
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 20 September 2022
Sec. Pediatric Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.990101
Aim: To detect the composition of the gut microbiota in biliary atresia after Kasai surgery.
Methods: Infants within six months after the Kasai operation who were diagnosed by cholangiography at Shanghai Children’s Hospital were enrolled in the study. Fecal samples were collected from diapers, placed into sterile tubes in the inpatient department or outpatient department and frozen at −80°C within half an hour. The gut microbiota was detected by 16S rRNA sequences. Then, the patients that were followed up to one year after the Kasai operation who suffered from cholangitis at least one time were grouped into the BAcho group, and the others were grouped into the BAnoncho group.
Results: Nine of 18 BA patients were grouped into the BAcho group, and the others were grouped into the BAnoncho group. In the BAcho group, AST, ALT and GGT were significantly increased compared to the BAnoncho group. The number of total OTUs (operational taxonomic units) in feces was more elevated in the BAnoncho group than in the BAcho group. In the BAnoncho group, the Chao index at the OTU level was significantly increased compared to that in the BAcho group (66.37 ± 21.5 vs. 45.64 ± 11.25, p = 0.02 < 0.05). Bifidobacterium was the most abundant genus in the BAnoncho group, accounting for 22.14%, and Klebsiella accounted for 22.74% in the BAcho group. Compared with the BAnoncho group, Bacteroides was significantly decreased in the BAcho group (p = 0.037).
Conclusion: The composition of the gut microbiota was different between BA with cholangitis and BA without cholangitis.
Biliary atresia (BA) is a progressive bile duct sclerosis disease with cholestasis that results in liver fibrosis (1). The incidence of BA ranges from 0.5–5:10,000 worldwide. The pathogenesis of BA, such as viral infection, gene mutation, autoimmunity, and abnormalities in bile duct development, is unclear (2). Kasai surgery, which was first performed by Kasai in Japan, is one of the main treatments for BA. However, approximately 70%–80% of children with liver cirrhosis after successful surgery still need liver transplantation (3–6). Reflux cholangitis, which is caused by the gut microbiota, is one of the risk factors for liver fibrosis after Kasai surgery. In addition, more than 60% of patients suffer from cholangitis within the first year after Kasai surgery (7, 8). Therefore, the purpose of the study was to investigate the distribution of the gut microbiota in cholangitis within the first year after Kasai surgery.
Type III biliary atresia infants from Shanghai Children’s Hospital were enrolled in the study. The biliary atresia was diagnosed by intraoperative cholangiography. After Kasai operation, cefoperazone sulbactam and metronidazole were given intravenously for 4 weeks, and then cefixime or sulfamethoxazol were taken orally in turn 5 mouths (change of oral antibiotic every week). All BA patients who were within 6 months after Kasai surgery with oral cefixime or cotrimoxazole were recruited to determine the gut microbiota between 2019.06 and 2020.06. All patients were followed up to one year after hepatic portoenterostomy. The exclusion criteria included intravenous antibiotics, oral probiotics and the loss of follow-up infants. The BA infants who suffered from at least one episode of cholangitis in the follow-up period were grouped as BAcho (BA patients with cholangitis), and the others were grouped as BAnoncho (BA patients without cholangitis). Meanwhile, the general characteristic information of all patients was collected. The definition of cholangitis is at least two of the following three points: (a) fever (temperature ≥38.5°C) with no other reasons, (b) jaundice or stool with lighter color, and (c) blood testing with elevated leukocytosis or C-reactive protein.
Fecal samples were collected from the diaper, placed into sterile tubes in the inpatient department or outpatient department and frozen at −80°C within half an hour. All samples were obtained from Shanghai Children’s Hospital in China. This study was approved by the ethics committee of the hospital. Genomic DNA extraction, PCR amplification, library preparation, and Illumina sequencing were performed according to methods described previously (9). In brief, total microbial DNA was extracted using a QIAamp DNA stool minikit (Qiagen, Germany). The extracted genomic DNA was PCR (polymerase chain reaction) amplified with barcoded primers (forward primer, 5′-ACT CCT ACG GGA GGC AGC AG-3′; reverse primer, 5′-GGA CTA CHV GGG TWT CTA AT-3′) targeting the 16S rRNA V3–V4 region. Water samples that had undergone the same procedures of DNA extraction and PCR amplification were used as a control. An equal amount of DNA from each sample was pooled and verified using an Agilent 2100 bioanalyzer (Agilent, USA). The data were analyzed on the free online Majorbio Cloud Platform (www.majorbio.com).
