management. Ann Coloproctol. (2019) 35(4):160–6. doi: 10.3393/ac.2019.08.10
43. Ziv Y, Zbar A, Bar-Shavit Y, Igov I. Low anterior resection syndrome (LARS): cause and effect and reconstructive considerations. Tech Coloproctol. (2013) 17(2):151–62. doi: 10.1007/s10151-012-0909-3
44. Chen TY, Wiltink LM, Nout RA, Meershoek-Klein Kranenbarg E, Laurberg S, Marijnen CA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. (2015) 14(2):106–14. doi: 10.1016/j.clcc.2014.12.007
45. Keramati MR, Abbaszadeh-Kasbi A, Keshvari A, Ahmadi-Tafti SM, Behboudi B, Kazemeini A, et al. Translation, validation and psychometric evaluation of the Persian (Farsi) version of the low anterior resection syndrome score (LARS-P). PLoS One. (2021) 16(2):e0247054. doi: 10.1371/journal.pone.0247054
46. Kaneko M, Nozawa H, Emoto S, Murono K, Sasaki K, Otani K, et al. Neoadjuvant imatinib therapy followed by intersphincteric resection for low rectal gastrointestinal stromal tumors. Anticancer Res. (2017) 37(9):5155–60.28870948
47. Kosugi C, Koda K, Tanaka K, Suzuki M, Yamazaki M, Shuto K, et al. Evaluation of preoperative chemotherapy with modified OPTIMOX-1 plus bevacizumab in patients with advanced rectal cancer with factors contraindicative of curative surgery. Hepatogastroenterology. (2015) 62(140):868–72.26902018
48. Suzuki N, Yoshida S, Tomochika S, Nakagami Y, Shindo Y, Tokumitsu Y, et al. Determining the protective characteristics and risk factors for the development of anastomotic leakage after low anterior resection for rectal cancer. Surg Today. (2021) 51(5):713–20. doi: 10.1007/s00595-020-02133-0
49. Neumann K, Randhawa N, Park J, Hochman DJ. Cost analysis of laparoscopic low anterior resection vs. transanal endoscopic microsurgery for rectal neoplasms. Curr Oncol. (2021) 28(3):1795–802. doi: 10.3390/curroncol28030167
50. Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. (2013) 15(9):1130–9. doi: 10.1111/codi.12244
51. Juul T, Ahlberg M, Biondo S, Emmertsen KJ, Espin E, Jimenez LM, et al. International validation of the low anterior resection syndrome score. Ann Surg. (2014) 259(4):728–34. doi: 10.1097/SLA.0b013e31828fac0b
52. Juul T, Elfeki H, Christensen P, Laurberg S, Emmertsen KJ, Bager P. Normative data for the low anterior resection syndrome score (LARS score). Ann Surg. (2019) 269(6):1124–8. doi: 10.1097/SLA.0000000000002750
53. Bondeven P, Emmertsen KJ, Laurberg S, Pedersen BG. Neoadjuvant therapy abolishes the functional benefits of a larger rectal remnant, as measured by magnetic resonance imaging after restorative rectal cancer surgery. Eur J Surg Oncol. (2015) 41(11):1493–9. doi: 10.1016/j.ejso.2015.07.003
54. Wells CI, Vather R, Chu MJ, Robertson JP, Bissett IP. Anterior resection syndrome–a risk factor analysis. J Gastrointest Surg. (2015) 19(2):350–9. doi: 10.1007/s11605-014-2679-x
55. Hain E, Manceau G, Maggiori L, Mongin C, Prost À la Denise J, Panis Y. Bowel dysfunction after anastomotic leakage in laparoscopic sphincter-saving operative intervention for rectal cancer: a case-matched study in 46 patients using the low anterior resection score. Surgery. (2017) 161(4):1028–39. doi: 10.1016/j.surg.2016.09.037
56. Carrillo A, Enríquez-Navascués JM, Rodríguez A, Placer C, Múgica JA, Saralegui Y, et al. Incidence and characterization of the anterior resection syndrome through the use of the LARS scale (low anterior resection score). Cir Esp. (2016) 94(3):137–43. doi: 10.1016/j.ciresp.2015.11.005
57. Sturiale A, Martellucci J, Zurli L, Vaccaro C, Brusciano L, Limongelli P, et al. Long-term functional follow-up after anterior rectal resection for cancer. Int J Colorectal Dis. (2017) 32(1):83–8. doi: 10.1007/s00384-016-2659-6
58. Hughes DL, Cornish J, Morris C. Functional outcome following rectal surgery-predisposing factors for low anterior resection syndrome. Int J Colorectal Dis. (2017) 32(5):691–7. doi: 10.1007/s00384-017-2765-0
59. Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, et al. Predicting the risk of bowel-related quality-of-life impairment after restorative resection for rectal cancer: a multicenter cross-sectional study. Dis Colon Rectum. (2016) 59(4):270–80. doi: 10.1097/DCR.0000000000000552
60. Sarcher T, Dupont B, Alves A, Menahem B. Anterior resection syndrome: what should we tell practitioners and patients in 2018? J Visc Surg. (2018) 155(5):383–91. doi: 10.1016/j.jviscsurg.2018.03.006
61. Nowakowski MM, Rubinkiewicz M, Gajewska N, Torbicz G, Wysocki M, Malczak P, et al. Defunctioning ileostomy and mechanical bowel preparation may contribute to development of low anterior resection syndrome. Wideochir Inne Tech Maloinwazyjne. (2018) 13(3):306–14. doi: 10.1016/j.jviscsurg.2018.03.006
62. Sun W, Dou R, Chen J, Lai S, Zhang C, Ruan L, et al. Impact of long-course neoadjuvant radiation on postoperative low anterior resection syndrome and quality of life in rectal cancer: post hoc analysis of a randomized controlled trial. Ann Surg Oncol. (2019) 26(3):746–55. doi: 10.1245/s10434-018-07096-8
63. Nuytens F, Develtere D, Sergeant G, Parmentier I, D'Hoore A, D'Hondt M. Perioperative radiotherapy is an independent risk factor for major LARS: a cross-sectional observational study. Int J Colorectal Dis. (2018) 33(8):1063–9. doi: 10.1007/s00384-018-3043-5
64. Rubinkiewicz M, Zarzycki P, Witowski J, Pisarska M, Gajewska N, Torbicz G, et al. Functional outcomes after resections for low rectal tumors: comparison of Transanal with laparoscopic Total Mesorectal excision. BMC Surg. (2019) 19(1):79. doi: 10.1186/s12893-019-0550-4
65. Miacci FLC, Guetter CR, Moreira PH, Sartor MC, Savio MC, Baldin Júnior A, et al. Predictive factors of low anterior resection syndrome following anterior resection of the rectum. Rev Col Bras Cir. (2020) 46(6):e20192361. doi: 10.1590/0100-6991e-20192361
66. Bolton WS, Chapman SJ, Corrigan N, Croft J, Collinson F, Brown JM, et al. The incidence of low anterior resection syndrome as assessed in an international randomized controlled trial (MRC/NIHR ROLARR). Ann Surg. (2020). doi: 10.1097/sla.0000000000003806
67. Dulskas A, Petrauskas V, Kuliavas J, Bickaite K, Kairys M, Pauza K, et al. Quality of life and bowel function following early closure of a temporary ileostomy in patients with rectal cancer: a report from a single-center randomized controlled trial. J Clin Med. (2021) 10(4). doi: 10.3390/jcm10040768
68. Rizzo G, Pafundi DP, Sionne F, D'Agostino L, Pietricola G, Gambacorta MA, et al. Preoperative chemoradiotherapy affects postoperative outcomes and functional results in patients treated with transanal endoscopic microsurgery for rectal neoplasms. Tech Coloproctol. (2021) 25(3):319–31. doi: 10.1007/s10151-020-02394-4
69. Benli S, Çolak T, Türkmenoğlu M. Factors influencing anterior/low anterior resection syndrome after rectal or sigmoid resections. Turk J Med Sci. (2021) 51(2):623–30. doi: 10.3906/sag-2007-145
Keywords: low anterior resection syndrome, colorectal cancer, total mesorectal excision, sphincter-preserving, risk factor
Citation: Ri H, Kang H, Xu Z, Kim K, Ren Y, Gong Z and Chen X (2022) The risk factors of low anterior resection syndrome after colorectal cancer surgery: A retrospective study of 566 patients in a single institution in China. Front. Surg. 9:990702. doi: 10.3389/fsurg.2022.990702
Received: 10 July 2022; Accepted: 5 August 2022;
Published: 25 August 2022.
Edited by:
Nicola Tartaglia, University of Foggia, ItalyReviewed by:
Juan José Segura-Sampedro, Hospital Universitario Son Espases, Spain© 2022 Ri, Kang, Xu, Kim, Ren, Gong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Chen Y2hlbnhpbmNqekBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID HyokJu Ri orcid.org/0000-0002-5172-7029 HaoNan Kang orcid.org/0000-0003-3893-3749 KunHyok Kim orcid.org/0000-0002-9740-2107 Xin Chen orcid.org/0000-0001-8122-4396
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.