
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 09 January 2023
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.982306
This article is part of the Research Topic Reproducibility and Rigour in Surgical Oncology View all 5 articles
 Saihua Chen1,†
Saihua Chen1,† Xiaofeng Tian2,†
Xiaofeng Tian2,† Guanjun Ju1,†
Guanjun Ju1,† Minxin Shi1
Minxin Shi1 Yibiao Chen1
Yibiao Chen1 Qing Wang1
Qing Wang1 Wencheng Dai3
Wencheng Dai3 Tinghua Li1
Tinghua Li1 Jing Pan1
Jing Pan1 Yihui Fan1*
Yihui Fan1*
Objective: This study aimed to investigate the feasibility of using indocyanine green (ICG) near-infrared (NIR) imaging during lymphadenectomy for oesophageal cancer.
Methods: Eighty-seven patients with primary oesophageal cancer were enrolled in this study. All the enrolled patients received an endoscopic injection of ICG between 40 min and 23 h before surgery. Nodal dissection during surgery was performed under fluorescence imaging visualisation, with the NIR signal shown in purple. ICG+ or ICG− nodes were recorded station by station and were microscopically evaluated.
Results: Endoscopic peritumoral ICG injection was successfully performed in all patients. Major post-surgery complications included wound infection, pleural effusion, dysphonia, pneumonia and anastomotic fistula. No patients experienced ICG-related adverse events. A total of 2,584 lymph nodes were removed, and the mean number of lymph nodes for each patient was 29.70 ± 9.24. Most of the removed nodes (97.83%) were ICG+, and 3.32% of the ICG+ nodes were metastatic. No metastatic nodes were ICG− or belonged to an ICG− lymph node station. The time from ICG injection to surgery did not affect the number of harvested lymph nodes.
Conclusions: The use of ICG-NIR imaging during oesophageal cancer surgery can enhance the visualisation of lymph nodes during surgery. It is a feasible, safe and helpful technique for lymphadenectomy.
Oesophageal cancer is one of the most aggressive gastrointestinal malignancies, with a 5-year overall global survival rate of 15%–25% (1, 2). It is the eighth most common cancer type and the sixth leading cause of death from cancer in the world (3). The incidence of oesophageal cancer is increasing year on year (4).
Surgical or endoscopic resection remains the mainstay of treatment for oesophageal cancer (5). It has been reported that lymphadenectomy can significantly improve the long-term survival and accuracy of tumour staging in patients with oesophageal cancer (6). Extensive lymphadenectomy was associated with improved prognosis (6). Currently, lymphadenectomy is often performed using a surgeon's experience without the aid of visual instruments. However, due to the complex lymphatic drainage around the oesophagus, it is a challenge for surgeons to distinguish which lymph nodes are at risk of metastatic disease. To remove as many lymph nodes as possible and decrease the risk of recurrence, the removal of ≥20 lymph nodes for T2 disease and ≥30 for T3/T4 disease has been suggested (7, 8). However, even an extensive 3-field lymphadenectomy may miss some key nodes containing occult metastases, resulting in a 5% risk of disease recurrence (6). Compared with 3-field lymphadenectomy, the commonly used 2-field lymphadenectomy technique has an even higher risk of recurrence (9). Thus, finding a method to dissect sufficient lymph nodes efficiently and accurately is important for the development of lymphadenectomy.
Near-infrared (NIR) fluorescence-guided imaging has been proven to enable surgical visualisation beyond that possible with the naked eye (10). Indocyanine green (ICG) is a Food and Drug Administration-approved NIR dye with a fluorescence emission wavelength ranging from 800 to 1,000 nm (11). The peak absorption and emission wavelengths of ICG are 785 and 810 nm, respectively (11). Indocyanine green-NIR is a surgical navigation technique that has achieved satisfactory results in the localisation of lymph nodes in patients with breast cancer (12), gastric cancer (13), non-small cell lung cancer (14) and other cancers (15). Some studies have been published on the application of ICG-NIR in oesophageal cancer. For example, Hachey et al. (6) conducted a first-in-human study to assess the safety and feasibility of an intraoperative ICG-NIR image-guided approach to lymphatic mapping in patients with oesophageal cancer. They found that NIR lymphatic mapping was safe and could accurately identify regional lymph nodes. Similarly, Wang et al. (16) found that ICG-NIR imaging could successfully perform regional lymph node mapping in thoracic oesophageal cancer. However, although the abovementioned studies had positive results, the feasibility of using ICG-NIR imaging for guiding radical lymphadenectomy in oesophageal cancer is still under evaluation.
