
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 09 August 2022
Sec. Thoracic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.981313
Background: For peripheral pulmonary nodules, the regularity of lymph node (LN) metastasis has not been studied. This study aimed to evaluate the metastasis pattern of intrapulmonary and relevant mediastinal lymph nodes in early-stage lung cancer, and further selected patients who were of low risk of LN metastasis as potential population to receive sub-lobectomy.
Methods: This study prospectively included consecutive patients with peripheral clinical T1N0M0 disease who underwent complete resection with LN dissection or sampling from August 2014 to July 2015. The patients were followed up to 15, May 2021. Univariable or multivariable Logistic analysis was used to identify the risk factors. Models predicting LN metastasis risk were conducted. The area under the curve for the receiver operating characteristic curves was used to evaluate the diagnostic value. Disease-free survival and overall survival were compared between groups.
Results: Finally, 201 patients were included in this study. For patients with negative tumor-bearing (TB) 13 and 14 station LNs, the positive rate of other lymph node stations was extremely low. Maximum CT value, pleural indentation and CEA level were risk factors for N1 station LNs metastasis. Besides, the factors above and lobulation sign were risk factors for skip metastasis beyond TB 13 and 14 station LNs. We constructed two scoring tables to predict N1 station metastasis and skip metastasis beyond TB 13 and 14 station. The AUC were 0·837 and 0·823, respectively. Based on the first table, 40·9% of patients suffered N1 station LNs metastasis and 27·3% had N2 disease in “high risk group” while the proportion was only 5·7% and 4·5% in “low risk group”. For patients with negative TB13 and TB14 station LNs, based on the latter table, 11·1% of patients had N1 stations LNs metastasis and 16·7% had pN2 disease in “high risk group” while only 2·3% patients in “low risk group” suffered this kind of metastasis.
Conclusion: For peripheral pulmonary nodules patients, stations 13 and 14 LNs may be the sentinel nodes. For patients with low risk of N1 metastasis and skip metastasis, sub-lobar resection might be sufficient for those who were of negative TB 13 and 14 station LNs.
Globally, lung cancer is the second most common cancer, and about 2·2 million new cases of lung cancer were diagnosed in 2020 (1). It's also the leading cause of cancer-related death with an estimated 1·8 million deaths worldwide (1). Non-small cell lung cancer (NSCLC) occurs with the highest frequency in lung cancer (2). Low-radiation-dose computed tomography (low-dose CT) can improve the likelihood of detection of lung cancer at an earlier stage (3–5) and most lung cancer that can be detected by CT develops in the periphery of the lungs (6). Nodules that are present beyond the visualized segmental bronchi are usually called peripheral pulmonary nodules (7).
Surgical resection is considered the standard treatment for patients with stage I or II lung cancer (8) and recent guideline recommended anatomic lung resection (9). While Takahiro Mimae and Morihito Okada reported that in early-stage NSCLC, segmentectomy has an advantage over lobectomy, with a comparable curability to and less toxicity than lobectomy (10).
Lymph node metastasis is the most common and major metastatic pathway of NSCLC as well as the most important factor which affect staging and prognosis. Accurate evaluation of lymph node metastasis is of great significance for guiding surgical methods and adjuvant therapy. Lymph node metastasis in lung cancer is associated with lymphatic drainage. Pulmonary lymph first flows to the lymph nodes around the segmental bronchi, then back to the second hilum of the lung, and thence to hilar lymph nodes (11). It is generally recognized that lymph node metastasis of lung cancer is affected by many factors, such as the location, size, pathological type, and differentiation of the primary tumor (12). Intrapulmonary lymph nodes are cleaned with the resection of a pulmonary lobe. Compared to lobectomy, lymph node dissection in other segments can be skipped during segmentectomy. Thus, intrapulmonary lymph nodes dissection may be missed in those patients with peripheral pulmonary nodules and a high risk of intrapulmonary lymph nodes metastasis. It's necessary for us to distinguish which patients are at low risk of intrapulmonary lymph node metastasis that they may benefit from segmentectomy. Until now, there is a lack of studies like that.
We conducted this study to research metastasis of intrapulmonary and mediastinal lymph nodes in early-stage lung cancer. Besides, we attempted to screen out those who are at low risk of intrapulmonary and mediastinal lymph node metastasis, to evaluate less extent of pulmonary resection for this population of patients.
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, the reference number is 2013-255-1. Every individual participant had signed an informed consent form for participating in the study.
