- 1Department of Orthopedics, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Institute of Orthopedics of Jiangxi Province, Nanchang, China
- 3Institute of Minimally Invasive Orthopedics of Nanchang University, Nanchang, China
Introduction: Acute epidural abscess after percutaneous endoscopic lumbar discectomy is a rare but grievous complication. When faced with a long-segment epidural abscess, open surgery has traditionally been performed which can lead to huge surgical trauma and unpredictable complications. For this reason, surgeons around the world are constantly looking for more minimally invasive and effective surgical methods.
Patient Concerns: Our patient was a 32-year-old woman who had been receiving percutaneous endoscopic interlaminar discectomy for L5/S1 lumbar disc herniation one week ago. She returned to our institution with a fever and lower back pain.
Diagnoses: Magnetic resonance imaging revealed a long segment epidural abscess accompanied by a paravertebral abscess, and staphylococcus aureus was detected in a bacterial culture of pyogenic fluids extracted from the paravertebral abscess.
Treatments: We performed percutaneous endoscopic drainage (PED) for the epidural abscess. Long-term sensitive antibiotic treatment after surgery.
Outcomes: Immediate pain relief was achieved and the inflammatory reaction subsided after 4 weeks of antibiotic therapy. Re-examination of the lumbar spine MRI after 1 month showed that the epidural abscess disappeared completely.
Conclusion: Percutaneous endoscopy allowed us to approach the epidural abscess directly, enabling the immediate drainage of the abscess with minimal trauma to the patient. The good results obtained show that percutaneous endoscopic drainage is a reliable way to treat a long-segment epidural abscess.
Introduction
Epidural abscess is a rare and severe complication of percutaneous endoscopic lumbar discectomy (PELD) (1) that can result in serious vascular and nerve injury if not detected and treated promptly (2–4). Antibiotic therapy alone is the first choice when confronted with this situation. Nevertheless, drainage is recommended for cases involving large abscesses or when antibiotic therapy is ineffective and open surgery has traditionally been performed (5, 6). Hereby We present a case of acute long segment epidural abscess following PELD that was successfully treated by percutaneous endoscopic drainage.
Case presentation
Chief complaints
A 32 years old young Asia woman initially presented with low back pain, left lower limb numbness and fever.
History of present illness
The patient had a fever for one week. Four days ago, the patient developed low back pain accompanied by numbness and severe pain in the left lower limb.
History of past illness
The patient had transforaminal endoscopic treatment for disc herniation one week ago (day surgery). There was no additional medical, family or genetic history.
Physical examination
Physical examination on admission, the patient's temperature was 38.1 °C, heart rate was 88 bpm, respiratory rate was 20 breaths per minute, blood pressure was 110/83 mmHg. The patient has significant tenderness in the lower back. The muscle strength of the left lower limb was grade III.
Laboratory examinations
Blood tests on admission: white blood cell count 19.01 × 109, hemoglobin 97 g/L, percentage of neutrophils 90%, red blood cell count and platelet count was normal. C-reactive protein greater than 200 mg/L, ESR was 78 mm/h. Albumin was 29.58 g/L, and the albumin ratio was 1.04. The electrocardiogram and chest X-ray results were normal.
Imaging examinations
A herniated L5-S1 disc with compression of the spinal cord and the left nerve root was found by magnetic resonance imaging (MRI) of the lumbar spine a week ago (Figure 1). Magnetic resonance imaging on admission demonstrated long-segment hyperintensity in the epidural and paravertebral. The pus ranges from L1 to S1 in sagittal and the dura mater was compressed to about 1/3 of its original value in axial. (Figures 2A,B).
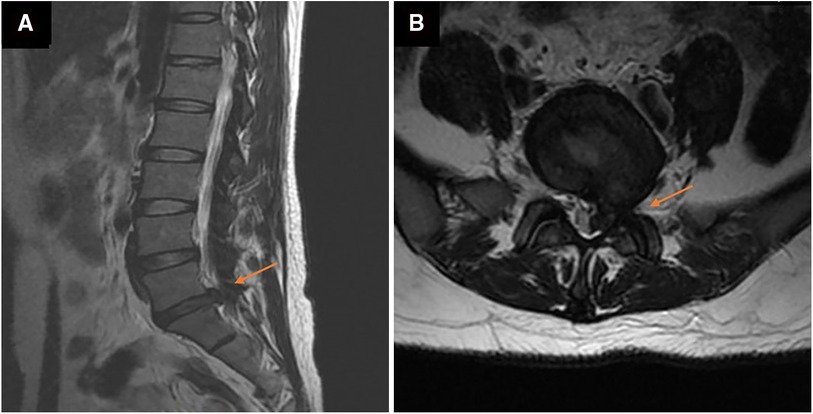
Figure 1. Magnetic resonance images of the lumbar before percutaneous endoscopic lumbar discectomy (PELD) in axial (A) and sagittal (B). a large disc herniation with compression of the spinal cord and nerve roots at the left of L5/S1.