In this study, we prospectively enrolled patients with primary oesophageal cancer who underwent lymphadenectomy under ICG-NIR-guided imaging. We aimed to detect the effectiveness of ICG-NIR imaging during lymphadenectomy for oesophageal cancer.
This prospective cohort study was approved by the ethics committee of The Affiliated Tumor Hospital of Nantong University in accordance with the Declaration of Helsinki. All the participants provided written informed consent before participating in the study.
The inclusion criteria were as follows: (1) patients who were diagnosed with primary oesophageal squamous cell carcinoma and were scheduled to undergo a minimally invasive Ivor Lewis esophagectomy, (2) patients aged 18–80 years, (3) patients with a tumour stage of cT1 to cT3, N−/+ and M0 at their preoperative evaluation and (4) patients with an ECOG score of 0 or 1.
The major exclusion criteria included being pregnant or breastfeeding, having a serious mental disorder, having a history of allergy to iodine or seafood and having a history of hyperthyroidism.
We purchased ICG powder from Dandong Yichuang Pharmaceutical Co. (Dandong, China) and diluted 25 mg of it in sterile water to create a 2.5-mg/ml solution. All the enrolled patients underwent endoscopic injection of ICG between 40 min and 23 h before surgery. A 0.5-ml volume of the prepared ICG solution was injected submucosally in the four quadrants around the tumour (Figure 1), providing a total volume of 2 ml. The ICG dose was determined based on previous studies (6, 17). The effectiveness of ICG-NIR fluorescence lymph node mapping was measured by comparing the positive rate of lymph nodes with fluorescence mapping to the overall positive rate of lymph nodes.
Preoperative examinations usually include blood routine, serum immunoglobulin, cellular immune test, liver function and renal function etc. All the enrolled patients underwent a minimally invasive Ivor Lewis esophagectomy and 2-field lymphadenectomy under the same surgical team. The extent of lymph node dissection was the extent in the conventional white light mode, and the number of lymph nodes was counted under the white light mode. Fluorescence-mapped lymph nodes were counted visually during fluorescence imaging. Nodal dissection was performed under fluorescence imaging visualisation, with the NIR signal shown in purple. Fluorescence activity was examined using an infrared camera. ICG+ or ICG− nodes were recorded station by station. Real-time NIR fluorescence images were obtained using a NOVADAQ fluorescence surgical system (storz) with a 10-mm 30° NIR thoracoscopic camera. Adverse events, such as anaphylaxis, were monitored after the administration of ICG. Lymphatic stations were sent separately to the pathology department of our hospital.
All surgical lymph node specimens were fixed in formalin, stained with haematoxylin/eosin and examined by pathologists. The ICG+ and ICG− nodes were microscopically evaluated and categorised as either metastatic or non-metastatic.
The data in this study were analysed using SPSS 22.0 software. Categorical data were described in numbers (percentages) and compared using the Chi-squared test or Fisher's exact test. Quantitative data were described as mean ± standard deviation and compared using a one-way analysis of variance. The diagnostic accuracy of ICG-NIR imaging for metastatic lymph nodes was determined using the standard epidemiological method of calculating the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). A value of P < 0.05 was considered statistically significant.
Eighty-seven patients with primary oesophageal cancer were enrolled in this prospective study. Among them, 64 were male, and 23 were female. The mean age was 68.45 ± 7.03 years, and the mean body mass index was 23.78 ± 2.70 kg/m2. The time from ICG injection to surgery ranged from 40 min to 23 h. Most of the patients (66.67%, 58/87) underwent endoscopic ICG injection 2–6 h before surgery. The characteristics of the enrolled patients are shown in Table 1. No patients experienced ICG-related adverse events (e.g., allergic reactions, rashes or bleeding associated with ICG injection), and no cases of chylothorax were observed.
Endoscopic peritumoral ICG injection was performed successfully in all patients. The 30-day mortality rate was 0%, and 20 (22.99%) patients experienced postoperative complications. Major complications included wound infection (5/87, 5.75%), pleural effusion (9/87, 10.34%), dysphonia (4/87, 4.60%), pneumonia (3/87, 3.45%) and anastomotic fistula (1/87, 1.15%).
