- 1Department of Colorectal Surgery, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Department of Gastroenterology, Rui Jin Hospital, Affiliate to Shanghai Jiao Tong University, School of Medicine, Shanghai, China
Background: The simple endoscopic score for Crohn's disease (SES-CD) is a widely used index to evaluate clinical and endoscopic activity. However, the association and predictive value of SES-CD for intestinal obstruction in Crohn's disease (CD) remains unclear. We aimed to establish the best cut-off indicators of SES-CD for early clinical intervention and subsequent prevention of intestinal obstruction in CD.
Methods: Data on patients with CD evaluated at our institute from January 2016 to January 2022 were retrospectively collected. The SES-CD and Crohn's Disease Activity Index scores used in the analysis indicated the results of the first clinical and colonoscopy evaluations after hospitalization. The primary outcome was the occurrence of intestinal obstruction during admission and follow-up.
Results: A total of 248 patients with a median follow-up time of 2 years [interquartile range: 1.0–4.0] were enrolled, of which 28.2% developed intestinal obstruction. An SES-CD score of 8 was the most significant threshold evaluation, and SES-CD ≥8 had the largest area under the receiver operating characteristic curve (0.705), with a sensitivity of 52.9% and specificity of 88.2% in predicting intestinal obstruction. Furthermore, SES-CD ≥8 had the greatest risk factor for intestinal obstruction (odds ratio: 7.731; 95% confidence interval: 3.901–15.322; p < 0.001) and significantly decreased the overall intestinal obstruction-free survival (p < 0.001).
Conclusion: The SES-CD endoscopic prediction model could be an effective predictor of intestinal obstruction in patients with CD. More frequent follow-up and colonoscopic surveillance should be considered in patients with SES-CD score ≥8 to prevent the development of intestinal obstruction.
Introduction
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) characterized by recurrent relapse and remission, which markedly affects the clinical prognosis of patients. Chronic proliferative inflammation and fibrosis of the intestinal wall are the characteristic pathological changes of CD, which promote several serious complications, such as intestinal stenosis and obstruction, various fistulas, sinuses, abscesses, and perforations. Intestinal obstruction is one of the most common complications within the first 10 years of CD diagnosis (1, 2). Previous studies reported that approximately 30% of CD patients eventually developed end-stage intestinal fibrosis (3) and nearly 80% of patients underwent colectomy for fibrotic intestinal segments due to intestinal obstruction (4). However, there is currently no effective treatment for CD intestinal fibrosis (5). Therefore, it is particularly important for clinicians to develop effective methods that can predict intestinal obstruction in the course of the disease to provide early intervention.
The Crohn's Disease Activity Index (CDAI) has been widely considered the primary measure of evaluating the clinical disease activity in patients with CD for over 40 years (6). Colonoscopy is necessary for evaluating endoscopic activity in each patient. Although there is a significant correlation between Crohn's Disease Index of Severity and simple endoscopic score for Crohn's disease (SES-CD), SES-CD is a simple, reproducible, and easy-to-use endoscopic scoring system that has been widely used in clinical practice (7, 8). However, the association and predictive value of SES-CD for intestinal obstruction in CD remains unclear.
In this study, we aimed to determine the association between SES-CD and the development of intestinal obstruction and to establish the best cut-off indicators of SES-CD for early clinical intervention in preventing the occurrence of intestinal obstruction in CD.
Methods
Patients
The clinical data of patients with CD who received regular medical treatment or surgery from January 2016 to January 2022 at our institute were retrospectively identified and collected from a prospectively maintained, institutional review board-approved database (Chinese Database System for IBD) (9, 10). Patients diagnosed with CD who received regular follow-up and provided complete clinical data were included in this study. Patients who were lost to follow-up or had incomplete data were excluded. In this study, patients who underwent surgery were re-examined within 3 months, including fecal calprotectin, colonoscopy, and imaging examinations. Nonsurgical patients were followed up in outpatient clinics every month to observe changes in clinical symptoms, and endoscopy and imaging examinations were performed within 6 months. When clinical symptoms developed during follow-up, early and frequent surveillance was performed. Follow-up data for each patient were collected continuously at each center over a period. Among the follow-up data, complications of CD were considered for further analysis in the present study. The ethics committee of Xinhua Hospital reviewed and approved the study protocol (approval no. XHEC-D-2022-094).
Evaluation of clinical and endoscopic activities
The CDAI and SES-CD scores of the first clinical and colonoscopic evaluations after hospitalization were used to assess the disease activity of CD (11). Detailed assessments of SES-CD and CDAI according to reports from previous research (7, 12) are presented in Supplementary Tables S1, 2. CDAI scores were evaluated by an independent IBD specialist according to the patients' physical signs (i.e., average daily temperature and extraintestinal manifestation), medication use (i.e., loperamide or opiate use for diarrhea), laboratory results (hematocrit), health conditions, and abdominal pain. CDAI-defined thresholds of 150, 221, and 450 were used for analysis, and CDAI ≥221 was considered as active CD based on the report of a previous study (12). The SES-CD scores were evaluated and recorded by two independent expert endoscopists who were blinded to the study. Higher endoscopic scores were used for further analysis when the endoscopic activity in the same affected area was inconsistent (13).