This prospective study initially included 331 consecutive patients with peripheral clinical T1N0M0 disease who underwent a complete resection in the department of thoracic, the First Affiliated Hospital from August 2014 to August 2015. Systematic mediastinal lymph node (LN) dissection or sampling was performed on each patient and intrapulmonary LNs (N1 LNs) were also systematically collected from the resected tissue samples by a trained thoracic surgeon cooperating with a pathologist. Systematic lymph node dissection (SLND), lobe-specific lymph node dissection (L-SLND) and selective lymph nodal sampling (SLNS) were conducted. Segmental bronchus and the intersegmental veins were carefully dissected and identified as segmental borders. The segmental nodes (station 13) and subsegmental nodes (station 14) were dissected and further divided as tumor-bearing (TB) or non-tumor-bearing (NTB) based on the location of the tumor.
Each patient enrolled in our study was followed up by telephone call and outpatient service every 3 months within the first year after the operation and every 6 months after the postoperative one year.
The exclusion criteria were as followed: (1) Patients have more than 1 lesion considered to be malignant; (2) patients with a history of another cancer or received any anti-tumor therapy preoperatively; (3) a selected lymph node biopsy was performed; (4) histological type was not invasive adenocarcinoma; (5) tumor invade all segments within a lobe; (6) patients with parietal pleura invasion; (7) Tumor-bearing station 13 and 14 LN were not evaluated; (8) loss to follow up; (9) suffered another malignancy during the follow-up procedure; (10) data was not complete (Supplementary Figure S1).
All tumors were staged according to the malignant tumor staging system in the eighth edition of the Union for International Cancer Control TNM Classification. Visceral pleural invasion (VPI) was defined as tumor cells invading beyond the elastic layer of the visceral pleura. If the invasion status was equivocal on hematoxylin-eosin–stained sections, an elastin stain was performed to confirm the presence or absence of VPI.
The outcomes of this study included overall survival (OS) and disease-free survival (DFS). The OS is the time from the operation to the death from any cause. DFS is the time from the operation to the first tumor recurrence/metastasis or death of the patients for any reason. The latest follow-up of the current study was performed on May 15, 2021.
Categorical variables were compared using the χ2 test, while continuous variables were analyzed using the t-test, Mann-Whitney U test. Univariable or multivariable Logistic analysis was used to identify the risk factors. The area under the curve (AUC) and its standard error (SE) for the receiver operating characteristic (ROC) curves were used to evaluate the diagnostic value. All endpoints were estimated using the Kaplan-Meier (KM) method and compared by the log-rank test. Two-sided p values of <0·05 were considered statistically significant. All analyses were performed using SPSS 22·0 software (IBM, Armonk, NY), GraphPad Prism 7·0 software (GraphPad Software, La Jolla, Ca).
Finally, 201 patients were included in this study. Most patients were female, younger than 65 years old with clinical T1b-1c disease. There were 14 (7·0%) patients who had pN1 disease and 19 (9·5%) participants suffered pN2 disease. The detailed characteristics of our study population were shown in Table 1.
Among all the patients, 33 (16·4%) patients had up-staging disease regardless of N1 or N2 lymph node metastasis. No lymph node metastasis was observed in patients with tumor diameters smaller than 1 centimeter.
18 (7·3%) patients had TB station 13 or 14 lymph node metastasis and among them 7 had only these two stations positive. For patients with negative TB 13 and 14 station lymph nodes, 2 (1·1%) cases had NTB station 13 and 14 lymph node metastasis and 14 patients had positive station 12 to N2 station lymph node. Among those N1 station lymph node metastasis patients, 71·4%(10/14) of them had only station 13 or 14 lymph node metastasis. Skip N2 metastasis was defined as the N2 station lymph nodes positive when all N1 station lymph nodes were negative. In our study, 6 (3·4%) cases showed skip N2 metastasis (Table 2). Survival analysis indicated that patients with pN0 disease had significantly better OS and DFS than pN1, general pN2 and skip pN2 disease while the survival outcomes of skip pN2 patients were better than general pN2 patients in both OS and DFS even though they were of the same pN stage (pN0 vs pN1 vs pN2 vs skip pN2: 79·03 vs 69·00 vs 47·92 vs 62·00 months for mean OS, p < 0·0001; 77·42 vs 66·71 vs 47·92 vs 62·00 months for mean DFS, p < 0·001) (Figure 1).