We successfully detected lymphatic drainage spreading from the ICG injection site in all 87 patients (Figure 2). Significant NIR background directly on the oesophageal specimen was observed, particularly around the injection site (Figure 3). Dye extravasation into the posterior mediastinum was observed in 4 of the 87 patients, making it difficult to assess the lymph nodes directly. However, the marked lymph nodes could be identified. Thus, these 4 patients were also included in the statistical analysis. Interestingly, all 4 patients underwent endoscopic ICG injection ≥6 h before surgery.
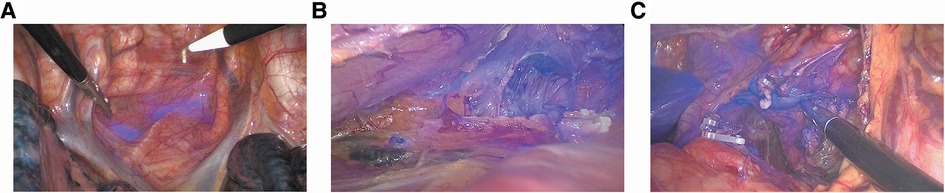
Figure 2. ICG-NIR image-guided identification of esophageal lymph nodes. (A) ICG+ periesophageal lymph nodes; (B) ICG+ paralaryngeal recurrent nerve lymph nodes and ICG+ lymphatic tract; (C) ICG+ left gastric lymph nodes and ICG+ lymphatic tract.
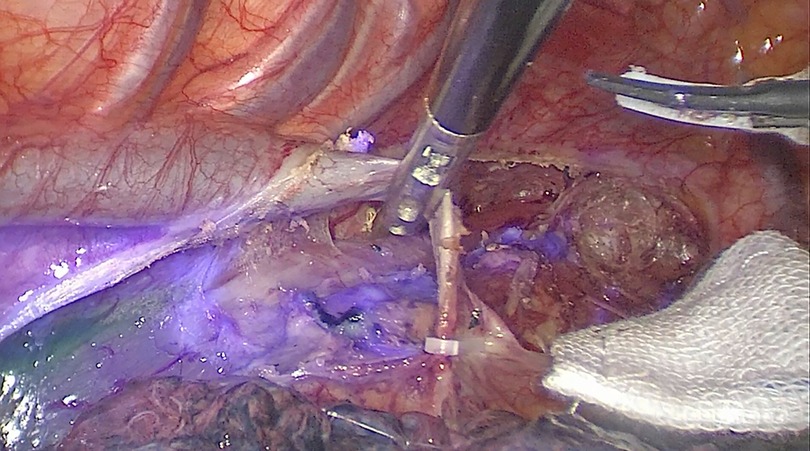
Figure 3. Significant NIR background directly on the esophageal specimen, particularly around the injection site.
A total of 576 lymph node stations were examined in the 87 patients. Among them, 557 (96.70%) stations showed at least one instance of positive ICG fluorescence. Of these 557 lymph node stations, 59 (10.59%) contained at least one metastatic node (Table 2). A total of 576 lymph node stations were detected with white light, of which 557 were fluorescent, and a total of 2,584 lymph nodes were removed. In all, 2,499 lymph nodes showed at least one positive instance of ICG fluorescence, and 85 lymph nodes showed negative ICG fluorescence. The mean number of lymph nodes for each patient was 29.70 ± 9.24 (median: 28, interquartile range: 23–35). Of the 2,584 lymph nodes, 2,528 (97.83%) were ICG+, among which, 84 (3.32%) were metastatic (Table 2). No metastatic nodes were ICG− or belonged to an ICG− lymph node station. The sensitivity of ICG-NIR imaging in the detection of the metastatic lymph nodes was 100%, and the specificity was only 2.24%. The overall PPV was only 3.32%, while the NPV was 100%.
The time from ICG injection to surgery did not affect the number of harvested lymph nodes. Patients who underwent ICG injection ≥6 h before surgery appeared to have higher numbers of harvested lymph nodes (32.90 ± 14.10) than those who received an injection of ICG ≤2 h before surgery (29.70 ± 7.95) or 2–6 h before surgery (29.14 ± 8.70). The difference between the three populations was statistically significant (P = 0.500).
