- 1Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Clinical Immunology Center, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Purposes: To compare perioperative outcomes of robotic pancreaticoduodenectomy (RPD) to open pancreaticoduodenectomy (OPD) using evidence from cohort studies.
Methods: Outcomes of interest include operative time, blood loss, R0 resection rate, lymph nodes harvested, overall complication rate, pancreatic fistula rate, delayed gastric emptying rate and 90-day mortality.
Results: 6 prospective studies and 15 retrospective studies were included. Five of these studies were limited to patients with pancreatic cancer. Operative time was significantly longer in RPD (WMD: 64.60 min; 95% CI: 26.89 to 102.21; p = 0.001). Estimated blood loss was lower in RPD (WMD: −185.44 ml; 95% CI: −239.66 to −131.21; p < 0.001). Overall complication rates (OR: 0.66; 95% CI: 0.44 to 0.97; p < 0.001) and pancreatic fistula rate (OR: 0.67; 95% CI: 0.55 to 0.82; p < 0.001) were both lower in RPD. Length of hospital stay was longer in OPD (WMD: −1.90; 95% CI: −2.47 to −1.33). 90-day mortality was lower in RPD [odds ratio (OR): 0.77; 95% CI: 0.45 to 0.95; p = 0.025].
Conclusion: At current level of evidence, RPD is a safer alternative than OPD with regard to post-operative outcomes and blood loss. However, in terms of oncological outcomes RPD show no advantage over OPD, and the cost of RPD was higher. In general, RPD is now considered a reliable technology, but high-quality randomized controlled trial (RCT) studies are still needed to support this conclusion.
1. Introduction
Pancreaticoduodenectomy (PD) has been universally accepted to be indicated in benign or malignant lesions of the pancreatic head, duodenum, and distal common bile duct. In 1994, Gagner reported the first laparoscopic pancreaticoduodenectomy, since when minimally invasive pancreaticoduodenectomy (MIPD) are increasingly being performed over the world (1). The development of the Da Vinci robotic platform takes MIPD a step further. Laparoscopic surgery has some shortcomings compared to robotic surgery, including limited vision and flexibility. And this contributed to the popularity of robotic surgery over the world (2). The first case of robotic-assistant pancreaticoduodenectomy (RAPD) was reported in 2007, and since then many studies have compared the safety and efficacy between open pancreaticoduodenectomy (OPD) and robotic pancreaticoduodenectomy (RPD). There have been several meta-analyses evaluating the effect between OPD and RPD. However, robotic surgery technology developed rapidly in these years, and the studies used in the existing meta-analyses are not new enough. Therefore, we focused on those studies published in the last 5 years (in or after 2016) to provide high-quality evidence for further clinical practice.
2. Methods
2.1. Literature-search strategy
A systematic review of the literature was performed in PubMed and Web of Science from January 2016 to October 2021. These key words were used: robot, robotic, robotic-assisted, open, and pancreaticoduodenectomy. Studies included should fulfill the following PICOS criteria in our meta-analyses. P (patients): Male or female patients with a benign or malignant disease that requires elective PD; I (intervention): RPD; C (control): OPD; O (outcome): At least 1 of the interested outcomes; S (study design): randomized controlled trials (RCTs) and observative studies.
References of the acquired articles were manually searched to broaden the search. When multiple researches describing the same population were published, the most complete or recent research was used.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as followed: (1) comparative study of RPD and OPD; (2) papers written in English; (3) papers published in or after 2016. Abstracts, case reports, reviews, letters to the editor, non-comparative studies, and articles without available data were excluded.
2.3. Data extraction and outcome of interest
All references were reviewed and evaluated by two researchers independently. Only full-length articles were eligible for extraction. The following data of included articles were extracted: first author, year of publication, study design, number of operated subjects, operative time, blood loss, R0 resection rate, lymph nodes harvested, overall complication rate, pancreatic fistula rate, delayed gastric emptying and 90-day mortality.
2.4. Quality assessment
The Newcastle–Ottawa Scale (NOS) was used to evaluate the methodological quality of non-randomized studies. Scores of each observational study range from 0 to 9, and studies having six or more stars were considered to be high-quality studies.
2.5. Statistical analysis
This meta-analysis was performed using Stata MP 16.0 software. The odds ratios (OR) and the weighted mean difference (WMD) with a 95% confidence interval (95% CI) were used to estimate dichotomous and continuous variables, respectively. p < 0.05 was considered statistically significant. For studies that reported continuous data as median and range values (or quartile and median), the standard deviations were calculated using the method described by Luo et al. (3). Heterogeneity was evaluated by the Chi-square test, and p < 0.100 was considered significant. I2 values were used for the evaluation of statistical heterogeneity. An I2 value of 50% or more indicated the presence of heterogeneity. The fixed effect model (FEM) and random effect model (REM) were used based on the value of I2. FEM was used in the case of I2 < 50% while REM was adopted in the case of I2 > 50%.