Differences in means were tested by the independent Student’s t test or the Wilcoxon rank-sum test between the two groups using SPSS 22. A p value of <0.05 was considered statistically significant. Bar graphs and photographs were processed by GraphPad Prism 8.
Eighteen BA patients were recruited for our study. Nine infants were grouped into BAcho, and the others were grouped into BAnoncho. Demographic data on clinical features are shown in Table 1. In the BAcho group, aspartate transferase, alanine transferase, and gamma-glutamyl transpeptidase levels in blood were significantly increased compared to those in the BAnoncho group. There were no differences between the two groups in terms of surgery age, collection time, direct bilirubin, total bilirubin, alkaline phosphatase or total bile acid. In the BAcho group, the yield of blood culture was 0. Five of nine patients in the BAcho group suffered from more than two cholangitis episodes within one year. Biochemical figures between BAcho group and BAnoncho group one mouth after Kasai surgery were also shown in Supplementary Table S2.
A total of 714,284 raw sequence reads were produced from 18 stool samples. The average number of reads per sample was 39682 ± 11062. The average length was 445 ± 4.9 bp (Supplementary Table S1). With the increasing number of samples, the number of total OTUs (operational taxonomic units) was more elevated in the BAnoncho group than in the BAcho group (Figure 1). In the BAnoncho group, the Chao index at the OTU level was significantly increased compared to that in the BAcho group (66.37 ± 21.5 vs. 45.64 ± 11.25, p = 0.02 < 0.05). There were 85 genera in the BAnoncho group and 61 in the BAcho group when less than 1% of genera were merged (Figure 2). In addition, 8 types of microbiota were detected at the genus level only in the BAcho group (shown in Supplementary Figure S1). The distribution of gut microbiota at the genus level in the BAcho and BAnoncho groups is shown in Figure 3. Bifidobacterium was the most abundant genus in the BAnoncho group, accounting for 22.14% (Figure 3). However, Klebsiella, which was the most abundant genus, occupied 22.74% in the BAcho group. Compared to the BAnoncho group, Bacteroides was significantly decreased in the BAcho group (0.0033 ± 0.0048 vs. 8.224 ± 20.48, p = 0.037). There was no difference in Klebsiella, Escherichia-Shigella, Bifidobacterium, Enterococcus, Enterobacter, or Clostridium-sensu-stricto-1 between the two groups (Figure 4).
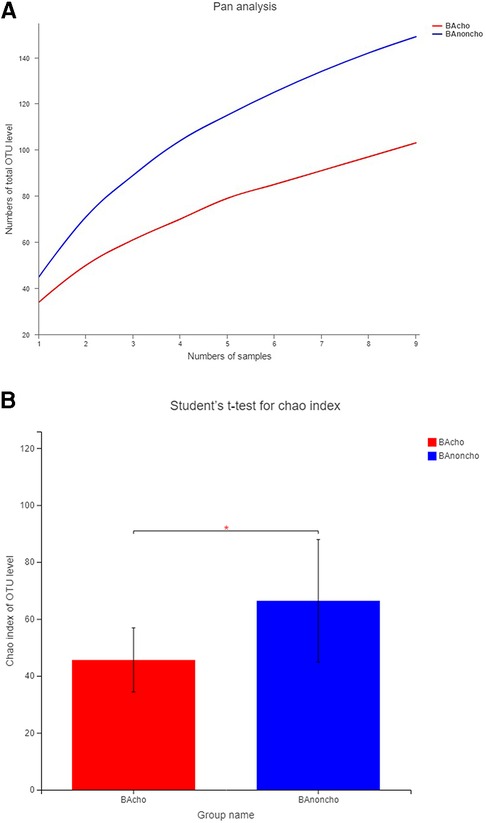
Figure 1. The OUT level in BAcho and BAnoncho. (A) The numbers of total OTU level were increasing with the numbers of samples. The numbers of OTU level in BAnoncho were more than those in BAcho in same numbers of samples. (B) In BAnoncho group, Chao index of OTU level was significantly increased compared to the BAcho group. (*p < 0.05, OTU: Operational Taxonomic Unit, defined by >97% 16S rRNA sequence similarity. Chao index:using for calculating the Community richness).