Data evaluation
The primary outcome was the occurrence of intestinal obstruction during admission and follow-up. The diagnosis of CD was strictly based on endoscopic and histological examinations. CD-associated complications (intestinal obstruction, abscess, cellulitis, and fistula) were diagnosed based on the identified clinical manifestations, abdominal signs, and radiological examinations. The extent of CD was monitored for involvement in the small intestine, ileocolon, colon, and upper gastrointestinal tract (14). The use of medications (mesalamine, biologics, steroids, immunomodulators, and antibiotics) and laboratory results were also recorded.
Statistical analysis
SPSS version 19.0 (IBM 2010, Chicago, IL, United States) and GraphPad Prism 8.0 (San Diego, CA, United States) were used for statistical analyses. Chi-square and Fisher's exact tests were used for univariate analyses. Multivariate logistic regression was used to determine the independent risk factors for intestinal obstruction. Receiver operating characteristic (ROC) curve analysis was performed to determine the predictive value of CDAI and SES-CD for intestinal obstruction. The Kaplan–Meier method with the log-rank test was used to compare the intestinal obstruction-free survival. All statistics were two-sided, with confidence intervals (CIs) set at 95%, and a p-value <0.05 was considered as significant.
Results
Baseline characteristics of patients
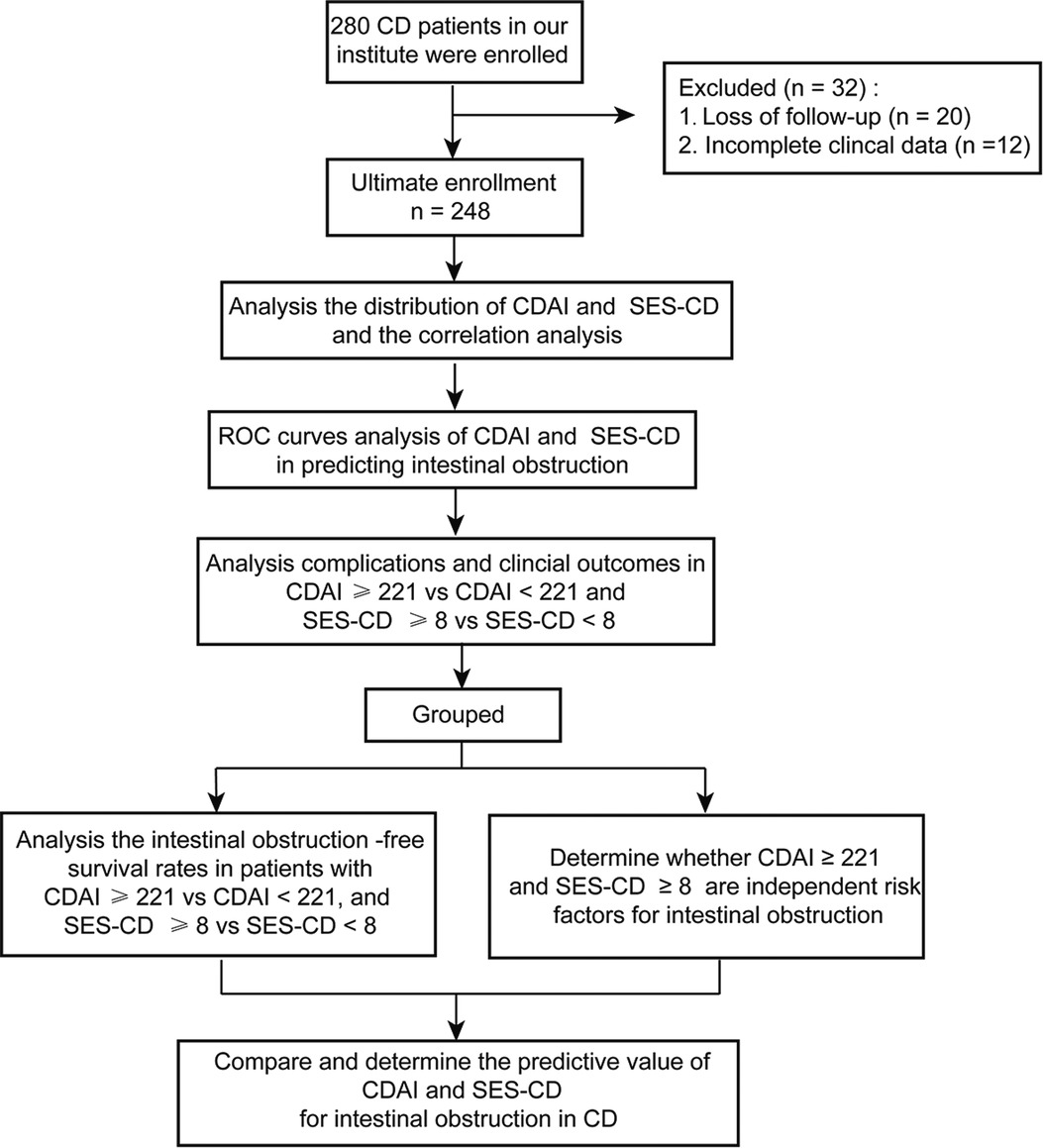
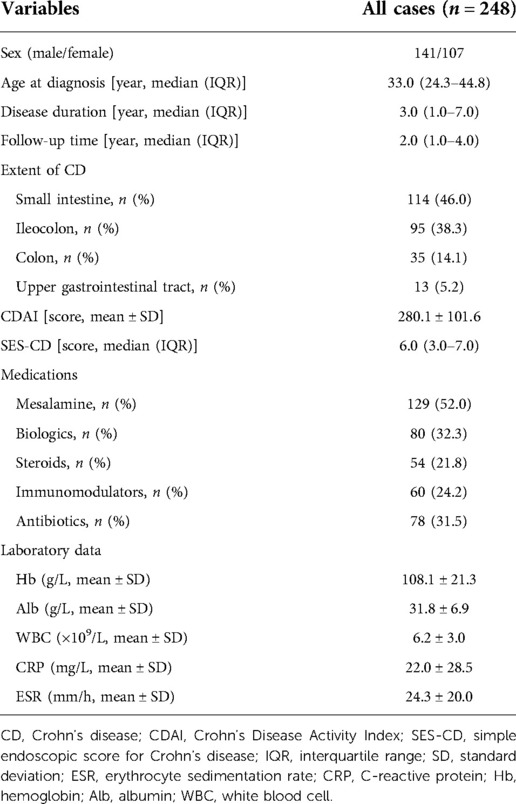
As shown in Figure 1, 280 patients with CD were evaluated at our institute. Among them, 12 patients had incomplete clinical data and 20 were lost to follow-up. In total, 248 eligible CD patients with a median age of diagnosis of 33.0 years [interquartile range (IQR): 24.3–44.8] and a median follow-up time of 2 years (IQR: 1.0–4.0) were enrolled in this study. In the whole cohort, 114 (46.0%) patients had small intestinal lesions, 95 (38.3%) developed ileocolon CD, 35 (14.1%) had colon lesions, and 13 (5.2%) had upper gastrointestinal tract involvement (Table 1).
We then analyzed the distribution of patients with different CDAI and SES-CD scores. We found that 180 (72.6%) patients were in the active CD stage (CDAI ≥ 221), and more than half of the patients (52.4%) had an SES-CD score of 6 or more (Figures 2A,B). We further demonstrated that the association between the CDAI and SES-CD scores was significant (R = 0.4717, p < 0.001, Figure 2C).
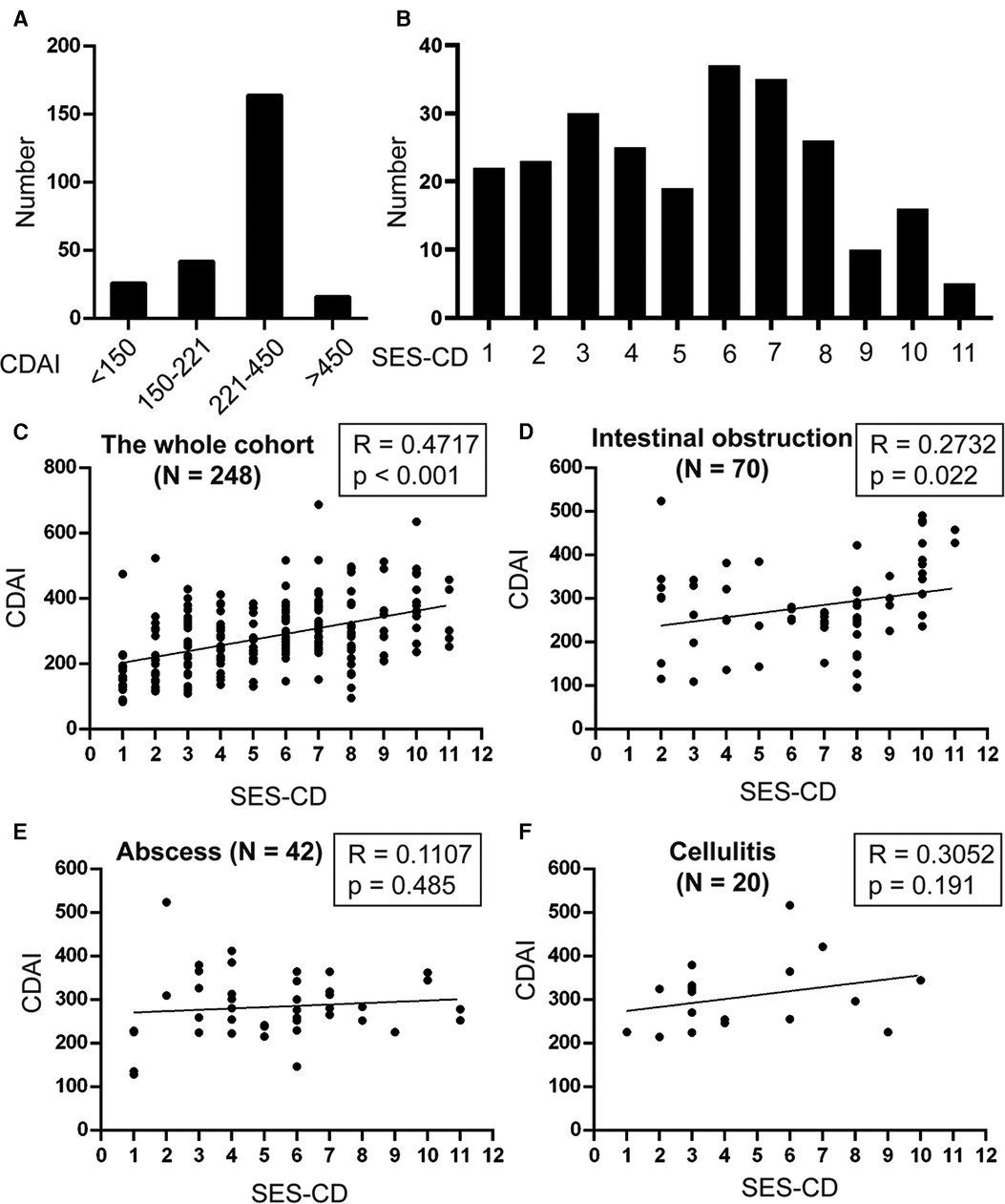
Figure 2. Analysis of the distribution and correlation of CDAI and SES-CD. The number of patients with different (A) CDAI and (B) SES-CD were analyzed. Spearman's correlation test was used to determine the correlation between CDAI and SES-CD in the (C) whole cohort, patients with (D) intestinal obstruction, (E) abscess, and (F) cellulitis.