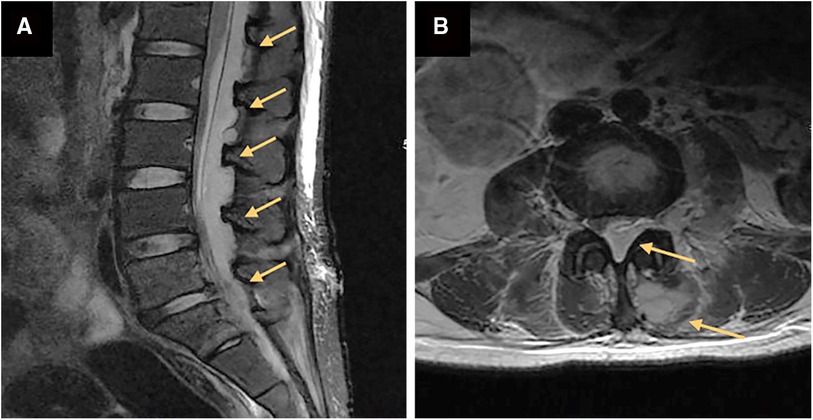
Figure 2. Magnetic resonance images of the lumbar before percutaneous endoscopic drainage in axial (A) and sagittal (B). (A) a long segment epidural abscess from L1 to S1 (orange arrow). (B) Massive epidural abscesses compressing the spinal cord and nerve roots with paravertebral involvement (orange arrow).
Microbiological identification of the causative agent
X-ray guided aspiration of pus and bacterial culture and drug sensitivity test was performed. The bacterial culture revealed a Staphylococcus aureus infection, and the drug sensitivity test showed that MOXIFLOXACIN is effective.
Final diagnosis
The final diagnosis of the presented case is Acute epidural abscess caused by Staphylococcus aureus infection after endoscopic discectomy.
Treatment
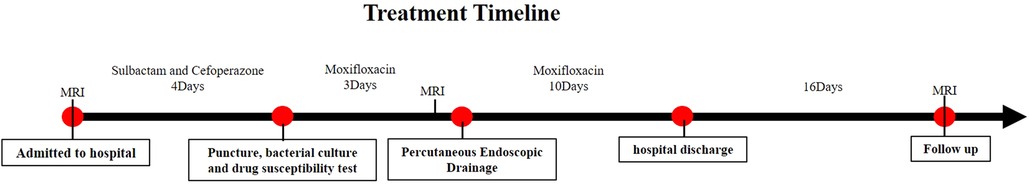
Antibiotic treatment: The whole antibiotic process was shown in Figure 3. The C-reactive protein was >200 mg/L, the white blood cell count was 19.01 × 109/L, the ESR was 78 mm/h, and the body temperature was 38.1 °C on admission. The patient was treated with cefoperazone sodium and sulbactam sodium. Moxifloxacin was replaced for antibacterial treatment four days later, depending on the results of bacterial culture and drug sensitivity test following a puncture. Three days later, the C-reactive protein was 173.9 mg/L, the white blood cell count was 27.71 × 109/L, the ESR was 117 mm/h, the body temperature was 38.5 °C and percutaneous endoscopic drainage was performed immediately. The pus extracted during the operation was subjected to bacterial culture and drug susceptibility test, and the results were the same as before (Moxifloxacin is sensitive, MIC≤0.25). Moxifloxacin was continued until discharge.