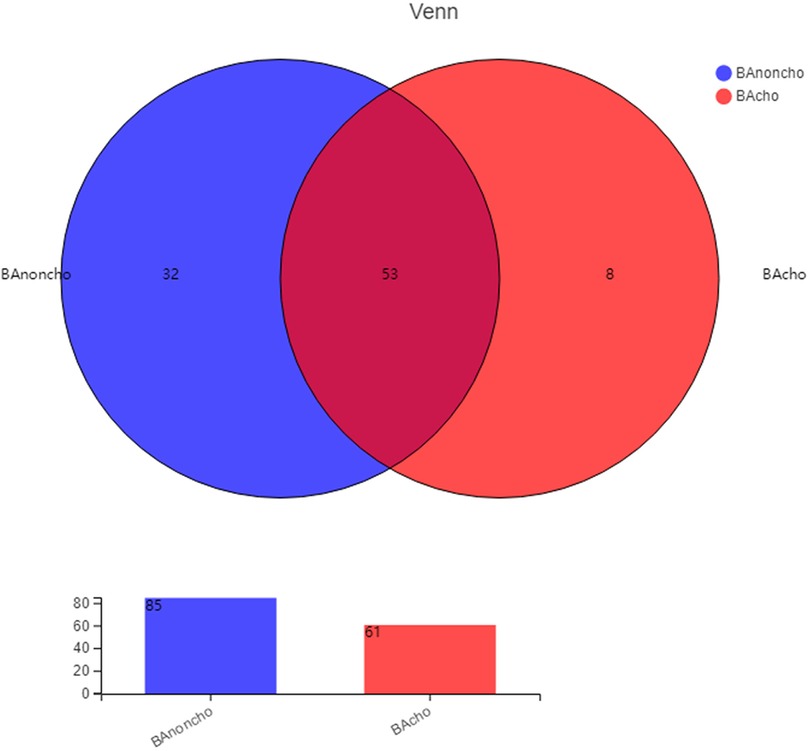
Figure 2. The kinds of gut microbiota in BAnoncho and BAcho group on genus level. There were 85 kinds of gut microbita in BAnoncho and 61 kinds in BAcho. There were 8 kinds of microbita on genus level only detected in BAcho group.
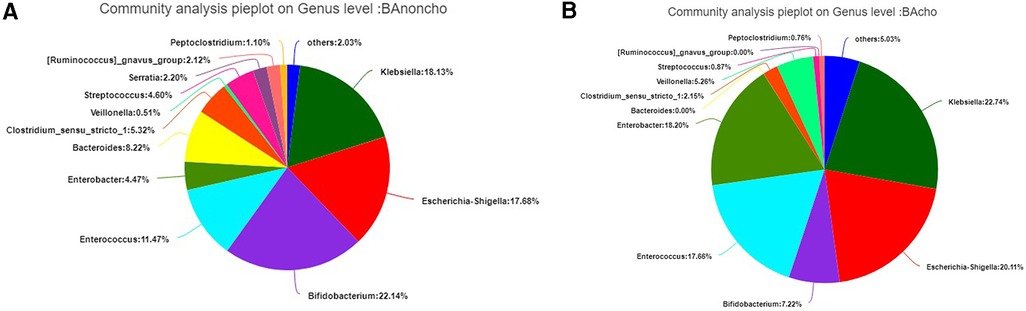
Figure 3. (A) The distribution of gut microbiota on genus level in BAnoncho. Bifidobacterium accounted for 22.14%. Klebsiella occupied 18.13%. Escherichia-shigella accounted for 17.68%. (B) The distribution of gut microbiota on genus level in BAcho. Klebsiella accounted for 22.74%. Escherichia-Shigella occupied 18.13%. Enterococcus accounted for 17.68%.