Analysis of the complications and surgical intervention in CD
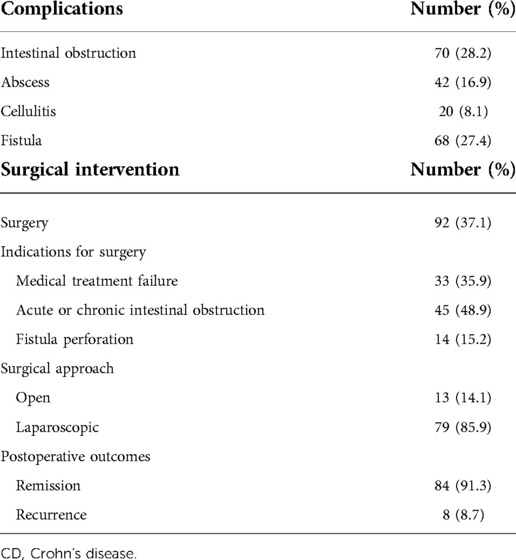
Intestinal obstruction was the most common complication in this study, which occurred in 70 (28.2%) CD patients. Furthermore, 42 (16.9%) patients developed abscesses, 20 (8.1%) had cellulitis, and 68 (27.4%) had fistulas (Table 2). A total of 92 (37.1%) patients received surgical intervention, of which 33 (35.9%) were due to medical treatment failure, 45 (48.9%) were due to acute or chronic intestinal obstruction, and 14 (15.2%) developed fistula perforation. In this study, the majority were laparoscopic surgeries, and more than 90% of patients achieved clinical remission after surgery (Table 2).
SES-CD threshold evaluation for predicting intestinal obstruction
To determine the best threshold value of SES-CD for the indication of intestinal obstruction, an ROC curve was generated. As shown in Figures 3A–F, SES-CD ≥8 had the most significant area under the ROC curve (AUC) (0.705), with a sensitivity of 52.9% and a specificity of 88.2% (p < 0.001), while CDAI showed no significance in predicting the occurrence of intestinal obstruction (p = 0.079). This result indicated that an SES-CD score of 8 can predict the occurrence of intestinal obstruction more effectively than clinical activity.