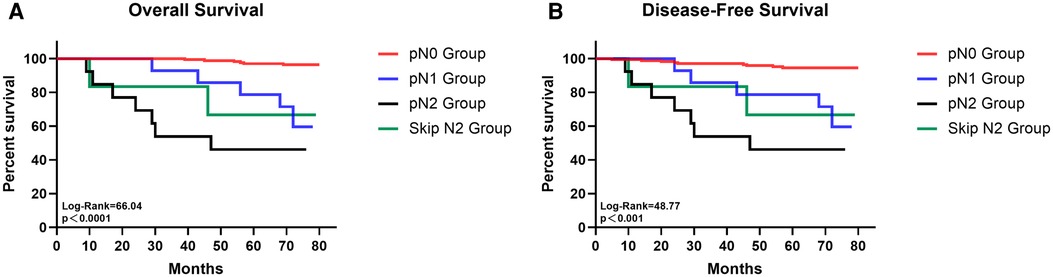
Figure 1. The survival curves of overall survival (A) and disease-free survival (B) for patients with pN0, pN1, pN2 and skip N2 disease.
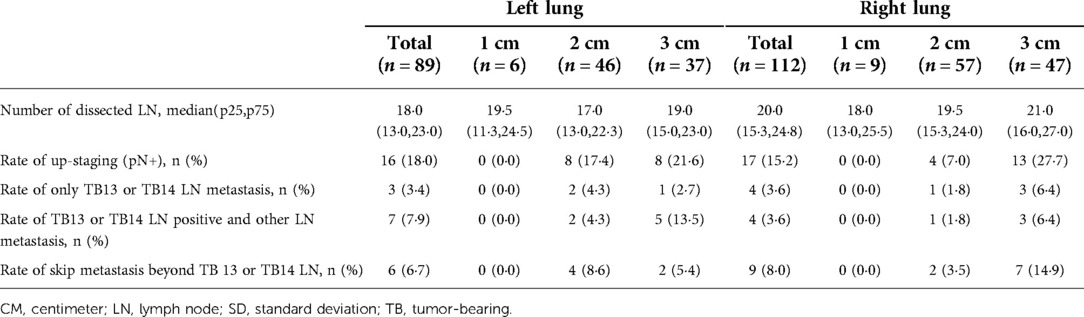
Table 2. Lymph node metastasis pattern for clinical stage IA (cT1N0M0) peripheral lung adenocarcinoma in left and right sides stratified by tumor size.
Totally, 27 (13·4%) patients suffered from N1 station lymph nodes metastasis. The factors that were significantly associated with the occurrence of N1 station lymph nodes metastasis were sizes of tumors, maximum CT value, the solid component of tumors, the occurrence of lobulation sign and pleural indentation, a rise of preoperative tumor markers, lower differentiation of tumors, VPI, solid, papillary or micropapillary growth component. Furthermore, multivariable regression analysis showed that maximum CT value, preoperative carcinoma embryonic antigen level, differentiation of tumors, VPI, and appearance of papillary growth component was an independent predictor of the metastasis of N1 station lymph nodes metastasis (Table 3).
To use preoperative characteristics to predict the metastasis of N1 station lymph nodes, extra regression analysis with only preoperative factors was performed, the results were shown in Supplementary Table S1. Based on the results of the regression analysis, an index that predicts which we called “N1LN” was formulated (Supplementary Table S2) The total score of the “N1LN” index was 10 points and drawing a ROC curve, the AUC of this index was 0·837 (Figure 2A). The cutoff value was 6·5 which was calculated by the ROC curve. Based on the cutoff value, patients were divided into “High N1LN risk Group” and “Low N1LN risk Group”. In the “High N1LN risk Group”, 40·9% (18/44) of patients suffered N1 station lymph nodes metastasis and 27·3% (12/44) had N2 disease while the proportion was only 5·7% (9/157) and 4·5% (7/157) in the “Low N1LN risk Group”, and the differences were of statistical significance (p < 0·001; p < 0·001, respectively). Moreover, the patients in the “High N1LN risk Group” had significantly worse prognosis in terms of not only OS (mean OS: 68·14 vs 78·55 months, log-rank = 22·82, p < 0·0001) but also DFS (mean DFS: 63·84 vs 78·05 months, log-rank = 27·14, p < 0·0001) (Figures 3A,B).