3. Result
3.1. Literature-search results
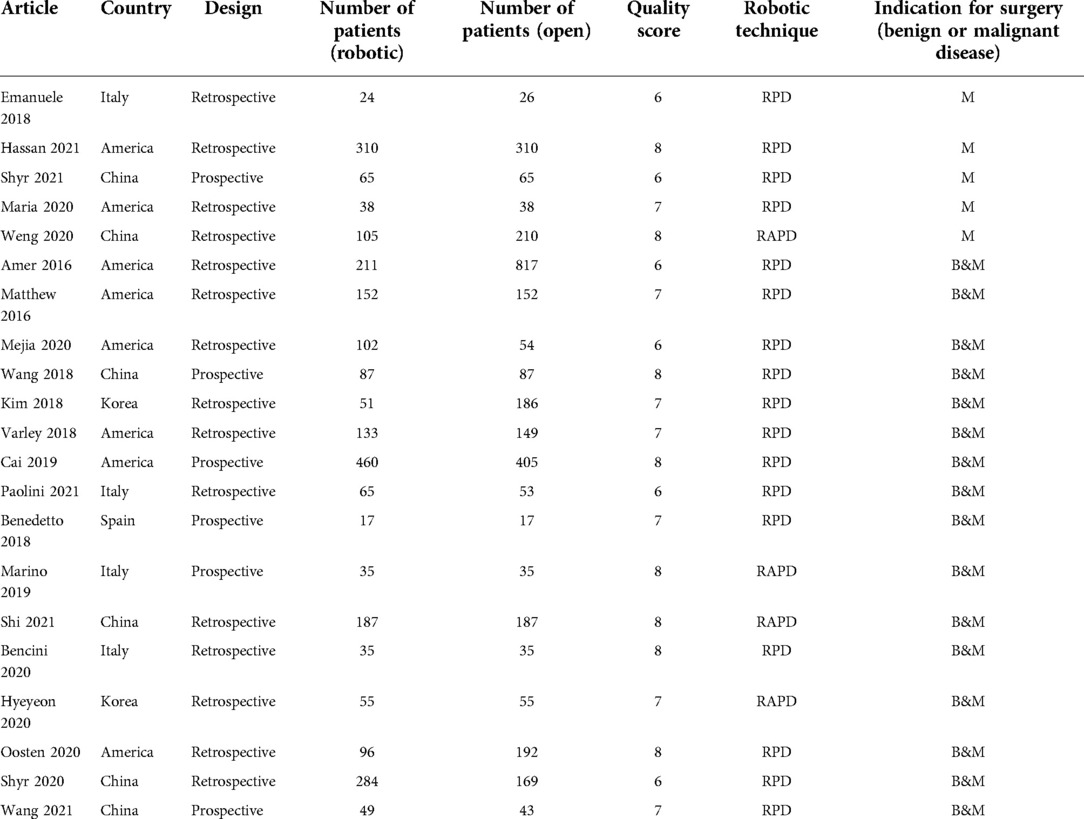
The first search strategy generated 518 studies. 21 articles including 5,756 patients (2,561 cases for RPD and 3,285 cases for OPD) fulfilled the predefined inclusion criteria and were included in this meta-analysis (4–24). All studies were non-RCTs, of which 6 studies were prospective while 15 studies were retrospective. The selection process is shown in Figure 1.
3.2. Study characteristics and quality assessment
The study characteristics and study quality are shown in Table 1. We screened articles published in 2016 and beyond. This is a worldwide meta-analysis, in which eight articles are from America, six articles are from China, four articles are from Italy, two articles are from Korea, and one article is from Spain. In most studies the robotic surgery group carried out RPD, while in four studies RAPD were used, where robots were only involved in some parts of the surgery. In five studies patients were limited to