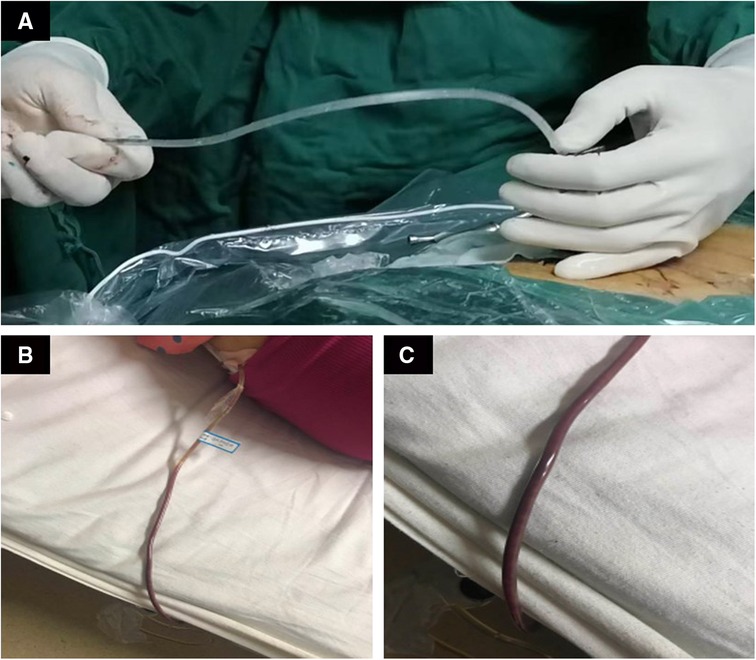
Surgery treatment: Patients were prone position, head high and feet low. Dezocine was given intravenously during the operation. The operation area was disinfected, covered with sterile cloth and pasted with skin film. Before the procedure, lidocaine was used for local anesthesia, and the guide wire was placed at the left intervertebral foramen of L5/S1 by cutting the skin 0.6 cm at the root of the guide wire. Also, establish the working channel along the guide wire. At this point, a small amount of pus can be seen flowing out of the pipeline (Figures 4A–C). Then the light source and the camera lens were connected, put the endoscope into the cannula, and adjusted with a proper water flow and pressure. The necrotic fibrous tissue around the abscess was removed with nucleus pulposus forceps, and the intervertebral foramen were appropriately enlarged, and a large amount of pus was seen gushing out (Figures 4D,E and Supplementary 1). Irrigation and drainage with normal saline, until no obvious pus outflow, visible dura swelling, nerve root relaxation. Than Place a drainage tube along the working channel, check pipe patency, exit the working channel, fix the drainage tube, suture the wound, and cover it with sterile dressing (Figure 5A). Check the patency of the drainage tube daily (Figures 5B,C) and observe the patient closely.
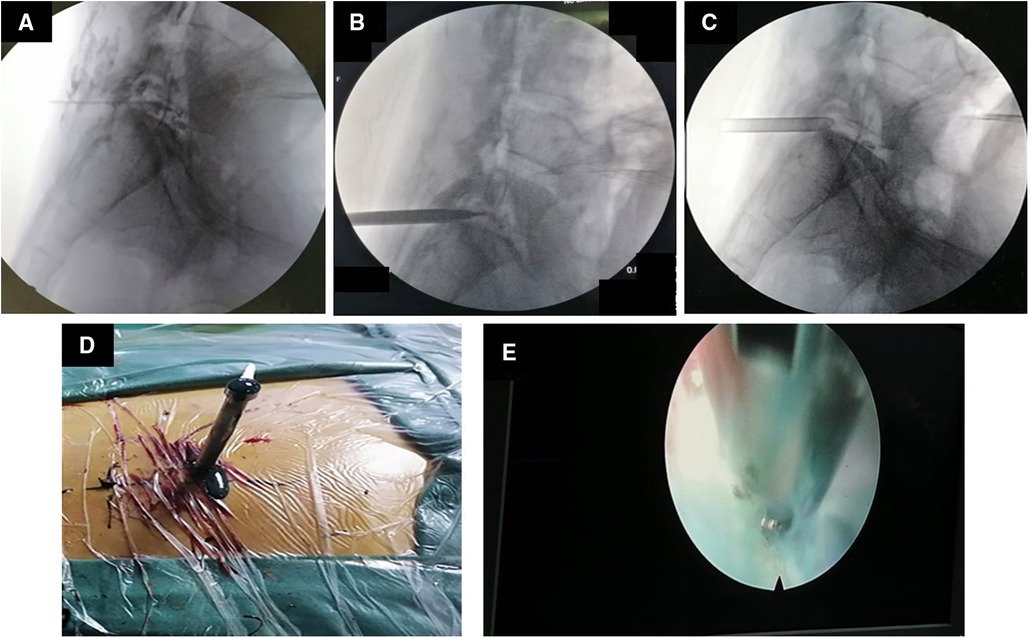
Figure 4. Images and video during percutaneous endoscopic drainage. (A) The guide wire is positioned at L5/S1. (B) Insert the channel along the guide wire. (C) The channel is placed at L5/S1. (D) After the passage is placed, pus begins to flow out. (E) Microscopic video of massive pus discharge.