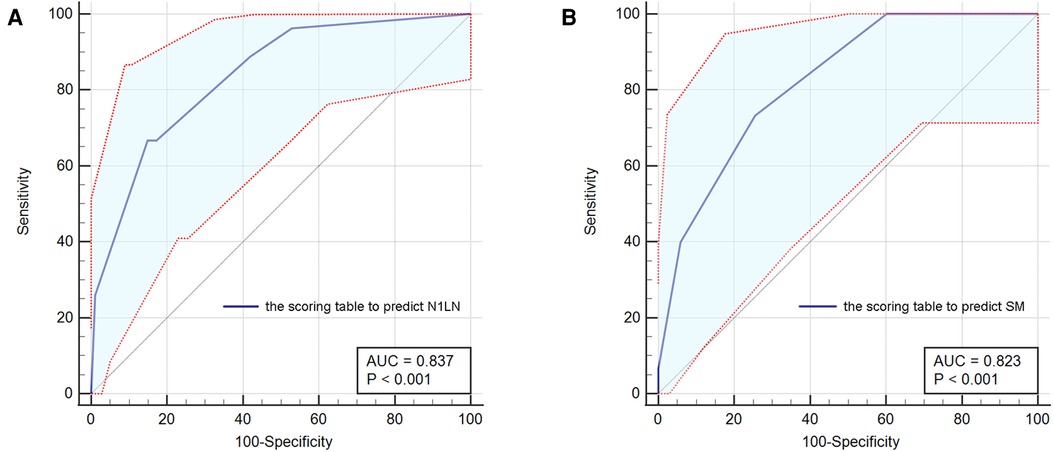
Figure 2. The ROC curves for the scoring table to predict N1LN (A) and the scoring table to predict SM (B). ROC, receiver operating characteristic; N1LN, N1 station lymph nodes metastasis; SM, skip metastasis beyond TB 13 and 14 station lymph nodes.
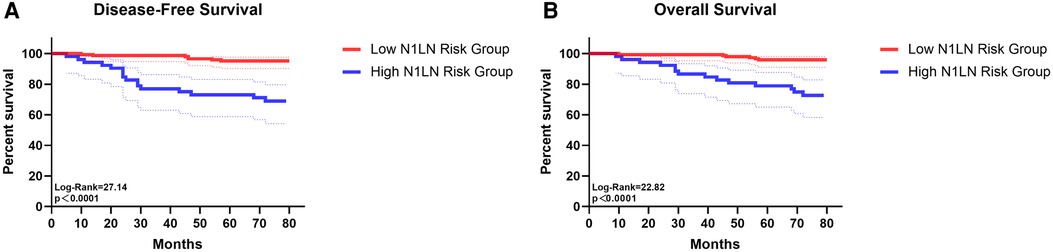
Figure 3. The survival curves of disease-free survival (A) and overall survival (B) for patients in low N1LN risk group and in high N1LN risk group.
Several preoperative parameters were enrolled in the univariable regression analysis. The diameter of tumors, maximum CT value, tumor consistency, the appearance of spicule sign, lobulation sign or pleural indentation, preoperative CEA, CA125, and CA199 were identified to be associated with the metastasis of other lymph node stations when TB 13 and TB 14 LNs were negative. And the results of the multivariable analysis indicated that maximum CT value larger than −75 Hu, the appearance of lobulation sign and pleural indentation, and the rise of preoperative CEA levels were independently associated with skip metastasis beyond TB 13 and TB 14 station lymph nodes (Table 4).
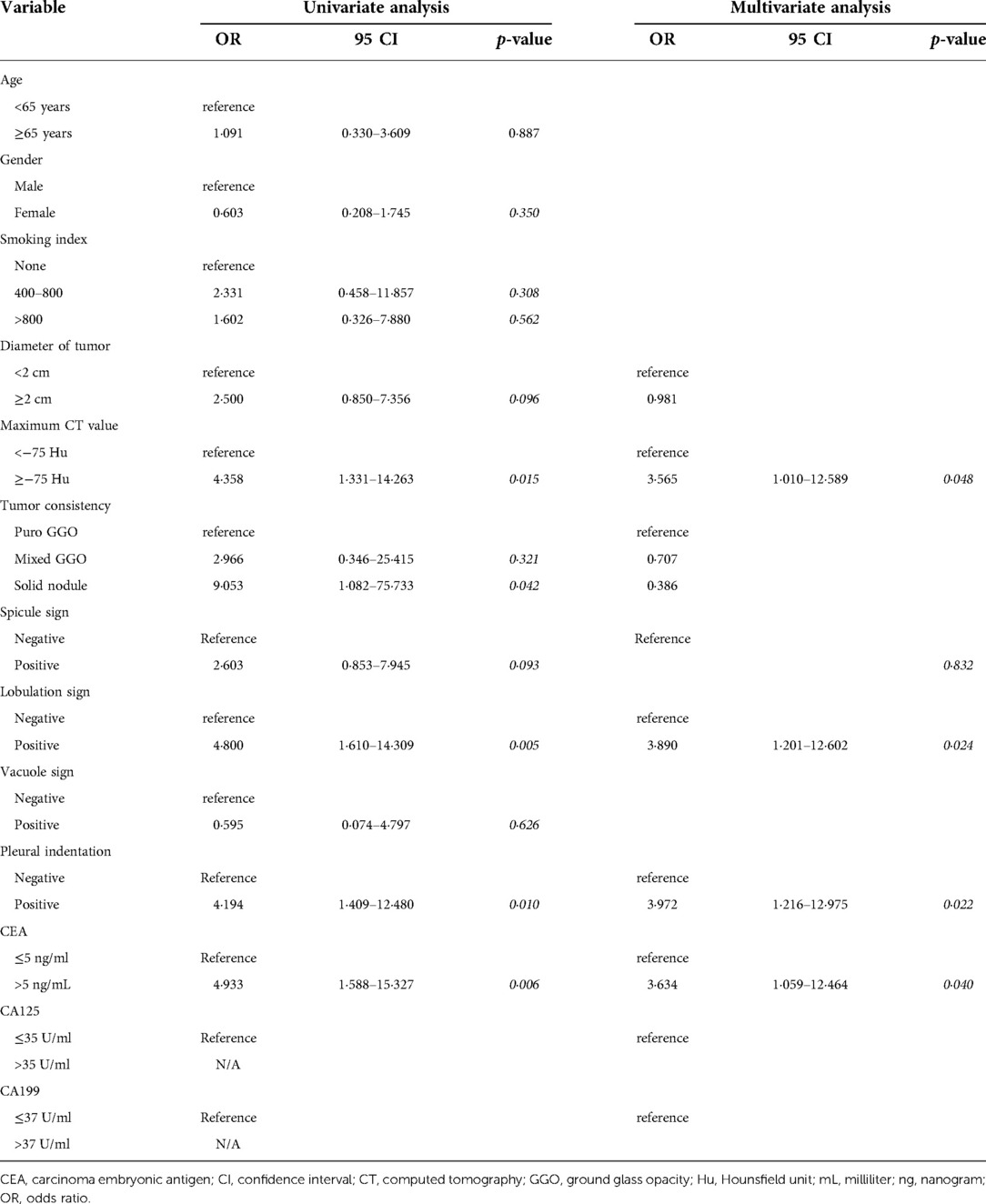
Table 4. Preoperative factors predicting skip metastasis beyond tumor-bearing 13 and tumor-bearing 14 lymph node.
