- 1Unit of Respiratory Diseases, “G. Mazzini” Hospital, Teramo, Italy
- 2Unit of Respiratory Diseases, “V. Fazzi” Hospital, Lecce, Italy
- 3Medical Oncology Unit, “Giuseppe Mazzini” Hospital, Teramo, Italy
- 4Radiotherapy Unit, “Giuseppe Mazzini” Hospital, Teramo, Italy
- 5Department of Life, Health and Environmental Sciences, University of L’Aquila, Thoracic Surgery Unit, “Giuseppe Mazzini” Hospital, Teramo, Italy
Several materials and techniques have been described for the procedure of chest wall reconstruction: the choice of using a technique or a material over another relies mainly on the surgeon's experience as well as thoracic defect localization and dimension, local availability of materials, and costs. From a technical point of view, autologous and alloplastic reconstruction are available, and, in both cases, rigid and non-rigid prostheses are found. Each material has its peculiarities, with advantages and disadvantages; thus, it is mandatory to be confident when planning the intervention to foresee possible complications and minimize them. We have reviewed the literature on chest wall reconstruction in chest wall tumors (both malignant and non malignant) with non-rigid prosthetic materials, focusing on safety outcomes.
Introduction
Reconstruction of the thoracic wall is indicated in case of cancers (either primary or metastatic), traumas, infections, and congenital defects: these conditions could result in functional and aesthetic impairments of the thoracic wall; thus, the aim of surgical operation is to protect intrathoracic structures, to preserve cardiac and respiratory functions, to avoid upper extremity instability, to receive radiotherapy when indicated, and to assure the best aesthetic result possible. In addition, in the case of cancer, the aim of surgery is to obtain the wider margins possible, free from malignant disease, or for palliative purposes (1, 2).
No strict indications are available about the size and the location of defects that need to be reconstructed nor about the material that has to be used; the reconstruction strategy is mainly up to the surgeon’s experience, to the local availability of materials, and to cost-effective analysis. Usually, defects smaller than 5 cm in any part of the thorax tend not to be reconstructed, as well as defects smaller than 10 cm and located in the posterior part of the thorax, because the protection and support given by the scapula do not alter thoracic and arm movements (3). All other chest wall defects need to be evaluated for surgical reconstruction, and part of the intervention plan consists in choosing the most suitable material for the chest wall reconstruction.
Methods for reconstruction of the thoracic wall could be categorized mainly into two groups: rigid and non-rigid prostheses. An ideal prosthetic material should be rigid enough to avoid chest paradoxical respiratory movements, should be inert and let the growth of fibrous tissue without the risk of infection, should be easily shaped during the operation, should be radiolucent to enable an anatomic reference for relapses detection during the follow-up, and should be not expensive (4).
The aim of this analysis is to review surgical outcomes of chest wall reconstruction, focusing on non-rigid materials in chest wall tumors in terms of mortality, quality of life, complications, and length of hospital stay.
Methods
A literature search was performed on PubMed using keywords “chest wall,” “reconstruction,” “semi-rigid,” “non-rigid,” “tumors,” “cancer,” “malignant,” “non malignant,” “benign,” and “diseases,” excluding case reports and studies with less than 10 patients.
Only articles in English were included and published from 2000 to this day.
Studies evaluating congenital defects, infections, and trauma as indications for chest wall reconstruction were excluded.