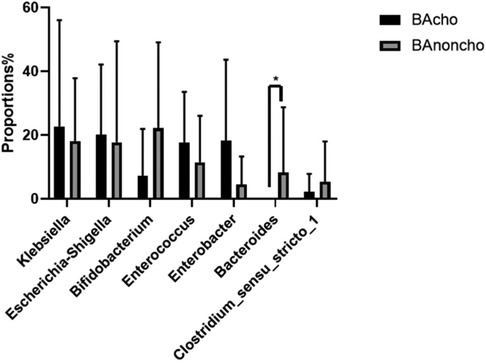
Figure 4. Wilcoxon rank-sum test bar plot on genus level between BAcho and BAnoncho. There was no difference on Klebsiella, Esherichia-Shigella, Bifidobacterium, Enterococcus, Enterobacter, Clostridium-sensu-stricto-1 between BAcho and BAnoncho group. Compare to BAcho, Bacterouides was significancely increased in BAnoncho. (*p < 0.05).
Cholangitis is the most common complication in BA after the Kasai operation. Katawaetee Decharun, MD (10) reported that the morbidity of cholangitis ranged from 15% to 62% with antibiotics. In most case series, nearly 90% of cholangitis patients experience episodes of cholangitis within the first year after the Kasai operation (11, 12). Qianfu Luo reported that serum bacteria such as Escherichia coli, Klebsiella pneumoniae, Shigella fexneri, and Enterobacteriaceae bacterium in cholangitis patients were similar to those in the gut (13). The main pathogenic mechanism of cholangitis may be bacterial translocation from the enteric tract, which may be caused by an insufficient volume of bile flow (14). In our study, there were 9 patients suffering from cholangitis, which accounted for 50%, and the incidence rate of cholangitis was similar to that in previous reports (15). Bacteria that cause cholangitis can be detected by blood culture, but the positivity rate is very low, ranging from 8.9% to 35.1% (16, 17). In our study, the yield of blood culture was zero. The main reason for the result is that the number of cases enrolled in the study was too small or because all of the patients were on oral antibiotics when the fecal samples were collected.
There are 100 trillion microorganisms of more than 1,000 types in the healthy human gut. The gastrointestinal microbial flora is affected by many factors, including diet, antibiotics, bile acid and delivery (18, 19). In turn, gut microbiota have a direct effect on human health by themselves and byproducts (18). Due to the diversity of affected factors, the gastrointestinal microbial flora in pediatric patients is very different from that in adults (20, 21). A study reported that in BA patients, the microbial diversity and gut primary and secondary bile acids were significantly reduced compared with those in healthy controls (22). It has been speculated that bile acid deficiency and abnormal bile acid metabolism may have altered the gut environment, leading to a shift in the gut microbiota composition (23). Yizhong Wang et al. reported that the diversity was significantly lower in infants with cholestatic jaundice than in healthy controls (9). In our study, the diversity of the fecal microbiota was significantly reduced in the BAcho group compared to the BAnoncho group. However, the blood total bile acid was similar between the BAcho group and BAnoncho group, and bile acid may not be the main factor affecting the diversity of the fecal microbiota in the BA after the Kasai operation.