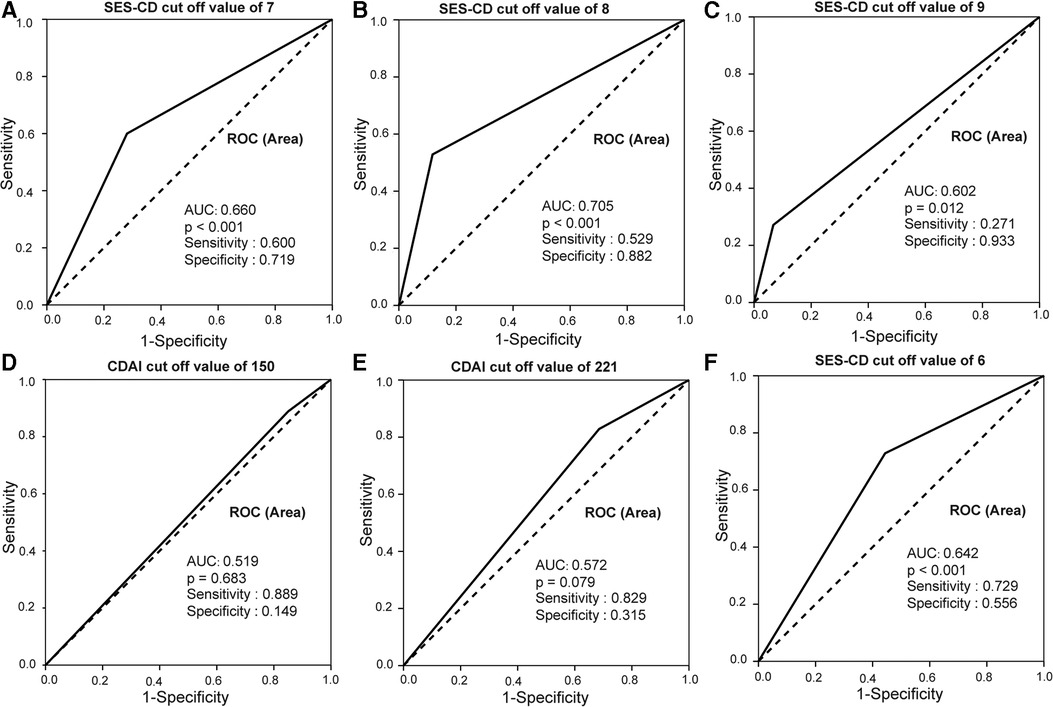
Figure 3. SES-CD and CDAI ROC curves in predicting intestinal obstruction in CD patients. (A–C) SES-CD ≥8 had the most significant AUC of 0.705 with a sensitivity of 52.9% and specificity of 88.2%. (D–F) CDAI ≥221 had the biggest area under AUC of 0.572 with a sensitivity of 82.9% and specificity of 31.5.
Analysis of the complications in patients with different endoscopic and clinical activity scores
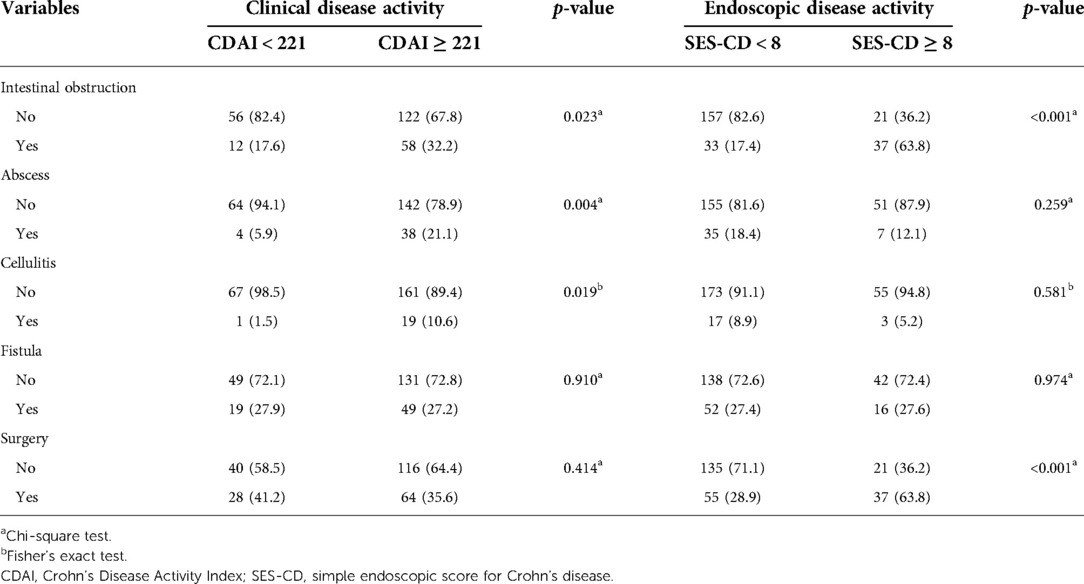
Based on the results, a CDAI score of 221 and an SES-CD score of 8 were used to further analyze the complications and outcomes of the patients. First, we indicated that patients with SES-CD ≥8 were more likely to develop intestinal obstruction (p < 0.001) and undergo surgical intervention (p < 0.001), although patients with active CD were also prone to develop intestinal obstruction (p = 0.023), abscess (p = 0.004), and cellulitis (p = 0.019) (Table 3). Furthermore, we found that there was a significant correlation between CDAI and SES-CD scores for intestinal obstruction (R = 0.2732, p = 0.022); however, no significant correlation was observed between these two for abscess and cellulitis (Figures 2D–F).