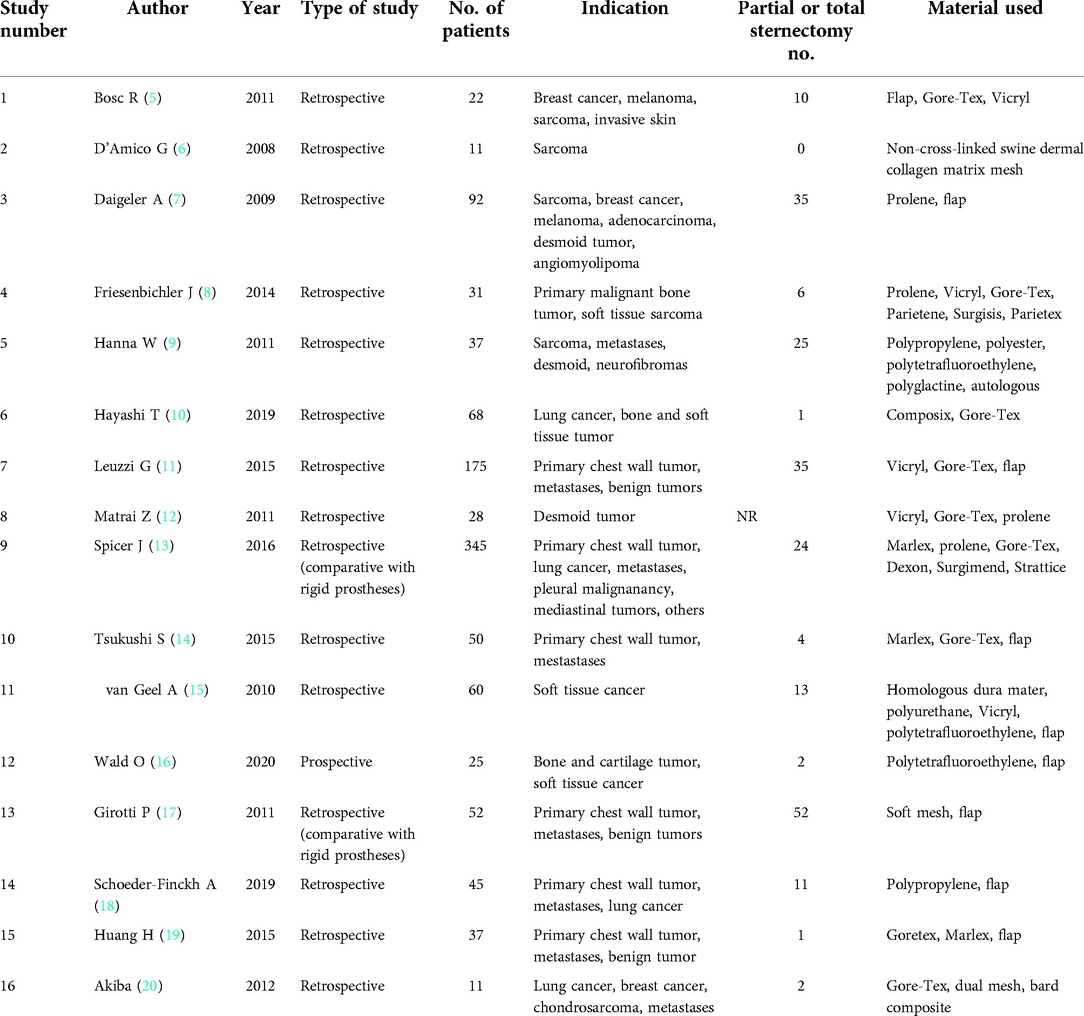
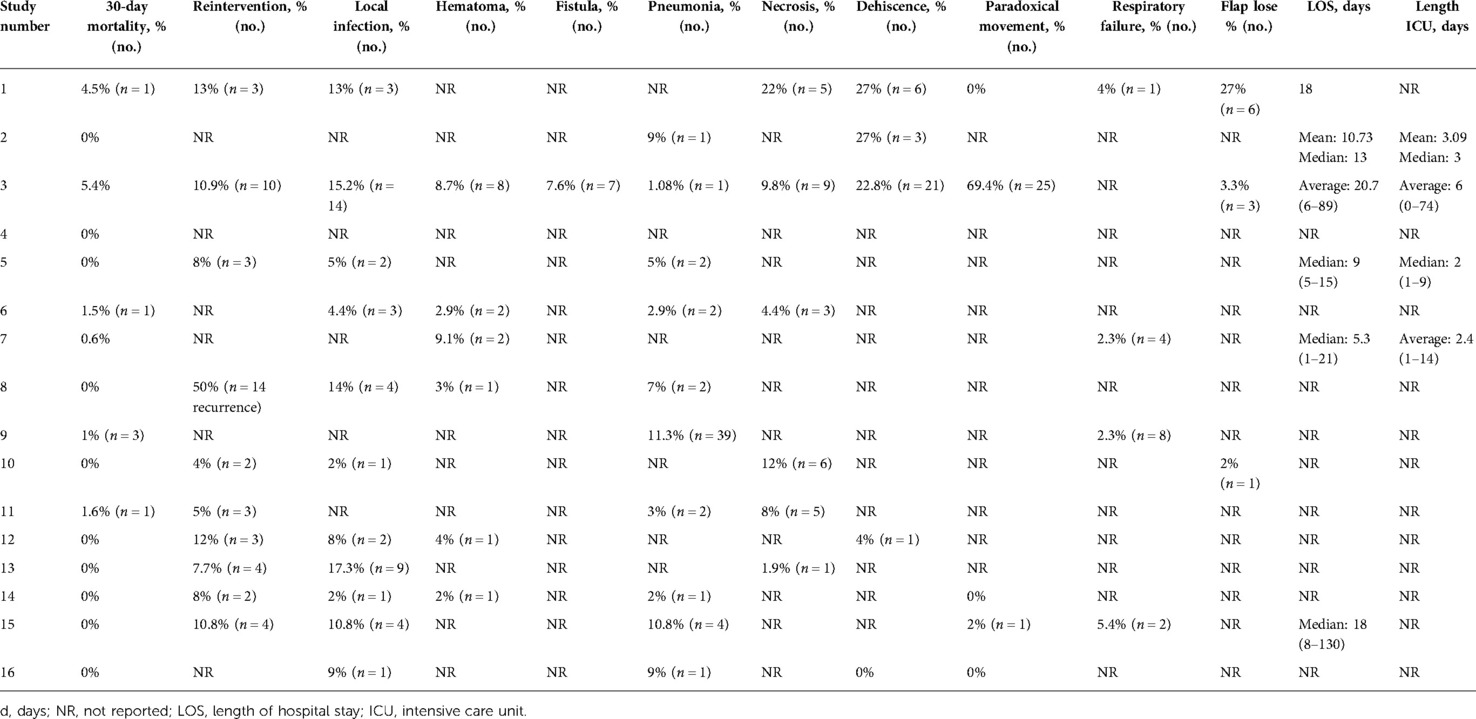
For each study, the following data were collected: type of the study, number of patients included, materials used for the reconstruction of the thoracic wall, if sternectomy was performed, 30-day mortality, need for reintervention, length of hospital stay, quality of life, and complications such as local infection, hematoma, fistula, pneumonia, necrosis, dehiscence of the surgical wound, paradoxical respiratory movement, and flap lose.
Results
After the first search, 1,343 records were found, and a second selection was made by the title, extracting 102 papers. Of these 102 papers, 16 were finally chosen for review (5–20). Studies evaluating the combination of rigid and semi-rigid prostheses were included only if a subgroup analysis for semi-rigid materials was done.
All but one were retrospective studies—one prospective noncomparative—; of these, two studies compared the outcomes of non-rigid prostheses with rigid ones retrospectively. The total amount of patients in the 16 studies was 1,089, ranging from 11 to 345. Indications for chest wall reconstruction were benign tumors (desmoid tumor, angiomyolipoma, and neurofibromas) and malignant tumors (sarcoma, lung cancer, metastases, breast cancer, melanoma, mediastinal tumors, and bone and cartilage tumors).
Materials used for the reconstruction of the thoracic wall were synthetic meshes (Gore-Tex, Vicryl, polypropylene, polytetrafluoroethylene, polyurethane, polyester, polyglycolic acid) in 16 studies and biologic meshes in 6 (acellular collagen matrix, homologous dura mater, swine dermal collagen matrix), associated or not with flaps. In 197 cases, patients also received a partial or total sternotomy.
Thirty-day mortality was reported in every study, ranging from 0% to 5.4% (average: 1.04%, 6 deaths/1,089).