Based on the results, we further constructed another scoring table (Supplementary Table S3) to predict the possibility of skip metastasis beyond TB 13 and 14 station lymph nodes which combined the aforementioned 4 independent risk factors and was called the “SM” index. The score was significantly correlated with the risk of skim metastasis beyond TB segmental or sub-segmental lymph nodes, and the AUC was 0·823 with the cut-off value of 3·75. For patients with negative TB13 and TB14 station lymph nodes, each of them was divided into “High SM risk Group” and “Low SM risk Group” according to the cut-off value.
Compared with the “Low SM risk Group”, the “High SM risk Group” harvested similar numbers of N1 and N2 station lymph nodes. However, in the “High SM risk Group”, 11·1% (6/54) of patients had N1 stations lymph nodes metastasis and 16·7% (9/54) had pN2 disease when the TB 13 and 14 station lymph nodes were negative while only 2·3% (3/129) patients in the “Low SM risk Group” suffered this kind of metastasis and the differences were of statistic significance (p = 0·033; p < 0·001, respectively) (Supplementary Table S4). The detailed lymph node metastasis status of each station between the high and low risk of “SM” index was shown in Supplementary Table S4. The K-M survival analysis and log-rank comparison revealed that the “Low SM risk Group” had better OS (mean OS: 78·23 vs 71·81 months, log-rank = 9·376, p = 0·002) and DFS (mean DFS: 77·02 vs 69·76 months, log-rank = 10·89, p = 0·001) than the “High SM risk Group” (Figures 4A,B).
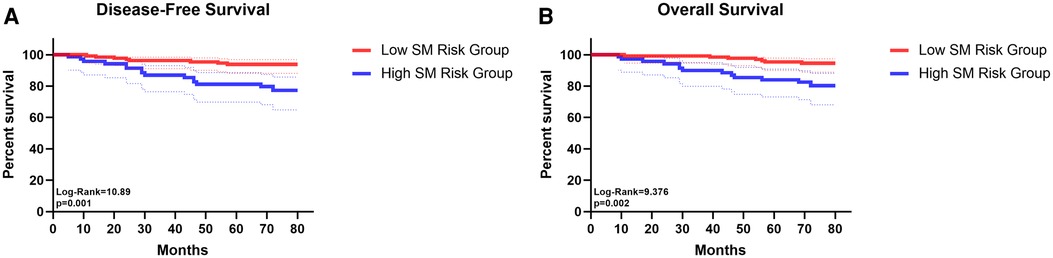
Figure 4. The survival curves of disease-free survival (A) and overall survival (B) for patients in low SM risk group and in high SM risk group.