SES-CD ≥8 as the most significant independent risk factor for intestinal obstruction
To further determine whether SES-CD ≥8 and clinical disease activity could contribute to the development of intestinal obstruction in CD, univariate analysis was performed. Disease duration (p = 0.028), CDAI score (p = 0.023), SES-CD (p < 0.001), use of immunomodulators (p = 0.046), and albumin level (Alb, p = 0.019) were significantly associated with intestinal obstruction (Table 4). Multivariate logistic regression further demonstrated that CDAI ≥221 [odds ratio (OR): 2.818; 95% CI: 1.218–6.519; p = 0.016], SES-CD ≥8 (OR: 7.731; 95% CI: 3.901–15.322, p < 0.001), and albumin < 35 g/L (OR: 2.501; 95% CI: 1.233–5.073, p = 0.011) were independent risk factors for intestinal obstruction. This result indicated that patients with SES-CD ≥8 were at the highest risk for the development of intestinal obstruction (Table 5).
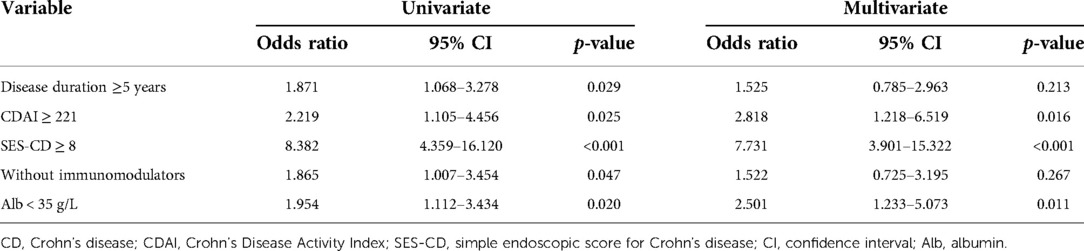
Table 5. Multivariate logistic regression analysis of risk factors for intestinal obstruction in CD patients.
We further analyzed the overall intestinal obstruction-free survival in patients with different endoscopic and clinical activity scores. The results of the Kaplan–Meier method with the log-rank test indicated that the overall intestinal obstruction-free survival was significantly lower in patients with SES-CD ≥8 than that in patients with SES-CD <8 (p < 0.001, Figure 4A), which demonstrated that patients with SES-CD ≥8 were more likely to develop intestinal obstruction earlier in the disease duration. However, there was no statistically significant difference in the intestinal obstruction-free survival in patients in remission or with a clinical activity status (Figure 4B). Taken together, these findings demonstrate that the endoscopic prediction model of SES-CD could be an effective predictor of intestinal obstruction in patients with CD.
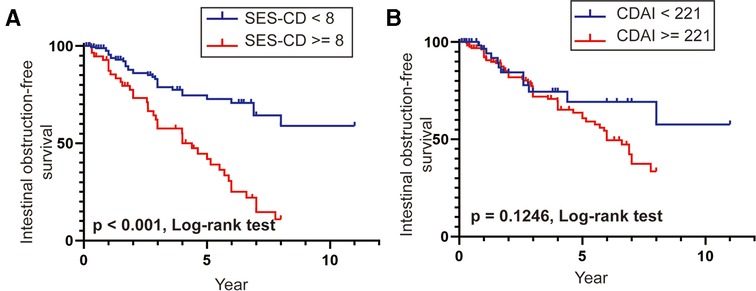
Figure 4. (A,B) Intestinal obstruction-free survival rates in patients with SES-CD ≥8 vs. SES-CD <8 and CDAI ≥221 vs. CDAI <221.
Discussion
The SES-CD indices are widely used by clinicians to evaluate the endoscopic disease activity of CD. Although stenosis is one of the items of SES-CD evaluation, whether SES-CD is an effective indicator of intestinal obstruction has not been clarified yet. Here, we demonstrated a significant correlation between intestinal obstruction and SES-CD. We then identified the most significant threshold evaluation for SES-CD and demonstrated that the SES-CD score of 8 had the best predictive value for intestinal obstruction. We further indicated that SES-CD ≥8 was the most risk contributing factor for intestinal obstruction and significantly decreased intestinal obstruction-free survival time. These data indicate that the endoscopic prediction model of SES-CD could be an effective predictor of intestinal obstruction in CD, outperforming the clinical disease activity. Therefore, more frequent follow-up and colonoscopic surveillance should be considered in patients with SES-CD ≥8, especially in those with mild clinical manifestations but high endoscopic activity.