pancreatic cancer, while in other studies the indication for surgery were wide, including benign and malignant disease. All the studies were of high quality according to the Newcastle–Ottawa Scale (NOS) (Supplementary Table S1).
Patients’ baseline characteristics are summarized in Table 2.
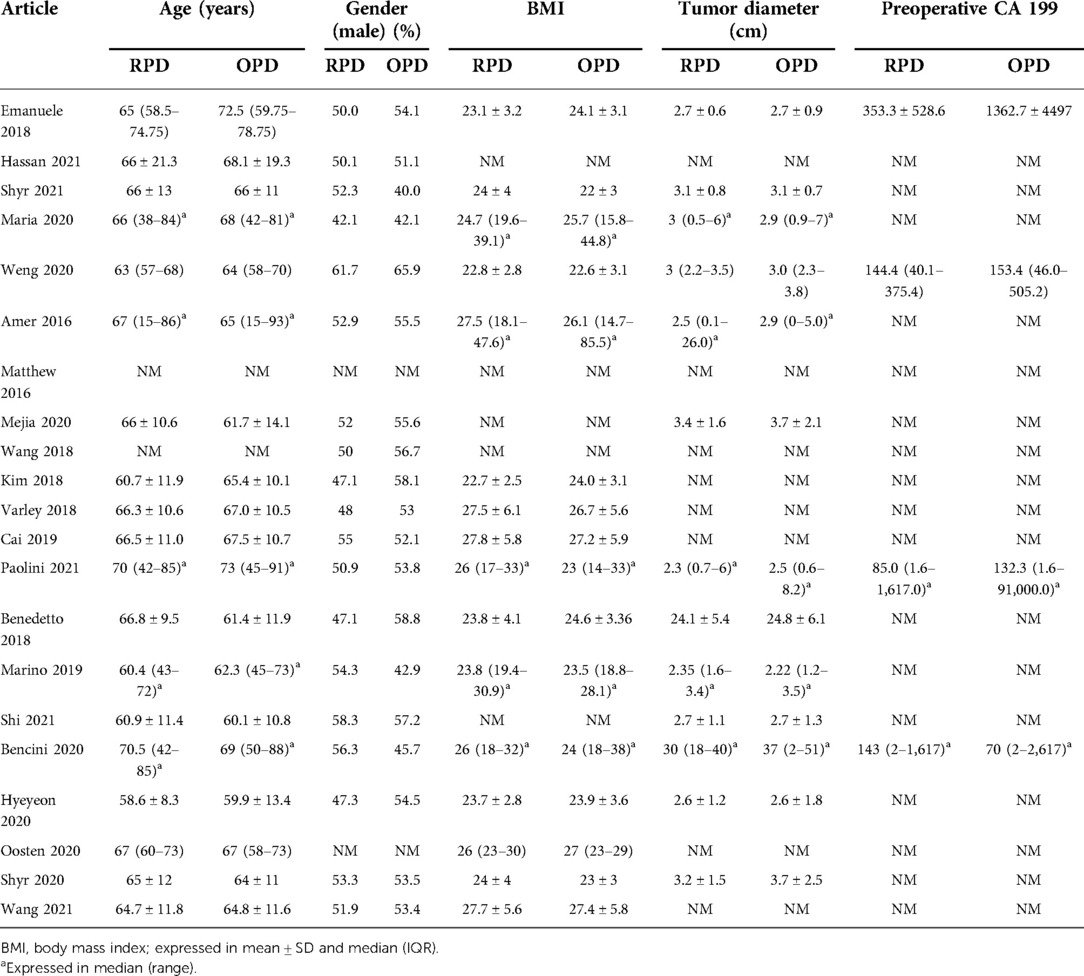
Table 2. Comparison of patients’ baseline characteristics in robotic vs. open pancreaticoduodenectomy.
3.3. Meta-analysis results
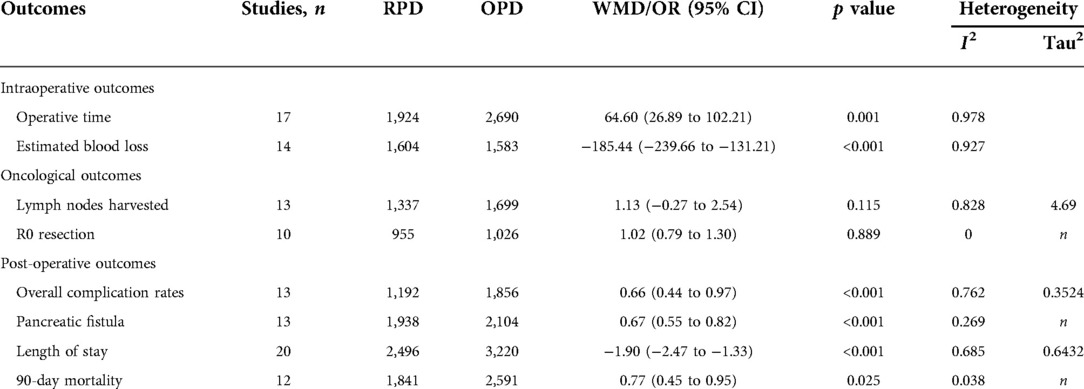
All 21 studies were included in this meta-analysis. The summarized result of meta-analysis is shown in Table 3.
3.3.1. Intraoperative outcomes
3.3.1.1. Operative time
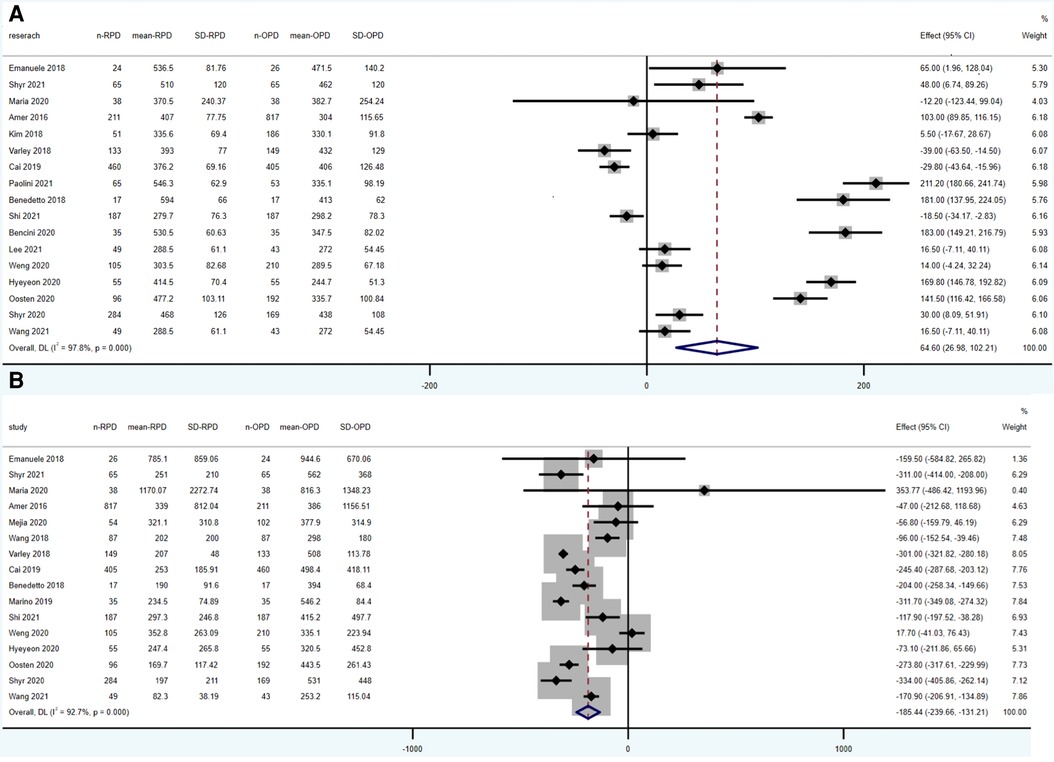
Operative time was reported in 17 studies (1,924 RPD vs. 2,690 OPD). According to the results of this meta-analysis, operative time was significantly longer in RPD group (WMD: 64.60 min; 95% CI: 26.89 to 102.21; p = 0.001), with high heterogeneity (I2 = 97.8%; Tau2 = 2133.45) in the REM (Figure 2A).