In this study, we analysed the feasibility and safety of using ICG-NIR imaging to guide surgeons in lymph node dissection in oesophageal cancer. As is well known, a complete lymphadenectomy is important to ensure correct staging in the surgical treatment of oesophageal cancer. Our study found ICG-NIR-guided lymphatic mapping during oesophageal cancer surgery to be a feasible, safe and helpful technique. Endoscopic peritumoral ICG injection was successfully performed in all the 87 patients enrolled, with 96.70% of lymph node stations and 97.83% of lymph nodes exhibiting fluorescence. All metastatic nodes were found to be ICG+ nodes.
Previously, ICG-NIR imaging was often used to detect sentinel lymph nodes in solid tumours, demonstrating that ICG was more effective than other tracers (18). For example, Kawakami et al. (19) found that the identification rate of ICG-NIR imaging for sentinel lymph nodes in lung cancer surgery was as high as 72.7%, and the high accuracy rate of this technique could be maintained throughout the follow-up period. Fujita et al. (20) examined the usefulness of ICG-NIR imaging in sentinel lymph node detection in gastric cancer surgery and found sentinel lymph node mapping using ICG fluorescence imaging to be highly accurate.
Numerous studies have attempted to use ICG-NIR imaging to guide radical lymphadenectomy for gastrointestinal tumours. Chen et al. (13) conducted a randomised study to investigate the safety and efficacy of ICG-NIR imaging during laparoscopic D2 lymphadenectomy in patients with gastric cancer. They found that ICG-NIR imaging significantly increased the number of lymph node dissections and reduced lymph node non-compliance (13). Kwon et al. (21) evaluated the role of ICG-NIR imaging as an intraoperative tool for achieving complete lymph node dissection in robotic radical gastrectomy in gastric cancer. In their study, the mean number of overall lymph nodes retrieved was higher in an NIR group than in a control group (48.9 vs. 35.2, P < 0.001), with a significantly higher number of lymph nodes retrieved at stations 2, 6, 7, 8 and 9 (21). Lan YT et al. (22) confirmed the feasibility of ICG-NIR imaging in robotic gastrectomy for gastric cancer. They found that all metastatic lymph nodes were located in the lymph node stations detected by ICG-NIR imaging. Similarly, a prospective trial by Baiocchi et al. (23) also discovered that ICG-NIR imaging-guided lymphadenectomy possessed a high feasibility rate, with considerable ease of use in gastric cancer. The safety and feasibility of ICG-NIR imaging-guided lymphadenectomy were also confirmed in patients with oesophageal cancer, as described in a previous study by Hachey et al. (6). Puccetti, F et al. (24) reported that the use of neoadjuvant radiotherapy had no significant effect on the percentage of ICG+ lymph node in the treatment of gastric cancer. And Zhang, L et al. (25) also reported that neoadjuvant radiotherapy had no effect on the percentage of ICG+ lymph node in breast cancer treatment.
In the present study, we also found that ICG-NIR imaging-guided lymphadenectomy was a safe method, and no ICG-related adverse event was observed. Major postoperative complications included wound infection, pleural effusion, dysphonia, pneumonia and anastomotic fistula, which was similar to previous studies (16, 26). Compared with previous research, the injection of ICG did not increase the rate of postoperative complications (26). Iwamoto, M. et al. (27) found that ICG could reduce the risk of complications such as anastomotic leakage in colorectal resection and Tokumaru, S. also reported that no complications were found using ICG and this method can be a powerful tool to avoid thoracic duct injury during esophageal cancer surgery (28).
Endoscopic peritumoral ICG injection was successfully performed in all 87 enrolled patients in this study, with 96.70% of lymph node stations and 97.83% of lymph nodes exhibiting fluorescence. In previous studies, the median number of lymph nodes retrieved in traditional 2-field lymphadenectomy for oesophageal cancer was 24 (interquartile range: 18–30) (29), while in the present study, ICG-NIR imaging-guided 2-field lymphadenectomy seemed to retrieve a higher number of lymph nodes (median: 28, interquartile range: 23–35). Thus, ICG-NIR imaging may increase the number of lymph node dissections during laparoscopic lymphadenectomy in patients with oesophageal cancer, as demonstrated by Chen et al. (13). However, this result should be confirmed by a larger two-arm randomised study. The key finding of our study is that all the retrieved metastatic nodes were ICG+ nodes; no metastatic nodes were ICG− or belonged to an ICG− lymph node station. Similar results were obtained by Baiocchi et al. (23), 22, Lan YT et al. (22) and Kwon et al. (21) in their respective studies.