Intestinal obstruction is one of the most common complications of CD. Activation of fibroblasts leads to abnormal deposition of the extracellular matrix in the mucosa and submucosa, which is an important cause of fibrosis and stenosis formation, leading to intestinal obstruction (15). In intestinal inflammation, various signaling pathways including TGF-b/SMAD, Wnt/β-catenin, and Rho-ROCK can promote the conversion of fibroblasts into myofibroblasts. Myofibroblasts have fibrotic phenotypes such as proliferation, matrix deposition, and anti-apoptosis, which can eventually drive intestinal fibrosis (16). However, currently, no effective antifibrotic therapies exist for intestinal fibrosis to prevent the development of intestinal obstruction. Thus, it is important to explore effective clinical indicators to perform earlier and more frequent surveillance of CD patients.
Previous studies have reported that SES-CD can indicate the endoscopic remission of vedolizumab and ustekinumab in CD patients (17–19). SES-CD has also been used to evaluate the efficacy and safety of the simultaneous treatment with two biological medications for refractory CD (20). Recently, a study reported that SES-CD >2 on the first postoperative ileocolonoscopy was a significant risk factor for clinical recurrence after surgery. Additionally, SES-CD of 1 and 5 were the appropriate cut-off values in predicting the clinical recurrence of small intestine-dominant and colon-dominant CD, respectively (21). However, data on the relationship between SES-CD and intestinal obstruction are limited. In this study, we first reported that SES-CD ≥8 had the best predictive value and was the most significant risk factor for intestinal obstruction.
The CDAI is commonly used in clinical practice to assess the disease activity in patients with CD. Although the validity, reliability, and responsiveness of the CDAI are well defined (6, 22, 23), it has several limitations as an outcome measurement system in clinical practice. The evaluation of some items in the CDAI depends on patients' subjectivity, and some items lack a clear definition in standard (12). Moreover, CDAI reflects systemic inflammation and lacks specificity, whereas SES-CD focuses on intestinal inflammation. Thus, patients with higher SES-CD scores are prone to more severe intestinal inflammation, leading to further activation of intestinal fibroblasts, which could, to some extent, explain why SES-CD outperforms CDAI in predicting intestinal obstruction. As we previously reported, Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), the transcriptional coactivators and effectors of the Hippo signaling pathway (24), activate intestinal fibroblasts and exacerbate their fibrotic phenotype in chronic intestinal inflammation. High expression of YAP/TAZ can contribute to intestinal obstruction in CD patients (25). Additionally, several inflammatory cytokines, such as IL-1β, TNF-α, PDGF, and IL-36, can stimulate intestinal fibroblasts and aggravate the fibrotic phenotype (26, 27). Therefore, we first constructed an endoscopic prediction model for SES-CD ≥8, which could reflect the degree of intestinal inflammation to provide a more direct and effective approach to predict intestinal obstruction to some extent.
This study has several limitations. First, missing follow-ups and incomplete clinical data existed because of the retrospective nature of the study. Second, further studies with larger sample size and longer follow-up period should be performed to confirm the results.
Conclusion
In this study, we identified the most significant threshold evaluation of SES-CD and demonstrated that an SES-CD score of 8 had the best predictive value for intestinal obstruction. We further indicated that SES-CD ≥8 was the most risk contributing factor for intestinal obstruction and significantly decreased the intestinal obstruction-free survival time. Thus, we constructed an endoscopic prediction model for SES-CD ≥8, which outperformed the clinical disease activity and could be an effective predictor of intestinal obstruction in CD. More frequent follow-up and colonoscopic surveillance should be considered in patients with SES-CD ≥8 to prevent the development of intestinal obstruction.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PD, LC, and YG conceived the study. WX analyzed the data and wrote the manuscript. ZH assisted with some analysis. YW collaborated to collect the patients’ information. JZ reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82000481, 81873547 and 82000494) and the Shanghai Sailing Program (No. 20YF1429400).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.984029/full#supplementary-material.