3.3.1.2. Estimated blood loss
Estimated blood loss was reported in 14 studies (1,604 RPD vs. 1,583 OPD) and was significantly lower in RPD (WMD: −185.44 ml; 95% CI: −239.66 to −131.21; p < 0.001) with high among-study statistical heterogeneity (I2 = 92.7%; Tau2 = 3152.37) (Figure 2B).
3.3.2. Oncological outcomes
3.3.2.1. Lymph nodes harvested
13 studies reported the results of lymph nodes harvested (1,337 RPD vs. 1,699 OPD). No statistically significant differences were found between the two groups (WMD: 1.13; 95% CI: −0.27 to 2.54; p = 0.115), with a high heterogeneity (I2 = 82.8%, Tau2 = 4.69) in the REM (Figure 3A).
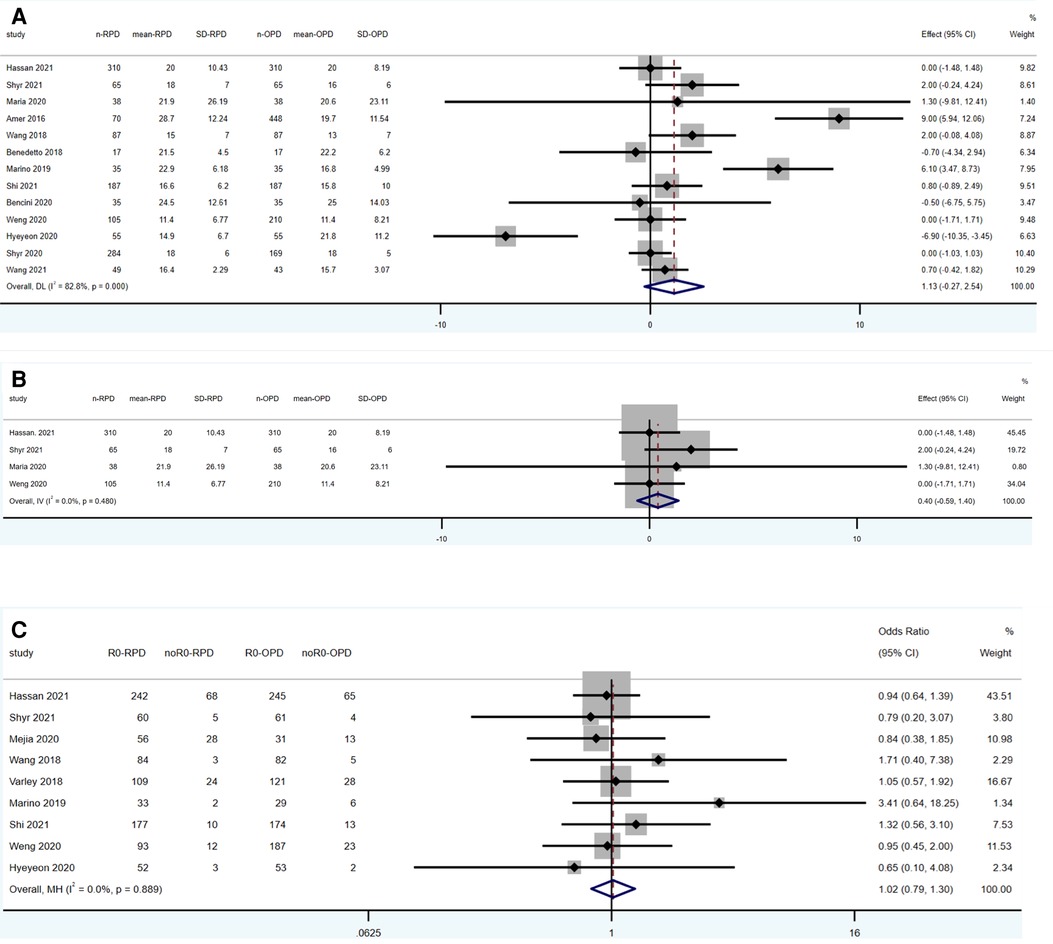
Figure 3. Meta-analysis of oncological outcomes: (A) lymph nodes harvested. (B) Lymph nodes harvested (in pancreatic cancer). (C) R0 resection.