To our knowledge, this is the first study to detect the composition of gut microbiota between the BAcho and BAnoncho groups by 16S rRNA sequencing. Zheng (22) reported that in BA, microbial dysbiosis was characterized by the enrichment of facultative anaerobes. Facultative anaerobes, such as Streptococcus, Klebsiella and Enterococcus, are considered potential pathogens that correlate with liver function indices in BA. In our study, Klebsiella was also increased in the two groups, which may be manipulated by antibiotics. The composition of Bacteroides accounted for 8.2% in the BAnoncho group compared with 0% in the BAcho group. The abundance of Bacteroides was very different in different alcohol feeding models, and it was found that the Bacteroides were relatively increased in intragastric feeding with alcohol, while Bacteroides were decreased in mice with chronic ad libitum ethanol feeding of the Lieber-DeCarli ethanol liquid (24–26). The abundance of Bacteroides was decreased in alcoholics. Ley considered that Bacteroides is decreased in human obesity (27). Due to the complexity of the fecal microbiota, the function of Bacteroides requires further study. We also found that Bidfidobacterium, which is considered a probiotic, had the highest proportion in the BAnoncho group. Tien-Hau Lien (28) reported that Lactobacillus casei Rhamnosu, a probiotic, was as effective as antibiotics in preventing cholangitis in BA. Recently, Ewa Orowska found (29) that the Lactobacillus casei Rhamnosu group had a lower rate of cholangitis after the Kasai operation than the placebo group, although the difference was not statistically significant in a randomized, double-blind, placebo-controlled trial. It is possible that probiotics for preventing cholangitis in BA with the Kasai operation may be a new therapy in the future.
The composition of the gut microbiota was different between BA with cholangitis and BA without cholangitis.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by ethics of committee of Shanghai Children's Hospital, Shanghai children's Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
ZL and WY wrote the paper. GZ collected stool and followed up the patient. LZ, XW, SQ, HX, LJ done the Kasai surgery. All authors contributed to the article and approved the submitted version.
The research was supported by Special Project for Clinical Research and cultivation from Shanghai Children’s Hospital (2021YLYM02).
The reviewer (ZLL) and the handling editor declared a shared affiliation at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.990101/full#supplementary-material.
1. McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. (2000) 355(9197):25–9. doi: 10.1016/S0140-6736(99)03492-3
2. Vij M, Rela M. Biliary atresia: pathology, etiology and pathogenesis. Future Sci OA. (2020) 6(5):FSO466. doi: 10.2144/fsoa-2019-0153
3. de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ, Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol. (2011) 9(12):1086–91. doi: 10.1016/j.cgh.2011.07.024
4. Gad EH, Kamel Y, Salem TA, Ali MA, Sallam AN. Short- and long-term outcomes after Kasai operation for type III biliary atresia: twenty years of experience in a single tertiary Egyptian center-A retrospective cohort study. Ann Med Surg. (2021) 62:302–14. doi: 10.1016/j.amsu.2021.01.052
5. Muraji T. Early detection of biliary atresia: past, present / future. Expert Rev Gastroenterol Hepatol. (2012) 6(5):583–9. doi: 10.1586/egh.12.37
6. Dübbers M. Gallenwegschirurgie im Kindesalte. Biliary tract surgery in childhood. Chirurg. (2020) 91(1):23–8 (in German). doi: 10.1007/s00104-019-01058-w
7. Baek SH, Kang JM, Ihn K, Han SJ, Koh H, Ahn JG. The epidemiology and etiology of cholangitis after Kasai portoenterostomy in patients with biliary atresia. J Pediatr Gastroenterol Nutr. (2020) 70(2):171–7. doi: 10.1097/MPG.0000000000002555
8. Ginström DA, Hukkinen M, Kivisaari R, Pakarinen MP. Biliary atresiaassociated cholangitis: the central role and effective management of bile lakes. J Pediatr Gastroenterol Nutr. (2019) 68(04):488–94. doi: 10.1097/MPG.0000000000002243