Outcome and follow-up
The patient's waist and left lower limb symptoms were significantly alleviated after the operation. One week later, the patient's body temperature dropped to normal (37.0 °C). About ten days later, the erythrocyte sedimentation rate (17 mm/h) and C-reactive protein(10 mg/L) decreased to normal. Two weeks later, the patient's symptoms disappeared, and she was discharged. Reexamination of lumbar magnetic resonance one month after surgery showed that the patient had complete disappearance of intraspinal and paravertebral abscesses (Figure 6).
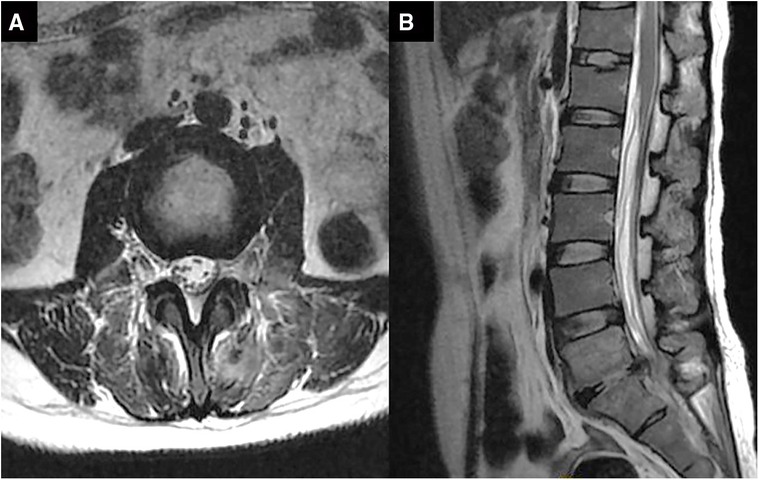
Figure 6. Magnetic resonance images of the lumbar vertebra after percutaneous endoscopic drainage for 1 month in axial (A) and sagittal (B). intraspinal and paravertebral abscesses have disappeared.
Discussion
Infection after PELD is a rare but serious complication, with an incidence of 0.1% to 0.7% (7), Such as Gu et al. treated 209 patients with lumbar disc herniation with minimally invasive surgery, and one patient developed a lumbar infection, with an infection rate of 0.47% (8). Fan et al. treated 738 patients with lumbar spinal stenosis with PELD, and 3 patients developed infection, with an infection rate of 0.41% (9). But Zhou et al. treated 426 patients with lumbar disc herniation with PELD and PEID without lumbar infection (1).
For lumbar epidural infection, the most important is active antimicrobial treatment and timely abscess removal. Antimicrobial therapy is the basis of treatment, and surgical decompression is always the key to the treatment of epidural abscess, especially when accompanied by neurological impairment (2, 10–13). David E Connor Jr et al. treated 77 patients with epidural abscesses surgically, with significant improvement in 56 patients and some improvement in symptoms in 6 patients (12). Amit R Patel et al., who treated 128 patients with epidural abscesses with medical therapy alone (51 patients) and surgery plus antibiotics (77 patients), showed that early surgery improved neurological outcomes, with more than 41% of patients requiring surgical decompression after medical therapy failed (14).
The treatment strategy for infected spine remains controversial. Conservative cases appear to be more likely to develop mechanical back pain and develop more deformities over time than surgical cases (15). However, open surgical intervention is always associated with more complications, despite lower overall mortality among surgical patients (16). It can cause huge surgical trauma, requiring the dissection of paravertebral muscles and the biting of lamina, seriously damaging the stability of the spine, and sometimes even requiring fusion surgery, which can reduce the patients' lumbar range of motion and greatly reduce the patients' quality of life (17). PED has the advantages of less complications and satisfactory clinical efficacy, which provides a minimally invasive surgical option for the treatment of spinal infection (18–20).