3.3.2.2. Lymph nodes harvested (in pancreatic cancer)
Four studies reporting the results of lymph nodes harvested are limited in pancreatic cancer patients (518 RPD vs. 623 OPD). No statistically significant differences were found between the two groups (WMD: 0.4; 95% CI: −0.59 to 1.40; p = 0.425), with a low heterogeneity (I2 = 0%) in the FEM (Figure 3B).
3.3.2.3. R0 resection
Ten studies reported the results of lymph nodes harvested (955 RPD vs. 1,026 OPD). No statistically significant differences were found between the two groups (OR: 1.02; 95% CI: 0.79 to 1.30; p = 0.889), with a low heterogeneity (I2 = 0%) in the FEM (Figure 3C).
3.3.3. Post-operative outcomes
3.3.3.1. Overall complication rates
Overall complication rate was reported in 13 studies (1,192 RPD vs. 1,856 OPD) and was significantly lower in RPD (OR: 0.66; 95% CI: 0.44 to 0.97; p < 0.001) with high among-study statistical heterogeneity (I2 = 76.2%; Tau2 = 0.3524) in the REM (Figure 4A).
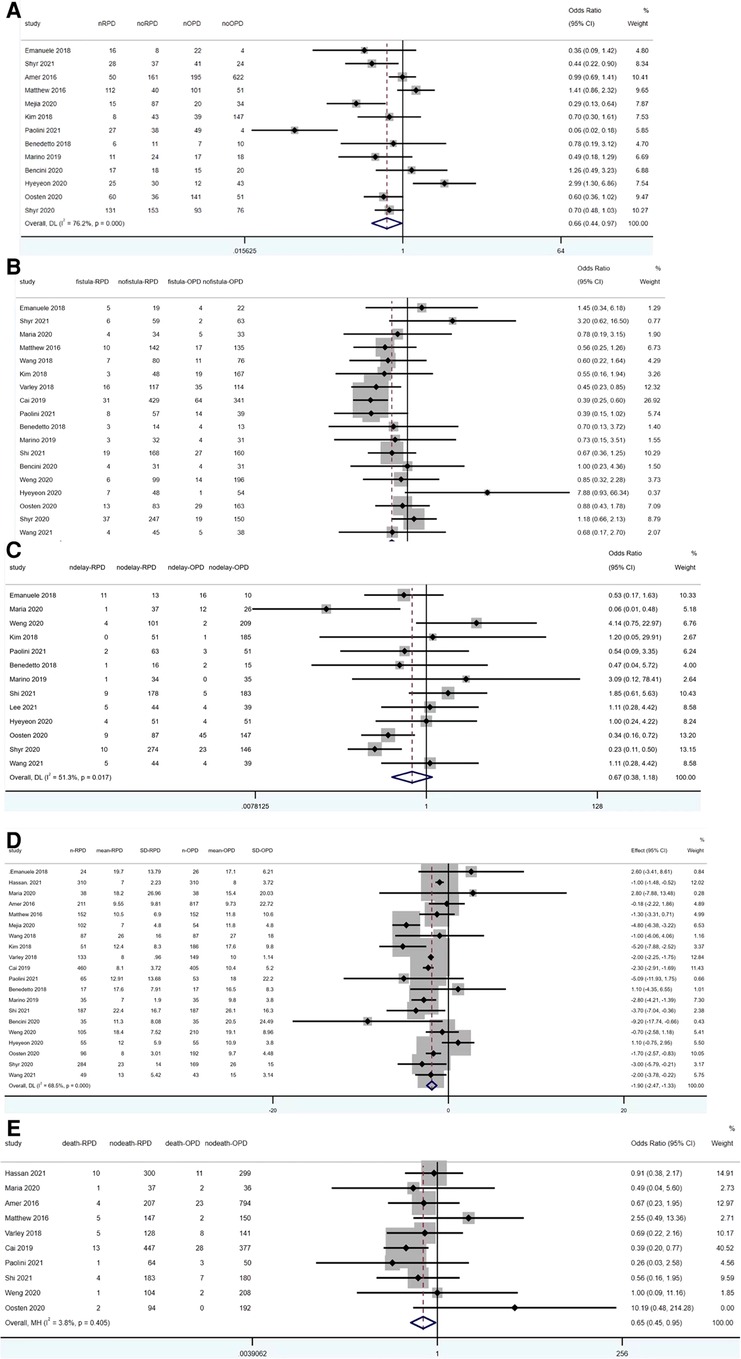
Figure 4. Meta-analysis of post-operative outcomes: (A) overall complication rates. (B) Pancreatic fistula. (C) Delayed gastric emptying. (D) Length of stay. (E) 90-day mortality.
3.3.3.2. Pancreatic fistula
Pancreatic fistula was reported in 13 studies (1,938 RPD vs. 2,104 OPD) and was significantly lower in RPD (OR: 0.67; 95% CI: 0.55 to 0.82; p < 0.001) with low among-study statistical heterogeneity (I2 = 26.9%) in the FEM (Figure 4B).
3.3.3.3. Delayed gastric emptying
Thirteen studies reported the results of delayed gastric emptying (1,055 RPD vs. 1,257 OPD). No statistically significant differences were found between the two groups (OR: 0.67; 95% CI: 0.38 to 1.18; p = 0.165), with a high heterogeneity (I2 = 51.3%, Tau2 = 0.4918) in the REM (Figure 4C).