References
1. Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El, Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. (2001) 49:777–82. doi: 10.1136/gut.49.6.777
2. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. (2002) 8:244–50. doi: 10.1097/00054725-200207000-00002
3. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. (2010) 139:1147–55. doi: 10.1053/j.gastro.2010.06.070
4. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn's disease complicated by strictures: a systematic review. Gut. (2013) 62:1072–84. doi: 10.1136/gutjnl-2012-304353
5. Gordon IO, Bettenworth D, Bokemeyer A, Srivastava A, Rosty C, de Hertogh G, et al. Histopathology scoring systems of stenosis associated with small bowel Crohn's disease: a systematic review. Gastroenterology. (2020) 158:137–50.e1. doi: 10.1053/j.gastro.2019.08.033
6. Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn's Disease Activity Index (CDAI). Gastroenterology. (1979) 77:843–6. doi: 10.1016/0016-5085(79)90384-6
7. Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. (2004) 60:505–12. doi: 10.1016/s0016-5107(04)01878-4
8. Sipponen T, Nuutinen H, Turunen U, Färkkilä M. Endoscopic evaluation of Crohn's disease activity: comparison of the CDEIS and the SES-CD. Inflamm Bowel Dis. (2010) 16:2131–6. doi: 10.1002/ibd.21300
9. Xu W, Ou W, Guo Y, Gu Y, Cui L, Zhong J, et al. Clinical outcomes and risk factors of secondary extraintestinal manifestation in ulcerative colitis: results of a multicenter and long-term follow-up retrospective study. PeerJ. (2019) 7:e7194. doi: 10.7717/peerj.7194
10. Xu W, Ding W, Gu Y, Cui L, Zhong J, Du P Risk factors of colorectal stricture associated with developing high-grade dysplasia or cancer in ulcerative colitis: a multicenter long-term follow-up study. Gut Liver. (2020) 14:601–10. doi: 10.5009/gnl19229
11. Xu W, Liu F, Tang W, Gu Y, Zhong J, Cui LC, et al. The Mayo endoscopic score is a novel predictive indicator for malignant transformation in ulcerative colitis: a long-term follow-up multicenter study. Front Surg. (2022) 9:832219. doi: 10.3389/fsurg.2022.832219
12. Khanna R, Zou G, D'Haens G, Feagan BG, Sandborn WJ, Vandervoort MK, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn's disease activity. Aliment Pharmacol Ther. (2015) 41:77–86. doi: 10.1111/apt.13001
13. Xu W, Tang W, Ding W, Hu H, Chen W, Qian Q, et al. Preoperative endoscopic activity predicts the occurrence of pouchitis after ileal pouch-anal anastomosis in ulcerative colitis: a multicenter retrospective study in China. Front Surg. (2021) 8:740349. doi: 10.3389/fsurg.2021.740349
14. Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. (2005) 19(Suppl A):5a–36a. doi: 10.1155/2005/269076
15. Chan WPW, Mourad F, Leong RW. Crohn's disease associated strictures. J Gastroenterol Hepatol. (2018) 33:998–1008. doi: 10.1111/jgh.14119
16. Rieder F, Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. (2009) 6:228–35. doi: 10.1038/nrgastro.2009.31
17. Danese S, Sandborn WJ, Colombel JF, Vermeire S, Glover SC, Rimola J, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn's disease. Gastroenterology. (2019) 157:1007–18.e7. doi: 10.1053/j.gastro.2019.06.038
18. Löwenberg M, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn's disease. Gastroenterology. (2019) 157:997–1006.e6. doi: 10.1053/j.gastro.2019.05.067
19. Löwenberg M, Vermeire S, Mostafavi N, Hoentjen F, Franchimont D, Bossuyt P, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn's disease. Gastroenterology. (2018) 155:1045–58. doi: 10.1053/j.gastro.2018.06.035
20. Yang E, Panaccione N, Whitmire N, Dulai PS, Vande Casteele N, Singh S, et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn's disease. Aliment Pharmacol Ther. (2020) 51:1031–8. doi: 10.1111/apt.15719
21. Akiyama S, Yamada A, Ollech JE, Komaki Y, Komaki F, Pekow J, et al. Predictability of simple endoscopic score for Crohn's disease for postoperative outcomes in Crohn's disease. J Gastroenterol Hepatol. (2021) 36:2785–93. doi: 10.1111/jgh.15540
22. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. (1976) 70:439–44. doi: 10.1016/S0016-5085(76)80163-1
23. Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. (1980) 1:514. doi: 10.1016/s0140-6736(80)92767-1
24. Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. (2019) 20:211–26. doi: 10.1038/s41580-018-0086-y
25. Ou W, Xu W, Liu F, Guo Y, Huang Z, Feng T, et al. Increased expression of yes-associated protein/YAP and transcriptional coactivator with PDZ-binding motif/TAZ activates intestinal fibroblasts to promote intestinal obstruction in Crohn's disease. EBioMedicine. (2021) 69:103452. doi: 10.1016/j.ebiom.2021.103452
26. Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis. (2001) 7:226–36. doi: 10.1097/00054725-200108000-00008
Keywords: Crohn’s disease, Crohn’s disease activity index, simple endoscopic score for Crohn’s disease, intestinal obstruction, predictive value
Citation: Xu W, Hua Z, Wang Y, Gu Y, Zhong J, Cui L and Du P (2022) The endoscopic prediction model of simple endoscopic score for Crohn’s disease (SES-CD) as an effective predictor of intestinal obstruction in Crohn’s disease: A multicenter long-term follow-up study. Front. Surg. 9:984029. doi: 10.3389/fsurg.2022.984029
Received: 1 July 2022; Accepted: 17 August 2022;
Published: 9 September 2022.
Edited by:
Gaetano Luglio, University of Naples Federico II, ItalyReviewed by:
Gianluca Pagano, University of Naples Federico II, ItalyMarco Frascio, University of Genoa, Italy
© 2022 Xu, Hua, Wang, Gu, Zhong, Cui and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Du ZHVwZW5nQHhpbmh1YW1lZC5jb20uY24= Long Cui Y3VpbG9uZ0B4aW5odWFtZWQuY29tLmNu Yubei Gu Z3liMTE4MDlAcmpoLmNvbS5jbg==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Weimin Xu
Weimin Xu Zhebin Hua1,†
Zhebin Hua1,†