Percutaneous endoscopic drainage is an attempt of spinal endoscopy in the treatment of spinal infections. Choi et al. used percutaneous endoscopic drainage to treat four different types of spinal infections, and most patients achieved satisfactory results (14/17). Among them, 4 cases were used to treat epidural abscesses, 2 patients achieved satisfactory results, 1 patient underwent minimally invasive drainage here (21), and 1 patient underwent open surgery here. In addition, Akira Iwata et al. used PED to successfully treat 4 patients with spinal fungal infection (18). Kai-Sheng Chang and Omar S Akbik respectively drainage of the cervical and thoracic epidural abscess through transforaminal endoscopy and received excellent result (22, 23).
Endoscopic treatment of epidural abscess has certain limitations. Endoscopic debridement of epidural abscesses may be limited in scope compared to traditional open surgery and may require reoperation to allow complete removal of pus.The success of PED treatment largely depends on the removal of pus that causes spinal cord compression and nerve damage. Endoscopic treatment alone requires more than a certain level of endoscopic surgical skill, so treatment outcomes may vary depending on the skill of the operator.
In this case, the long-stage abscess compressed the spinal cord and nerves, and the patient had severe neurological symptoms, so immediate spinal decompression was necessary. We planned to perform PED drainage first. If the neurological symptoms did not improve after endoscopic treatment of the epidural abscess, additional posterior spinal open surgery could be considered at any time. Fortunately, the patient's symptoms improved significantly after the operation, and the validation index gradually decreased. At the last follow-up, the collapse of the L5/S1 intervertebral space was observed, but this patient did not have any local or global symptoms.
Nowadays, minimally invasive surgery is gradually replacing the conventional open surgery field, and then open surgery is needed in the field of epidural infection, minimally invasive surgery can also achieve better surgical results. This means that the area in which endoscopic technology can be used has been widened. For some skilled surgeons in endoscopic surgery, this technique may be one of the good treatment options.
Conclusion
We could approach the epidural abscess directly with percutaneous endoscopy, allowing for immediate drainage of the abscess with less burden on the patient. The success of this case demonstrates that transforaminal endoscopy can be used to treat long segment spinal epidural abscess, which can be used as a reference for clinicians when developing a surgical plan for the treatment of acute long segment spinal epidural abscess.
Care checklist (2013) statement
The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Second Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XC and TW provided the idea of the project and completed the surgery; TL and HW completed the collection of case data and the writing of the paper; JY and JJ provided relevant suggestions for the writing of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science of Jiangxi Province (No. 708082060172).
Acknowledgements
The authors would like to thank Dr Chao Zhang (Department of Orthopedics, Second Affiliated Hospital of Nanchang University, Nanchang, China) for providing help for writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.985666/full#supplementary-material.
Abbreviations
PELD, percutaneous endoscopic lumbar discectomy, PED, percutaneous endoscopic drainage.
References
1. Zhou C, Zhang G, Panchal RR, Ren X, Xiang H, Xuexiao M, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Physician. (2018) 21(2):E105–E12. PMID: 29565953
2. Darouiche RO. Spinal epidural abscess. N Engl J Med. (2006) 355(19):2012–20. doi: 10.1056/NEJMra055111
3. Vakili M, Crum-Cianflone NF. Spinal epidural abscess: a series of 101 cases. Am J Med. (2017) 130(12):1458–63. doi: 10.1016/j.amjmed.2017.07.017
4. Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. (2004) 26(3):285–91. doi: 10.1016/j.jemermed.2003.11.013
5. Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurg. (1990) 27(2):177–84. doi: 10.1227/00006123-199008000-00001