In Wang's (16) research, the PPV of ICG-NIR imaging in metastatic lymph node detection was 5.89%, and the NPV was 94.38% in oesophageal cancer. In Park's study, the NPV of ICG-NIR imaging in the detection of nodal metastasis was 100% for the right recurrent laryngeal nerve chain and 98.2% for the left recurrent laryngeal nerve chain in T1 oesophageal cancer (30). Consistent with these studies, we also demonstrated that ICG-NIR imaging in metastatic lymph node detection had very high sensitivity and NPV, indicating that ICG− lymph nodes were not the dominant region of tumour lymphatic drainage. The low PPV and specificity observed in this study indicated that ICG-NIR imaging was unable to identify metastatic lymph nodes specifically. Thus, ICG-NIR imaging could only reflect lymphatic drainage and could not improve the detection of metastatic lymph nodes.
The most suitable ICG injection time before surgery for regional lymph node identification in gastrointestinal tumours has not been determined. In Chen's (13) study, patients underwent endoscopic ICG injection 1 day before surgery, while in Wang's (16) research, ICG injection was administered 30 min before surgery (16). In a study by Romanzi et al. (31), patients underwent esophagogastroduodenoscopy for peritumoral submucosal ICG injection 18 h before surgery. In the present study, the time from ICG injection to surgery ranged from 40 min to 23 h. According to the time from ICG injection to surgery, we divided the enrolled patients into three groups (i.e., ≤2, 2–6 and ≥6 h) and found that the time from injection to surgery did not affect the number of harvested lymph nodes. However, most of the patients (66.67%) in this study underwent endoscopic ICG injection 2–6 h before surgery, which might have caused selection bias. Thus, further prospective studies are needed to confirm the optimal time for ICG injection.
In future studies, we believe that research on the effectiveness of ICG-NIR fluorescence lymph node localisation should be conducted extensively in a variety of tumours. To establish an optimised protocol for ICG injection, different doses, concentrations and times of ICG injection should be tested to determine the optimal times and doses in different tumours. At the same time, the ICG-NIR fluorescence lymph node localisation technique will be further improved to achieve more effective detection of metastatic lymph nodes.
The present study has several limitations. First, it was a single-centre study with a small sample size. A multi-centre with a larger sample size is needed to confirm the conclusions of this research. Second, most of the patients in this study received an endoscopic injection of ICG 2–6 h before surgery. This might have caused a selection bias in determining the optimal ICG injection time.
This study revealed that ICG-NIR imaging during oesophageal cancer surgery can enhance lymph node visualisation during surgery. It is a feasible, safe and helpful technique for lymphadenectomy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by ethics committee of Tumor Hospital Affiliated to Nantong University. The patients/participants provided their written informed consent to participate in this study.
Conception and design of the work: CSH, TXF and JGJ; Data collection: SMX, CYB, WQ, DWC, LTH and PJ; Supervision: CSH, TXF and JGJ; Analysis and interpretation of the data: SMX, CYB, WQ, DWC, LTH, PJ and YF; Statistical analysis: CSH, TXF, JGJ and YF; Drafting the manuscript: CSH, TXF and JGJ; Critical revision of the manuscript: CSH, TXF, JGJ and YF. All authors contributed to the article and approved the submitted version.