A reintervention was necessary in 34 cases in the overall population due to complications related to the first operation, and in the study of Matrai et al. (12), 14 patients were reoperated because of recurrent disease.
Regarding complications, local infection was investigated in 11 studies, ranging from 2% to 17.3% (42 patients/1,089); hematoma was found in 6 studies, ranging from 2% to 9.1% (15 patients/1,089); fistula was reported only in the study of Daigeler et al. (7), being found in 7 out of 92 (7.6%) patients. Paradoxical movements of the chest wall were reported in 5 cases: in the studies by Daigeler et al. (7) and Huang et al. (19), 25 out of 92 (69.4%) cases and 1 out of 37 (2%) cases were reported; in the other 3 studies, the percentage of these complications was 0%. Pneumonia was considered in 10 studies, ranging from 1.08% to 11.3% (53 patients/1,089); necrosis in 6 studies, ranging from 1.9% to 22% (29 patients/1,089); dehiscence in 5 studies, ranging from 0% to 27% (31 patients/1,089); respiratory failure in 4 studies, ranging from 2.3% to 5.4% (15 patients/1,089); and flap lose in 3 studies, ranging from 2% to 27% (10 patients/1,089).
The only study investigating the quality of life and patients' satisfaction was of Daigeler et al. (7): the aesthetic appearance was considered sufficient or more by the majority of patients, whereas, compared to before the operation, the quality of life was described much better in the 24% of cases, slightly better in the 14%, unchanged in the 19%, slightly worse in the 35%, and much worse in the 8%.
Six studies reported the length of hospital stay, ranging from 1 to 130 days in the ward and from 1 to 74 days in the intensive care unit.
Tables 1 and 2 provide a detailed description of each study.
Discussion
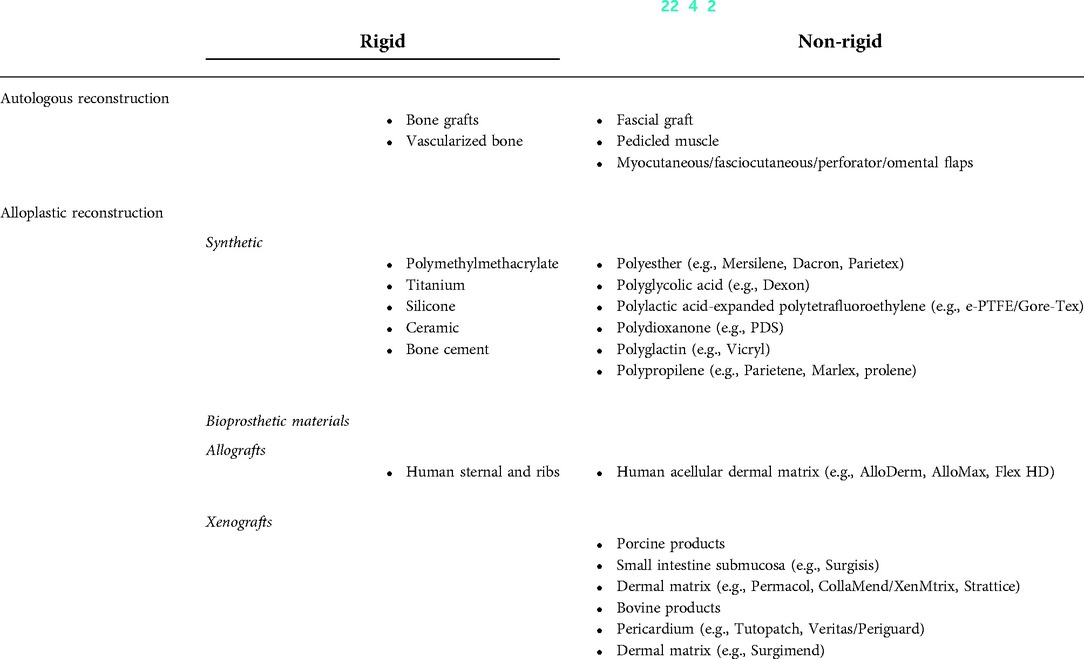
A number of prosthetic materials are available for chest wall reconstruction and could be grouped mainly into two categories, rigid and non-rigid materials.