3.3.3.4. Length of stay
20 studies reported the data of length of stay (2,496 RPD vs. 3,220 OPD). The meta-analysis showed OPD has significant longer length of stay than RPD (WMD: −1.90; 95% CI: −2.47 to −1.33; p < 0.001), with a high heterogeneity (I2 = 68.5%, Tau2 = 0.6432) in the REM (Figure 4D).
3.3.3.5. 90-day Mortality
12 studies reported the data of 90-day mortality (1,841 RPD vs. 2,591 OPD). The meta-analysis showed RPD has significant lower 90-day mortality than OPD (OR: 0.65; 95% CI: 0.45 to 0.95; p = 0.025), with a low heterogeneity (I2 = 3.8%) in the FEM (Figure 4E).
4. Discussion
Since first RAPD was reported in 2007, RPD technology has developed rapidly. With the improvement of equipment and doctors gradually through the learning curve, the safety and efficiency of RPD comparing to OPD is gradually improved. Hence, the relevant research results have timeliness. Therefore, although there have been previous meta-analyses comparing clinical outcomes between OPD and RPD, these meta-analyses contained some former studies and can't sufficiently represent current situation. So, we screened articles published after 2016 in our meta-analyses and contained several new studies in this year in order to show the latest RPD development as far as possible.
4.1. Findings in our meta-analyses
According to the result of our meta-analysis, RPD has a longer operative time and lower blood loss comparing to OPD, which is also supported by previous researches. As a significant advantage of robotic surgery, RPD showed a lower blood loss. And it may be explained by high-quality three-dimensional (3-D), optical 10–15 magnification vision, and greater precision (25). Multiple factors may lead to the longer operative time in RPD. On one hand, the long time for preparation of machine before operation resulted in a longer operative time. On the other hand, surgeons in these studies not passing through the learning curve may also contribute to longer operative time. What deserve attention is that the result of operative time and estimated blood loss showed high heterogeneity. According to the forest plot of operative time (Figure 2A), most studies raised up that operative time was higher in RPD group, but four studies reached the opposite conclusion (7, 14, 15, 19). Many factors can affect the operation time, of which the most important factor is the proficiency of the surgeon. In addition, the equipment of the center and the surgery team also influence the operative time. The heterogeneity of estimated blood loss was also high (I2 = 0.927). One study showed obvious different conclusion comparing with other studies (7). It's obvious that the exclusion of this study will not influence the conclusion of our article. Blood loss can be affected by the proficiency of the surgeon and the condition of the patients (e.g., the location and kind of cancer).
Lymph nodes harvested and margin status are considered to be consistent with prognosis of pancreatic cancer. Although various methods of margin quantification in different studies increase the complexity to assessment, margin status is still recognized to have prognostic significance for overall survival of pancreatic ductal adenocarcinoma (PDAC) in PD (26). Similarly, the number of lymph nodes harvested also plays a role in the reveal of prognostic performance (27). Historically, a mass of researches on OPD and RPD compared their differences in margin status, and previous meta-analysis also counted the oncological outcomes of the two groups. Except the meta-analysis of Dong et al. demonstrated that the RPD group has a larger number of lymph nodes harvested and a lower resection margin involvement rate, another two meta-analyses early and this meta-analysis all reveal that there is no difference of those oncological outcomes in the two groups (28–30). The heterogeneity of lymph nodes harvested and overall complication rate is high. The composition of patients’ tumor varies in different studies, which may lead to the heterogeneity of lymph nodes harvested. Besides, different operation centers may have different diagnostic criteria and definition for post-operative complications, causing the heterogeneity of overall complication rate.
Furthermore, we analyzed the oncological outcomes in studies limited to pancreatic cancer. Five studies analyzed patients with only pancreatic cancer, and other studies contained patients with kinds of disease which accepted RPD or OPD. In most studies, patients accepted PD because of different diseases, including pancreatic cancer, ampullary adenocarcinoma, and neuroendocrine tumor. Obviously, the malignancy of these tumors is different, reducing the credibility of the comparison of the prognosis indicator between OPD and RPD.
Of the five studies limited to pancreatic cancer, four involved lymph nodes harvested, and analysis of these four articles also showed no difference in RPD and OPD groups. Only two articles limited in pancreatic cancer mentioned R0 resection which is too few to analyze. Comparing the results of the two meta-analyses, no different conclusions were reached.
The safety of RPD has been proved in previous studies. As expected, our meta-analysis revealed that clinical outcomes favor RPD, including overall complication rates, pancreatic fistula rate, and length of hospital stay. Besides, different from the previous meta-analysis, this meta-analysis demonstrated that 90-day mortality also favors RPD.
4.2. Strengths
The safety and efficiency of RPD comparing to OPD is gradually improved, owing to the improvement of equipment and doctors gradually through the learning curve. This is the latest meta-analyses that included all eligible studies published in these 5 years. The number of studies is one strength of our article. Furthermore, to our knowledge, this is the first meta-analyses and systematic review that included all studies limited to patients with pancreatic cancer.
4.3. Limitations
Although we found 5 studies researching RPD and OPD in pancreatic cancer patients, most studies mix patients with various diseases together for analysis, making it impossible to conduct subgroup analysis. Besides, lack of RCTs in our meta-analysis is another limitation.