This study was supported by the Mandatory project of Nantong Science and Technology Bureau (MS12020027)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. (2020) 50(1):12–20. doi: 10.1007/s00595-019-01878-7
2. Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. (2018) 41(3):210–5. doi: 10.1016/j.asjsur.2016.10.005
3. Wang Y, Cheng J, Xie D, Ding X, Hou H, Chen X, et al. NS1-binding Protein radiosensitizes esophageal squamous cell carcinoma by transcriptionally suppressing c-Myc. Cancer Commun (Lond). (2018) 38(1):33. doi: 10.1186/s40880-018-0307-y
4. Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. (2020) 5(1):229. doi: 10.1038/s41392-020-00323-3
5. Huang TX, Fu L. The immune landscape of esophageal cancer. Cancer Commun (Lond). (2019) 39(1):79. doi: 10.1186/s40880-019-0427-z
6. Hachey KJ, Gilmore DM, Armstrong KW, Harris SE, Hornick JL, Colson YL, et al. Safety and feasibility of near-infrared image-guided lymphatic mapping of regional lymph nodes in esophageal cancer. J Thorac Cardiovasc Surg. (2016) 152(2):546–54. doi: 10.1016/j.jtcvs.2016.04.025
7. Herrera LJ. Extent of lymphadenectomy in esophageal cancer: how many lymph nodes is enough? Ann Surg Oncol. (2010) 17(3):676–8. doi: 10.1245/s10434-009-0824-7
8. Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. (2008) 112(6):1239–46. doi: 10.1002/cncr.23309
9. Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg. (1998) 175(1):47–51. doi: 10.1016/s0002-9610(97)00227-4
10. Ekman M, Girnyi S, Marano L, Roviello F, Chand M, Diana M, et al. Near-Infrared fluorescence image-guided surgery in esophageal and gastric cancer operations. Surg Innov. (2022) 29(4):540–49. doi: 10.1177/15533506211073417
11. Blanco-Colino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol. (2018) 22(1):15–23. doi: 10.1007/s10151-017-1731-8
12. Yin R, Ding LY, Wei QZ, Zhou Y, Tang GY, Zhu X. Comparisons of ICG-fluorescence with conventional tracers in sentinel lymph node biopsy for patients with early-stage breast cancer: a meta-analysis. Oncol Lett. (2021) 21(2):114. doi: 10.3892/ol.2020.12375
13. Chen QY, Xie JW, Zhong Q, Wang JB, Lin JX, Lu J, et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: a randomized clinical trial. JAMA Surg. (2020) 155(4):300–11. doi: 10.1001/jamasurg.2019.6033
14. Digesu CS, Hachey KJ, Gilmore DM, Khullar OV, Tsukada H, Whang B, et al. Long-term outcomes after near-infrared sentinel lymph node mapping in non-small cell lung cancer. J Thorac Cardiovasc Surg. (2018) 155(3):1280–91. doi: 10.1016/j.jtcvs.2017.09.150
15. Cabrera S, Barahona-Orpinell M, Almansa-González C, Padilla-Iserte P, Bebia V, Martí L, et al. Combined use of ICG and technetium does not improve sentinel lymph node detection in endometrial cancer: results of the COMBITEC study. Gynecol Oncol. (2021) 162(1):32–7. doi: 10.1016/j.ygyno.2021.05.002
16. Wang X, Hu Y, Wu X, Liang M, Hu Z, Gan X, et al. Near-infrared fluorescence imaging-guided lymphatic mapping in thoracic esophageal cancer surgery. Surg Endosc. (2022) 36(6):3994–4003. doi: 10.1007/s00464-021-08720-7
17. Gilmore DM, Khullar OV, Jaklitsch MT, Chirieac LR, Frangioni JV, Colson YL. Identification of metastatic nodal disease in a phase 1 dose-escalation trial of intraoperative sentinel lymph node mapping in non-small cell lung cancer using near-infrared imaging. J Thorac Cardiovasc Surg. (2013) 146(3):562–70; discussion 569–70. doi: 10.1016/j.jtcvs.2013.04.010
18. Sun WYL, Dang JT, Modasi A, Nasralla A, Switzer NJ, Birch D, et al. Diagnostic accuracy of sentinel lymph node biopsy using indocyanine green in lung cancer: a systematic review and meta-analysis. Gen Thorac Cardiovasc Surg. (2020) 68(9):905–13. doi: 10.1007/s11748-020-01400-8