Rigid prostheses, such as methyl methacrylate, silicone, and titanium, have the advantage of ensuring chest wall stability, but on the other hand, rupture, displacement, infection, seroma, and disorders of physiologic respiratory movements were reported (4).
Non-rigid prostheses are meshes and patches, either synthetic or biologic: among synthetic ones are polypropylene, polytetrafluoroethylene, and Vicryl, and among biologic meshes are human bioprosthetic material and bovine pericardium. Advantages of using these consist of the easy manipulation of the material that can be stretched uniformly and sutured easily, providing a scaffold for connective tissue in-growth without significant foreign-body reaction; this is especially true for biologic meshes that allow regeneration stimulating regrowth and revascularization with a lower risk of infection compared to other techniques. Disadvantages are minor protection of intrathoracic organs compared to rigid prostheses since the strength could not be enough to protect them, the cosmetic results, and the higher costs in the case of biologic meshes (21, 4).
Table 3 summarizes rigid and non-rigid prosthetic materials (22, 2, 4).
According to the results of our review, non-rigid materials for reconstruction of the thoracic wall in tumors have a good safety profile: despite not every study specifically reporting complications such as local infection or necrosis, the mortality rate and the overall risk of complications are always acceptable, also considering the underlying diseases. Furthermore, a common limitation of the reviewed studies is that they rarely report paradoxical respiratory movement, the patient's quality of life, and the patient's satisfaction. We think that these parameters are important outcomes in this kind of surgical procedure since the goals of chest wall reconstruction—apart from the cancer curative intent—are to preserve the cardiac and respiratory function, provide a satisfactory cosmetic result, and, as a direct consequence, improve patients' quality of life.
Interestingly, some studies have compared in a non-randomized way the outcomes of rigid and non-rigid materials. Spicer et al. (13) reviewed 427 patients that underwent chest wall reconstruction, 82 with rigid prostheses and 345 with non-rigid prostheses. No significant difference was found between the two groups in terms of complications and mortality. Complications seem to be related to the extent of resection (number of resected ribs, lobectomy, and pneumonectomy) rather than to the surgical technique used. Similar results were reported by Weyant et al. (23): median length of hospital stay, mortality, and complications did not differ between the rigid and non-rigid prosthesis groups, but a significant clinical difference was found in patients with larger chest wall defects. Kilic et al. (24) compared methylmethacrylate “sandwich” with polytetrafluoroethylene in 59 consecutive patients with chest wall defects larger than 5 cm and located in the anterior part of the thorax: 21 underwent reconstruction with polytetrafluoroethylene and 38 with methylmethacrylate “sandwich.” No significant differences were found in mortality, but paradoxical respiratory movement was higher in the polytetrafluoroethylene group as well as the mean length of hospital stay was longer.
A comparison between different non-rigid materials was performed in a few studies. Huang et al. (19) retrospectively compared patients that underwent chest wall reconstruction with Gore-Tex (n = 18), with autologous flaps (n = 14), or other meshes (Marlex, Medifit) (n = 5). They found a higher chest drainage time in the Gore-Tex group and one empyema in the Marlex group, and no differences were found in the shrinkage of the materials among the three groups. Pneumonia was described in two patients treated with Gore-Tex and in one patient with autologous flap and mesh. Regarding paradoxical movement, one case in the Gore-Tex group was reported. Two cases of infections were found in the flap and in the Gore-Tex group.
Other similar pieces of evidence come out from the study by Hanna et al. (9): postoperative outcomes of non-rigid meshes and autologous flaps were similar between those with a chest wall defect <60 cm2 and those with a defect >60 cm2; in the subgroup analysis of the large-defect cohort, the incidence of admission in intensive care unit was higher in the mesh group; conversely the rate of reoperation and local infection was higher in the mesh group, but none of the above-mentioned parameteres reached statistical significance.