4.4. Implications for clinical practice
This meta-analysis found that RPD showed lower blood loss, overall complication rates, pancreatic fistula, and 90-day mortality compared with OPD. Besides, length of hospital stay was shorter in RPD. Although the operative time is longer in RPD group, and there were no differences in R0 resection and lymph nodes harvested, RPD has shown benefits over OPD and seemed to be proposed as an equivalent alternative to OPD. However, all the current studies about OPD and RPD are not RCTs, and high-quality studies are still needed. In addition, centers with the ability to perform a sufficient number of surgeries and professional surgeons who have overcome the learning curve are essential for successful implementation of RPD. What's more, RPD costs much higher than OPD, which is also an important factor in the choice of surgical methods.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
Conception and design of the work: YF and JQ. Data acquisition and analysis: YF and YY. Interpretation of the data: YF and YY. Drafting the manuscript: YF and JQ. Critical revision of the manuscript: YY and DW. Agreement to be accountable for all aspects of the work: YF, JQ, YY, DW, and TZ. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Key R/D Program of China (2018YFE0118600); National Natural Science Foundation of China (No. 81972258); National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.989065/full#supplementary-material.
References
1. Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. (1994) 8(5):408–10. doi: 10.1007/BF00642443
2. Kornaropoulos M, Moris D, Beal EW, Makris MC, Mitrousias A, Petrou A, et al. Total robotic pancreaticoduodenectomy: a systematic review of the literature. Surg Endosc. (2017) 31(11):4382–92. doi: 10.1007/s00464-017-5523-z
3. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
4. Kauffmann EF, Napoli N, Menonna F, Iacopi S, Lombardo C, Bernardini J, et al. A propensity score-matched analysis of robotic versus open pancreatoduodenectomy for pancreatic cancer based on margin status. Surg Endosc. (2019) 33(1):234–42. doi: 10.1007/s00464-018-6301-2
5. Aziz H, Khan M, Khan S, Serra GP, Goodman MD, Genyk Y, et al. Assessing the perioperative complications and outcomes of robotic pancreaticoduodenectomy using the national cancer database: is it ready for prime time? J Robot Surg. (2022)16(3):687–94. doi: 10.1007/s11701-021-01296-3
6. Shyr BU, Shyr BS, Chen SC, Shyr YM, Wang SE. Propensity score-matched comparison of the oncological feasibility and survival outcomes for pancreatic adenocarcinoma with robotic and open pancreatoduodenectomy. Surg Endosc. (2022) 36(2):1507–14. doi: 10.1007/s00464-021-08437-7
7. Baimas-George M, Watson M, Murphy KJ, Iannitti D, Baker E, Ocuin L, et al. Robotic pancreaticoduodenectomy may offer improved oncologic outcomes over open surgery: a propensity-matched single-institution study. Surg Endosc. (2020) 34(8):3644–9. doi: 10.1007/s00464-020-07564-x
8. Weng Y, Jiang Y, Fu N, Jin J, Shi Y, Huo Z, et al. Oncological outcomes of robotic-assisted versus open pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a propensity score-matched analysis. Surg Endosc. (2021) 35(7):3437–48. doi: 10.1007/s00464-020-07791-2
9. Zureikat AH, Postlewait LM, Liu Y, Gillespie TW, Weber SM, Abbott DE, et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. (2016) 264(4):640–9. doi: 10.1097/SLA.0000000000001869
10. McMillan MT, Zureikat AH, Hogg ME, Kowalsky SJ, Zeh HJ, Sprys MH, et al. A propensity score-matched analysis of robotic vs open pancreatoduodenectomy on incidence of pancreatic fistula. JAMA Surg. (2017) 152(4):327–35. doi: 10.1001/jamasurg.2016.4755
11. Mejia A, Shah J, Vivian E, Acharya P. Analysis of 102 fully robotic pancreaticoduodenectomies: clinical and financial outcomes. Pancreas. (2020) 49(5):668–74. doi: 10.1097/MPA.0000000000001545
12. Wang SE, Shyr BU, Chen SC, Shyr YM. Comparison between robotic and open pancreaticoduodenectomy with modified blumgart pancreaticojejunostomy: a propensity score-matched study. Surgery. (2018) 164(6):1162–7. doi: 10.1016/j.surg.2018.06.031
13. Kim HS, Han Y, Kang JS, Kim H, Kim JR, Kwon W, et al. Comparison of surgical outcomes between open and robot-assisted minimally invasive pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. (2018) 25(2):142–9. doi: 10.1002/jhbp.522
14. Varley PR, Zenati MS, Klobuka A, Tobler J, Hamad A, Hogg ME, et al. Does robotic pancreaticoduodenectomy improve outcomes in patients with high risk morphometric features compared to the open approach. HPB. (2019) 21(6):695–701. doi: 10.1016/j.hpb.2018.10.016