9. Wang Y, Gao X, Zhang X, Xiao Y, Huang J, Yu D, et al. Gut Microbiota dysbiosis is associated with altered bile acid metabolism in infantile cholestasis. mSystems. (2019) 4(6):e00463–19. doi: 10.1128/mSystems.00463-19
10. Decharun K, Leys CM, West KW, Finnell SM. Prophylactic antibiotics for prevention of cholangitis in patients with biliary atresia status post-Kasai portoenterostomy: a systematic review. Clin Pediatr. (2016) 55(1):66–72. doi: 10.1177/0009922815594760
11. Bowles BJ, Abdul-Ghani A, Zhang J, Shim WK. Fifteen years’ experience with an antirefluxing biliary drainage valve. J Pediatr Surg. (1999) 34(11):1711–4. doi: 10.1016/s0022-3468(99)90651-6
12. Ogasawara Y, Yamataka A, Tsukamoto K, Okada Y, Lane GJ, Kobayashi H, et al. The intussusception antireflux valve is ineffective for preventing cholangitis in biliary atresia: a prospective study. J Pediatr Surg. (2003) 38(12):1826–9. doi: 10.1016/j.jpedsurg.2003.08.025
13. Luo Q, Hao F, Zhang M, Guo C. Serum bacterial DNA detection in patients with cholangitis after Kasai procedure. Pediatr Int. (2015) 57(5):954–60. doi: 10.1111/ped.12737
14. Chuang JH, Lee SY, Chen WJ, Hsieh CS, Chang NK, Lo SK. Changes in bacterial concentration in the liver correlate with that in the hepaticojejunostomy after bile duct reconstruction: implication in the pathogenesis of postoperative cholangitis. World J Surg. (2001) 25(12):1512–8. doi: 10.1007/s00268-001-0162-9
15. Hertel PM, Estes MK. Rotavirus and biliary atresia: can causation be proven? Curr Opin Gastroenterol. (2012) 28(1):10–7. doi: 10.1097/MOG.0b013e32834c7ae4
16. Hung PY, Chen CC, Chen WJ, Lai HS, Hsu WM, Lee PH, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr. (2006) 42(2):190–5. doi: 10.1097/01.mpg.0000189339.92891.64
17. Ernest van Heurn LW, Saing H, Tam PK. Cholangitis after hepatic portoenterostomy for biliary atresia: a multivariate analysis of risk factors. J Pediatr. (2003) 142(5):566–71. doi: 10.1067/mpd.2003.195
18. Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. (2018) 15(7):397–411. doi: 10.1038/s41575-018-0011-z
19. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. (2010) 107(26):11971–5. doi: 10.1073/pnas.1002601107
20. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. (2012) 486(7402):222–7. doi: 10.1038/nature11053
21. Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta TA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. (2015) 3:36. doi: 10.1186/s40168-015-0101-x
22. Wang J, Qian T, Jiang J, Yang Y, Shen Z, Huang Y, et al. Gut microbial profile in biliary atresia: a case-control study. J Gastroenterol Hepatol. (2020) 35(2):334–42. doi: 10.1111/jgh.14777
23. Molinaro A, Wahlstrom A, Marschall HU. Role of bile acids in metabolic control. Trends Endocrinol Metab. (2018) 29:31–41. doi: 10.1016/j.tem.2017.11.002
24. Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. (2011) 53(1):96–105. doi: 10.1002/hep.24018
25. Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. (2013) 8(1):e53028. doi: 10.1371/journal.pone.0053028
26. Canesso MCC, Lacerda NL, Ferreira CM, Gonçalves JL, Almeida D, Gamba C, et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol. (2014) 14:240. doi: 10.1186/s12866-014-0240-4
27. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. (2006) 444(7122):1022–3. doi: 10.1038/4441022a
28. Lien TH, Bu LN, Wu JF, Chen HL, Chen AC, Lai MW, et al. Use of Lactobacillus casei rhamnosus to prevent cholangitis in biliary atresia after Kasai operation. J Pediatr Gastroenterol Nutr. (2015) 60(5):654–8. doi: 10.1097/MPG.0000000000000676
Keywords: biliary atresia, composition, gut microbiota, Kasai operation, cholangitis
Citation: Zheng L, Wu Y, Gong Z, Lv Z, Xu W, Sheng Q, Huang X and Liu J (2022) The composition of the gut microbiota is altered in biliary atresia with cholangitis. Front. Surg. 9:990101. doi: 10.3389/fsurg.2022.990101
Received: 9 July 2022; Accepted: 5 September 2022;
Published: 20 September 2022.
Edited by:
Alessandro Inserra, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Zoe Larghi Laureiro, Bambino Gesù Children's Hospital (IRCCS), Italy© 2022 Zheng, Wu, Gong, Lv, Xu, Sheng, Huang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wu Yibo eWlib193dTEwMDdAMTYzLmNvbQ==
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.