Lobectomy had been generally accepted as a standard cure for NSCLC, a few authors had doubts as to whether lobectomy is necessary for treatment of small lesions (13). Hisashi Saji et al suggested that for patients with small-sized (≤2 cm, consolidation-to-tumor ratio >0·5) peripheral NSCLC, segmentectomy should be the standard surgical procedure instead of lobectomy (14). However, a few potential high-risk lymph nodes may be ignored during segmentectomy. Therefore, it's of great significance to determine the risk factors of lymph nodes metastasis, and those peripheral pulmonary nodules patients at low risk of intrapulmonary lymph nodes metastasis may be proper to undergo segmentectomy.
The aim of this study was to investigate the pattern of intrapulmonary lymph nodes metastasis and corresponding mediastinal lymph node metastases in patients with peripheral NSCLC. And based on the law that pulmonary lymph first flows to the lymph nodes around the segmental bronchi, then back to the second hilum of the lung, and thence to hilar lymph nodes (11), we try to determine whether TB station 13 or 14 lymph node metastasis can be a predictor for other lymph nodes metastasis. Then we can construct a model to distinguish patients at high or low risk of lymph node metastasis and discover the best surgical method and lymph node dissection. This study suggests that the cervical node involvement of lung adenocarcinoma is spread mostly orderly, and the incidence of skip metastasis is low. Certainly, there are a small number of skip pN2 patients, but their survival outcomes were better than general pN2 patients in both OS and DFS.
Stiles et al. compared lobectomy and sublobar resections on the basis of the extent of LN dissection in stage I NSCLC patients (15). When patients with at least 1 lymph node removed were examined, the survival rate of sublobar resections was lower than lobectomy (15). While when patients with ≥9 lymph nodes were examined, the overall survival and cancer-specific survival seemed to be similar (15). These findings show that only enough LN assessment is performed, might the oncological outcomes of sublobar resections be as effective as those of lobectomy (15). Lymph node metastasis is an important factor in treatment decisions. We can hardly find prospective and large-sample studies on clinical data of intrapulmonary lymph nodes especially stations 13 and 14 (16). In our study, among those N1 station lymph node metastasis patients, most of them had only station 13 or 14 lymph node metastasis. So we think it's necessary to perform station 13 and 14 lymph node dissection. However, as far as I know, many surgeons didn't dissect station 13 and 14 lymph nodes. Based on the pattern of lymph node metastasis, we constructed a table to evaluate N1 Station lymph node metastasis. Additionally, we found that patients with negative 13 and 14 station lymph nodes were less likely to have other station lymph nodes positive, which indicated that maybe they can act as the sentinel nodes. Further, we constructed another table to predict skip metastasis beyond TB 13 and TB 14 station lymph nodes. With the help of the tables, we can differentiate the high risk of lymph node metastasis from the low risk, and then we can point out which patient can take less extent of pulmonary resection. We can see significant differences in overall survival and disease-free survival between high-risk and low-risk groups.
Our tables consisted of maximum CT value, pleural indentation, lobulation sign, and CEA level. Preoperative imaging CT or PET-CT has limited ability to detect lymph nodes (17), thus people turn to research serum biomarkers. Carcinoembryonic antigen (CEA) is the most commonly used one in tumor markers (18–21), it was an important predictor for lymph node metastasis (22). In lung adenocarcinoma, the expression of CEA was correlated with lymph node metastasis (23). Ding et al.'s study showed that lobulated margins were associated with invasiveness (24). In pure GGNs, invasive pulmonary adenocarcinoma was more lobulated than preinvasive lesions (25). In the comparison of CT findings between invasive tumor and noninvasive tumor, they showed differences in terms of pleural indentation and lobulation (26). Ma et al. also found that the 5-year survival rate of patients with slick margin was a little higher than those with lobulation sign (27). They indicated that pleura indentation was one of the risk factors for poor prognosis of stage I NSCLC (27). Mean CT value was significantly associated with invasive extent (28). Likewise, maximum CT value was also a significant predictor of histologic invasiveness (29). The invasive component of pure GGO was present in the site with high CT value (30). In our study, multivariate analysis revealed that lower CT value, lower CEA level, negative pleural indentation and nonlobulated sign were important differentiators of low lymph nodes metastasis risk group (p < 0·01), with excellent differentiating accuracy (area under ROC curve, 0·823). By studying the pattern of intrapulmonary and mediastinal lymph nodes metastasis and finding the risk factors for lymph nodes metastasis, we tried to point out which patient can take less extent of pulmonary resection.