15. Cai J, Ramanathan R, Zenati MS, Al Abbas A, Hogg ME, Zeh HJ, et al. Robotic pancreaticoduodenectomy is associated with decreased clinically relevant pancreatic fistulas: a propensity-matched analysis. J Gastrointest Surg. (2020) 24(5):1111–8. doi: 10.1007/s11605-019-04274-1
16. Paolini C, Bencini L, Gabellini L, Urciuoli I, Pacciani S, Tribuzi A, et al. Robotic versus open pancreaticoduodenectomy: is there any difference for frail patients? Surg Oncol. (2021) 37:101515. doi: 10.1016/j.suronc.2020.12.009
17. Ielpo B, Caruso R, Duran H, Diaz E, Fabra I, Malavé L, et al. Robotic versus standard open pancreatectomy: a propensity score-matched analysis comparison. Updates Surg. (2019) 71(1):137–44. doi: 10.1007/s13304-018-0529-1
18. Marino MV, Podda M, Gomez Ruiz M, Fernandez CC, Guarrasi D, Gomez Fleitas M. Robotic-assisted versus open pancreaticoduodenectomy: the results of a case-matched comparison. J Robot Surg. (2020) 14(3):493–502. doi: 10.1007/s11701-019-01018-w
19. Shi Y, Jin J, Qiu W, Weng Y, Wang J, Zhao S, et al. Short-term outcomes after robot-assisted vs open pancreaticoduodenectomy after the learning curve. JAMA Surg. (2020) 155(5):389–94. doi: 10.1001/jamasurg.2020.0021
20. Bencini L, Tofani F, Paolini C, Vaccaro C, Checcacci P, Annecchiarico M, et al. Single-centre comparison of robotic and open pancreatoduodenectomy: a propensity score-matched study. Surg Endosc. (2020) 34(12):5402–12. doi: 10.1007/s00464-019-07335-3
21. Kim H, Park SY, Park Y, Kwon J, Lee W, Song KB, et al. Assessment of learning curve and oncologic feasibility of robotic pancreaticoduodenectomy: a propensity score-based comparison with open approach. J Hepatobiliary Pancreat Sci. (2022) 29(6):649–58. doi: 10.1002/jhbp.837
22. van Oosten AF, Ding D, Habib JR, Irfan A, Schmocker RK, Sereni E, et al. Perioperative outcomes of robotic pancreaticoduodenectomy: a propensity-matched analysis to open and laparoscopic pancreaticoduodenectomy. J Gastrointest Surg. (2021) 25(7):1795–804. doi: 10.1007/s11605-020-04869-z
23. Shyr BU, Shyr BS, Chen SC, Shyr YM, Wang SE. Robotic and open pancreaticoduodenectomy: results from Taipei veterans general hospital in Taiwan. Updates Surg. (2021) 73(3):939–46. doi: 10.1007/s13304-020-00899-z
24. Wang W, Liu Q, Zhao ZM, Tan XL, Wang ZZ, Zhang KD, et al. Comparison of robotic and open pancreaticoduodenectomy for primary nonampullary duodenal adenocarcinoma: a retrospective cohort study. Langenbecks Arch Surg. (2022) 407(1):167–73. doi: 10.1007/s00423-021-02303-9
25. Shyr YM, Wang SE, Chen SC, Shyr BU, Shyr BS. Robotic pancreaticoduodenectomy for pancreatic head cancer and periampullary lesions. Ann Gastroenterol Surg. (2021) 5(5):589–96. doi: 10.1002/ags3.12457
26. Pine JK, Haugk B, Robinson SM, Darne A, Wilson C, Sen G, et al. Prospective assessment of resection margin status following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma after standardisation of margin definitions. Pancreatology. (2020) 20(3):537–44. doi: 10.1016/j.pan.2020.01.004
27. You MS, Lee SH, Choi YH, Shin BS, Paik WH, Ryu JK, et al. Lymph node ratio as valuable predictor in pancreatic cancer treated with R0 resection and adjuvant treatment. BMC Cancer. (2019) 19(1):952. doi: 10.1186/s12885-019-6193-0
28. Da Dong X, Felsenreich DM, Gogna S, Rojas A, Zhang E, Dong M, et al. Robotic pancreaticoduodenectomy provides better histopathological outcomes as compared to its open counterpart: a meta-analysis. Sci Rep. (2021) 11(1):3774. doi: 10.1038/s41598-021-83391-x
29. Yan Q, Xu LB, Ren ZF, Liu C. Robotic versus open pancreaticoduodenectomy: a meta-analysis of short-term outcomes. Surg Endosc. (2020) 34(2):501–9. doi: 10.1007/s00464-019-07084-3
Keywords: robotic pancreaticoduodenectomy, open pancreaticoduodenectomy, pancreatic cancer, outcome, meta-analysis
Citation: Fu Y, Qiu J, Yu Y, Wu D and Zhang T (2022) Meta-analysis of robotic versus open pancreaticoduodenectomy in all patients and pancreatic cancer patients. Front. Surg. 9:989065. doi: 10.3389/fsurg.2022.989065
Received: 8 July 2022; Accepted: 20 September 2022;
Published: 11 October 2022.
Edited by:
Andrew Gumbs, Centre Hospitalier Intercommunal de Poissy, FranceReviewed by:
Xiaohang Li, The First Affiliated Hospital of China Medical University, ChinaXu Che, Cancer Hospital Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China
© 2022 Fu, Qiu, Yu, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taiping Zhang dHBpbmd6aGFuZ0B5YWhvby5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Yibo Fu
Yibo Fu Jiangdong Qiu
Jiangdong Qiu Yiqi Yu1
Yiqi Yu1 Taiping Zhang
Taiping Zhang