19. Kawakami Y, Kondo K, Kawakita N, Matsuoka H, Toba H, Takizawa H, et al. Long-term outcomes of sentinel node identification using indocyanine green in patients with lung cancer. Thorac Cancer. (2021) 12(2):165–71. doi: 10.1111/1759-7714.13737
20. Fujita T, Seshimo A, Kameoka S. Detection of sentinel nodes in gastric cancer by indocyanine green fluorescence imaging. Hepatogastroenterology. (2012) 59(119):2213–6. doi: 10.5754/hge12023
21. Kwon IG, Son T, Kim HI, Hyung WJ. Fluorescent lymphography-guided lymphadenectomy during robotic radical gastrectomy for gastric cancer. JAMA Surg. (2019) 154(2):150–8. doi: 10.1001/jamasurg.2018.4267
22. Lan YT, Huang KH, Chen PH, Liu CA, Lo SS, Wu CW, et al. A pilot study of lymph node mapping with indocyanine green in robotic gastrectomy for gastric cancer. SAGE Open Med. (2017) 5:2050312117727444. doi: 10.1177/2050312117727444
23. Baiocchi GL, Molfino S, Molteni B, Quarti L, Arcangeli G, Manenti S, et al. Fluorescence-guided lymphadenectomy in gastric cancer: a prospective western series. Updates Surg. (2020) 72(3):761–72. doi: 10.1007/s13304-020-00836-0
24. Puccetti F, Cinelli L, Genova L, Battaglia S, Barbieri LA, Treppiedi E, et al. Applicative limitations of indocyanine green fluorescence assistance to laparoscopic lymph node dissection in total gastrectomy for cancer. Ann Surg Oncol. (2022) 29(9):5875–82. doi: 10.1245/s10434-022-11940-3
25. Zhang L, Cheng M, Lin Y, Zhang J, Shen B, Chen Y, et al. Ultrasound-assisted carbon nanoparticle suspension mapping versus dual tracer-guided sentinel lymph node biopsy in patients with early breast cancer (ultraCars): phase III randomized clinical trial. Br J Surg. (2022) 109(12):1232–38. doi: 10.1093/bjs/znac311
26. Wang X, Li X, Cheng H, Zhang B, Zhong H, Wang R, et al. Single-Port inflatable mediastinoscopy combined with laparoscopic-assisted small incision surgery for radical esophagectomy is an effective and safe treatment for esophageal cancer. J Gastrointest Surg. (2019) 23(8):1533–40. doi: 10.1007/s11605-018-04069-w
27. Iwamoto M, Ueda K, Kawamura J. A narrative review of the usefulness of indocyanine green fluorescence angiography for perfusion assessment in colorectal surgery. Cancers (Basel). (2022) 14(22):5623. doi: 10.3390/cancers14225623
28. Tokumaru S, Kitazawa M, Nakamura S, Koyama M, Soejima Y. Intraoperative visualization of morphological patterns of the thoracic duct by subcutaneous inguinal injection of indocyanine green in esophagectomy for esophageal cancer. Ann Gastroenterol Surg. (2022) 6(6):873–9. doi: 10.1002/ags3.12594
29. Li B, Zhang Y, Miao L, Ma L, Luo X, Zhang Y, et al. Esophagectomy with three-field versus two-field lymphadenectomy for middle and lower thoracic esophageal cancer: long-term outcomes of a randomized clinical trial. J Thorac Oncol. (2021) 16(2):310–7. doi: 10.1016/j.jtho.2020.10.157
30. Park SY, Suh JW, Kim DJ, Park JC, Kim EH, Lee CY, et al. Near-Infrared lymphatic mapping of the recurrent laryngeal nerve nodes in T1 esophageal cancer. Ann Thorac Surg. (2018) 105(6):1613–20. doi: 10.1016/j.athoracsur.2018.01.083
Keywords: indocyanine green, near infrared, esophageal cancer, lymphadenectomy, metastatic nodes
Citation: Chen S, Tian X, Ju G, Shi M, Chen Y, Wang Q, Dai W, Li T, Pan J and Fan Y (2023) Indocyanine green near-infrared imaging-guided lymph node dissection during oesophageal cancer surgery: A single-centre experience. Front. Surg. 9:982306. doi: 10.3389/fsurg.2022.982306
Received: 30 June 2022; Accepted: 13 December 2022;
Published: 9 January 2023.
Edited by:
Dimitrios Schizas, National and Kapodistrian University of Athens, GreeceReviewed by:
Efstahios Kotidis, Aristotle University of Thessaloniki, Greece© 2023 Chen, Tian, Ju, Shi, Chen, Wang, Dai, Li, Pan and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yihui Fan ZmFueWlodWlfZmZAMTI2LmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.