This is the first study that constructed two scoring tables to evaluate N1 station lymph nodes metastasis and predict skip metastasis beyond TB 13 and TB 14 station lymph nodes. With the two tables, doctors can find out low-risk patients and make optimal surgery plan for them. There are several limitations of this study. First, it was conducted at a single center. Second, the number of the sample may be not enough to support a strong level of evidence of its clinical application. Further multicenter studies and more cases are therefore needed to verify these results. Third, we didn't compare segmentectomy and lobectomy in the “Low SM risk Group”. Further study is needed to determine whether segmentectomy can be a perfect substitute for lobectomy in low lymph nodes metastasis risk group.
In conclusion, different clinical features lead to the different probability of lymph node metastasis. For patients with peripheral pulmonary nodules, stations 13 and 14 lymph nodes may be the sentinel nodes. Therefore, for patients with high risk of N1 metastasis and skip metastasis, lobectomy is appropriate for those who were of positive TB 13 and 14 station lymph nodes. As for patients in the low-risk group, those who have negative TB 13 and 14 station lymph nodes may be the potential population who are proper to take sublobar resection. In addition, station 13 and 14 lymph node dissection should be performed in both lobectomy and sublobar resection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University. The patients/participants provided their written informed consent to participate in this study.
LK and HM made the concept and design. QZ, YW, PX, and LY obtained and analyzed the clinical data. LK and HM drafted the manuscript. LK, HM, QZ, YW, PX, LY, WL, and JH critically revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
The paper was supported by the Major Science and Technology Project of Zhejiang Province (2020C03058) and the Lung Tumor Diagnosis and Treatment Technology Research Center of Zhejiang Province (JBZX-202007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.981313/full#supplementary-material.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. (2008) 83(5):584–94. doi: 10.4065/83.5.584
3. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. (2011) 365(5):395–409. doi: 10.1056/NEJMoa1102873
4. de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. (2020) 382(6):503–13. doi: 10.1056/NEJMoa1911793
5. Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early lung cancer action project: overall design and findings from baseline screening. Lancet. (1999) 354(9173):99–105. doi: 10.1016/S0140-6736(99)06093-6
6. Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJM, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. (2013) 187(8):848–54. doi: 10.1164/rccm.201209-1651OC
7. Narula T, Machuzak MS, Mehta AC. Newer modalities in the work-up of peripheral pulmonary nodules. Clin Chest Med. (2013) 34(3):395–415. doi: 10.1016/j.ccm.2013·06·001
8. Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. (2021) 32(12):1637–42. doi: 10.1016/j.annonc.2021·08·1994
9. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28(suppl_4):iv1–iv21. doi: 10.1093/annonc/mdx222
10. Mimae T, Okada M. Are segmentectomy and lobectomy comparable in terms of curative intent for early stage non-small cell lung cancer? Gen Thorac Cardiovasc Surg. (2020) 68(7):703–6. doi: 10.1007/s11748-019-01219-y
11. Lu Y, Ma T, Wang L, Xue T. Advances in lymph node metastasis and lymph node dissection in early non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2019) 22(8):520–5. doi: 10.3779/j.issn.1009-3419·2019·08·07
12. Pan T, Zheng Z, Li J, Tang Y, Pan Y, Wei X, et al. Relationship between tumor size and lymph node metastasis in squamous cell carcinoma and adenocarcinoma of the lung. Zhongguo Fei Ai Za Zhi. (2006) 9(3):267–9. doi: 10.3779/j.issn.1009-3419·2006·03·12
13. Okada M, Yoshikawa K, Hatta T, Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. (2001) 71(3):956–60; discussion 961. doi: 10.1016/S0003-4975(00)02223-2
14. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. (2022) 399(10335):1607–17. doi: 10.1016/S0140-6736(21)02333-3
15. Stiles BM, Mao J, Harrison S, Lee B, Port JL, Sedrakyan A, et al. Extent of lymphadenectomy is associated with oncological efficacy of sublobar resection for lung cancer ≤2 cm. J Thorac Cardiovasc Surg. (2019) 157(6):2454–65. doi: 10.1016/j.jtcvs.2019·01·136