6. Behmanesh B, Gessler F, Quick-Weller J, Dubinski D, Konczalla J, Seifert V, et al. Early versus delayed surgery for spinal epidural abscess : clinical outcome and health-related quality of life. J Korean Neurosurg Soc. (2020) 63(6):757–66. doi: 10.3340/jkns.2019.0230
7. Pan M, Li Q, Li S, Mao H, Meng B, Zhou F, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician. (2020) 23(1):49–56. PMID: 32013278
8. Gu Y-T, Cui Z, Shao H-W, Ye Y, Gu A-Q. Percutaneous transforaminal endoscopic surgery (PTES) for symptomatic lumbar disc herniation: a surgical technique, outcome, and complications in 209 consecutive cases. J Orthop Surg Res. (2017) 12(1):25. doi: 10.1186/s13018-017-0524-0
9. Fan N, Yuan S, Du P, Wu Q, Wang T, Wang A, et al. Complications and risk factors of percutaneous endoscopic transforaminal discectomy in the treatment of lumbar spinal stenosis. BMC Musculoskelet Disord. (2021) 22(1):1041. doi: 10.1186/s12891-021-04940-z
10. Baker AS, Ojemann RG, Swartz MN, Richardson EP. Spinal epidural abscess. N Engl J Med. (1975) 293(10):463–8. doi: 10.1056/NEJM197509042931001
11. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. (2000) 23(4):175–204. doi: 10.1007/PL00011954
12. Connor DE, Chittiboina P, Caldito G, Nanda A. Comparison of operative and nonoperative management of spinal epidural abscess: a retrospective review of clinical and laboratory predictors of neurological outcome. J Neurosurg Spine. (2013) 19(1):119–27. doi: 10.3171/2013.3.SPINE12762
13. Ghobrial GM, Beygi S, Viereck MJ, Maulucci CM, Sharan A, Heller J, et al. Timing in the surgical evacuation of spinal epidural abscesses. Neurosurg Focus. (2014) 37(2):E1. doi: 10.3171/2014.6.FOCUS14120
14. Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. (2014) 14(2):326–30. doi: 10.1016/j.spinee.2013.10.046
15. Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). (2018) 160(3):487–96. doi: 10.1007/s00701-018-3467-2
16. Wang Y-C, Wong C-B, Wang IC, Fu T-S, Chen L-H, Chen W-J. Exposure of prebiopsy antibiotics influence bacteriological diagnosis and clinical outcomes in patients with infectious spondylitis. Med (Baltimore). (2016) 95(15):e3343. doi: 10.1097/MD.0000000000003343
17. Schwab JH, Shah AA. Spinal epidural abscess: diagnosis, management, and outcomes. J Am Acad Orthop Surg. (2020) 28(21):e929–e38. doi: 10.5435/JAAOS-D-19-00685
18. Iwata A, Ito M, Abumi K, Sudo H, Kotani Y, Shono Y, et al. Fungal spinal infection treated with percutaneous posterolateral endoscopic surgery. J Neurol Surg A Cent Eur Neurosurg. (2014) 75(3):170–6. doi: 10.1055/s-0032-1329268
19. Yang S-C, Fu T-S, Chen L-H, Chen W-J, Tu Y-K. Identifying pathogens of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res. (2008) 466(12):3086–92. doi: 10.1007/s11999-008-0441-y
20. Yang S-C, Fu T-S, Chen H-S, Kao Y-H, Yu S-W, Tu Y-K. Minimally invasive endoscopic treatment for lumbar infectious spondylitis: a retrospective study in a tertiary referral center. BMC Musculoskelet Disord. (2014) 15:105. doi: 10.1186/1471-2474-15-105
21. Choi E-J, Kim S-Y, Kim H-G, Shon H-S, Kim T-K, Kim K-H. Percutaneous endoscopic debridement and drainage with four different approach methods for the treatment of spinal infection. Pain Physician. (2017) 20(6):E933–E40. PMID: 28934797
22. Chang K-S, Sun L-W, Cheng C-Y, Chang S-W, Chen C-M. Full endoscopic removal of cervical spinal epidural abscess: case report and technical note. Neurospine. (2020) 17(Suppl 1):S160–S5. doi: 10.14245/ns.2040218.109
Keywords: epidural abscess, percutaneous endoscopic drainage, PELD = percutaneous endoscopic lumbar discectomy, case report, minimal invasive
Citation: Li T, Wu H, Yuan J, Jia J, Wu T and Cheng X (2022) Percutaneous endoscopic drainage for acute long segment epidural abscess following endoscopic lumbar discectomy: A case report. Front. Surg. 9:985666. doi: 10.3389/fsurg.2022.985666
Received: 4 July 2022; Accepted: 13 September 2022;
Published: 30 September 2022.
Edited by:
Panagiotis Korovessis, Olympion Medical Center, GreeceReviewed by:
Dingjun Hao, Xi'an Honghui Hospital, ChinaBin Zhu, Second Hospital of Anhui Medical University, China
© 2022 Li, Wu, Yuan, Jia, Wu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xigao Cheng MjI4MjA2ODQ2QHFxLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Tao Li
Tao Li Hui Wu
Hui Wu Jinghong Yuan
Jinghong Yuan Jingyu Jia
Jingyu Jia Tianlong Wu3
Tianlong Wu3 Xigao Cheng
Xigao Cheng