16. Bi L, Zhang H, Ge M, Lv Z, Deng Y, Rong T, et al. Intrapulmonary lymph node (stations 13 and 14) metastasis in peripheral non-small cell lung cancer. Medicine (Baltimore). (2021) 100(27):e26528. doi: 10.1097/MD.0000000000026528
17. Schmidt-Hansen M, Baldwin DR, Hasler E, Zamora J, Abraira V, Roqué I, et al. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev. (2014) 2014(11):CD009519. doi: 10.1002/14651858.CD009519.pub2
18. Concannon JP, Dalbow MH, Hodgson SE, Headings JJ, Markopoulos E, Mitchell J, et al. Prognostic value of preoperative carcinoembryonic antigen (CEA) plasma levels in patients with bronchogenic carcinoma. Cancer. (1978) 42(3 Suppl):1477–83. doi: 10.1002/1097-0142(197809)42:3+<1477::AID-CNCR2820420818>3.0.CO;2-E
19. Hanagiri T, Sugaya M, Takenaka M, Oka S, Baba T, Shigematsu Y, et al. Preoperative CYFRA 21-1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer. Lung Cancer. (2011) 74(1):112–7. doi: 10.1016/j.lungcan.2011·02·001
20. Hsu W-H, Huang C-S, Hsu H-S, Huang W-J, Lee H-C, Huang B-S, et al. Preoperative serum carcinoembryonic antigen level is a prognostic factor in women with early non-small-cell lung cancer. Ann Thorac Surg. (2007) 83(2):419–24. doi: 10.1016/j.athoracsur.2006.07.079
21. Kuo Y-S, Zheng M-Y, Huang M-F, Miao C-C, Yang L-H, Huang T-W, et al. Association of divergent carcinoembryonic antigen patterns and lung cancer progression. Sci Rep. (2020) 10(1):2066. doi: 10.1038/s41598-020-59031-1
22. Haruki T, Aokage K, Miyoshi T, Hishida T, Ishii G, Yoshida J, et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol. (2015) 10(6):930–6. doi: 10.1097/JTO.0000000000000546
23. Chen Z-Q, Huang L-S, Zhu B. Assessment of seven clinical tumor markers in diagnosis of non-small-cell lung cancer. Dis Markers. (2018) 2018:9845123. doi: 10.1155/2018/9845123
24. Ding H, Shi J, Zhou X, Xie D, Song X, Yang Y, et al. Value of CT characteristics in predicting invasiveness of adenocarcinoma presented as pulmonary ground-glass nodules. Thorac Cardiovasc Surg. (2017) 65(2):136–41. doi: 10.1055/s-0036-1587592
25. Lee SM, Park CM, Goo JM, Lee H-J, Wi JY, Kang CH. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology. (2013) 268(1):265–73. doi: 10.1148/radiol.13120949
26. Yanagawa M, Tsubamoto M, Satoh Y, Hata A, Miyata T, Yoshida Y, et al. Lung adenocarcinoma at CT with 0·25-mm section thickness and a 2048 matrix: high-spatial-resolution imaging for predicting invasiveness. Radiology. (2020) 297(2):462–71. doi: 10.1148/radiol.2020201911
27. Ma J, Yang Y-L, Wang Y, Zhang X-W, Gu X-S, Wang Z-C. Relationship between computed tomography morphology and prognosis of patients with stage I non-small cell lung cancer. Onco Targets Ther. (2017) 10:2249–56. doi: 10.2147/OTT.S114960
28. She Y, Zhao L, Dai C, Ren Y, Zha J, Xie H, et al. Preoperative nomogram for identifying invasive pulmonary adenocarcinoma in patients with pure ground-glass nodule: a multi-institutional study. Oncotarget. (2017) 8(10):17229–38. doi: 10.18632/oncotarget.11236
29. Ichinose J, Kawaguchi Y, Nakao M, Matsuura Y, Okumura S, Ninomiya H, et al. Utility of maximum CT value in predicting the invasiveness of pure ground-glass nodules. Clin Lung Cancer. (2020) 21(3):281–7. doi: 10.1016/j.cllc.2020·01·015
30. Koezuka S, Mikami T, Tochigi N, Sano A, Azuma Y, Makino T, et al. Toward improving prognosis prediction in patients undergoing small lung adenocarcinoma resection: radiological and pathological assessment of diversity and intratumor heterogeneity. Lung Cancer. (2019) 135:40–6. doi: 10.1016/j.lungcan.2019·06·023
Keywords: peripheral pulmonary nodules (PPNs), lymph node, sentinel node (SN), risk prediction models, pattern of lymph node metastasis
Citation: Ke L, Ma H, Zhang Q, Wang Y, Xia P, Yu L, Lv W and Hu J (2022) The pattern of lymph node metastasis in peripheral pulmonary nodules patients and risk prediction models. Front. Surg. 9:981313. doi: 10.3389/fsurg.2022.981313
Received: 29 June 2022; Accepted: 25 July 2022;
Published: 9 August 2022.
Edited by:
Mong-Wei Lin, National Taiwan University Hospital, TaiwanReviewed by:
Pei-Hsing Chen, National Taiwan University Hospital, Taiwan© 2022 Ke, Ma, Zhang, Wang, Xia, Yu, Lv and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Lv eHgwMDEzOUAxMjYuY29t Jian Hu ZHJfaHVqaWFuQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.