The study from Girotti and colleagues (17) described specifically the use of semi-rigid material after sternectomy: the mortality was 0%, but the rate of infection was higher reported among the selected studies. It was not specified which type of rigid material was used, although data from the literature suggested that Gore-Tex dual mesh performs well in sternal reconstruction (20).
Thus, given the paucity and the design of the studies on the topic, no strong conclusions could be drawn about the best material to be used.
Moreover, it has to be underlined that in the majority of reports a combination of non-rigid and rigid prostheses is reported, with the rationale of exploiting the different features of materials, especially in the case of reconstruction of large chest wall defects. One of the most common composites is the combination of polymethylmethacrylate in two layers of the propylene mesh, placed as a “sandwich” (21), but several materials could be mixed, such as rigid+non-rigid synthetic prostheses, autologous + synthetic prostheses, xenograft + synthetic prostheses, and so on. The report by Heo et al. (25) is an example of how rigid and non-rigid synthetic prosthetic meshes along with xenografts could be combined at the same time: in six patients, after the removal of the chest wall tumor, the visceral pleura was repaired with Gore-Tex; the first layer of the acellular dermal matrix was placed on Gore-Tex, then bone cement was used as a substitute for ribs, and finally the second layer of the acellular dermal matrix was sutured with the first one, preventing the contact of the cement with the soft tissue. No major complications occurred, and no perioperative mortality was reported.
This strengthens what was stated before about the choice of the prosthetic material, which has to be related to the patient's chest wall defect in terms of the dimension and location in the thorax and to the experience and preference of the operator, rather than on a proven superiority of a technique.
Finally, soft tissue replacement is part of the procedure of chest wall reconstruction, but it is seldom used alone as a technique of reconstruction because of the lack of rigidity, especially in large defects: it consists of transporting/transplanting muscles or part of the greater omentum along with the skin for the closure of thoracic defects. Muscles commonly used are the pectoralis major, latissimus dorsi, and rectus abdominis muscles. Also, omentoplasty has been used due to its good vascularization pattern and immunological property; it also requires a skin graft as coverage (26).
Conclusions
In conclusion, to date, no “gold standard” procedure is recommended for chest wall reconstruction. The surgical procedure should be tailored to the patient's clinical status, the underlying disease, and the dimension and the location of the chest wall defect, as well the use of prosthetic materials should be decided on the basis of local availability, cost, and, more importantly, the surgical experience of the operator. Non-rigid prostheses, used alone or in combination with other materials, offer good outcomes from either a clinical or a safety point of view. Further studies are necessary to investigate the patients' quality of life and patient's satisfaction with aesthetic results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Author contributions
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.976463/full#supplementary-material.
References
1. Isaac KV, Elzinga K, Buchel EW. The best of chest wall reconstruction: principles and clinical application for complex oncologic and sternal defects. Plast Reconstr Surg. (2022) 149(3):547e–62e. doi: 10.1097/PRS.0000000000008882
2. Sanna S, Brandolini J, Pardolesi A, Argnani D, Mengozzi M, Dell'Amore A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg. (2017) 3:95. doi: 10.21037/jovs.2017.06.10
3. Deschamps C, Tirnaksiz BM, Darbandi R, Trastek VF, Allen MS, Miller DL, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg. (1999) 117(3 ):588–96. doi: 10.1016/S0022-5223(99)70339-9
4. Thomas PA, Brouchet L. Prosthetic reconstruction of the chest wall. Thorac Surg Clin. (2010) 20(4):551–8. doi: 10.1016/j.thorsurg.2010.06.006.20974439
5. Bosc R, Lepage C, Hamou C, Matar N, Benjoar MD, Hivelin M, et al. Management of chest wall reconstruction after resection for cancer: a retrospective study of 22 consecutive patients. Ann Plast Surg. (2011) 67(3):263–8. doi: 10.1097/SAP.0b013e3181f9b292
6. D'Amico G, Manfredi R, Nita G, Poletti P, Milesi L, Livraghi L, et al. Reconstruction of the thoracic wall with biologic mesh after resection for chest wall tumors: a presentation of a case series and original technique. Surg Innov. (2018) 25(1):28–36. doi: 10.1177/1553350617745954
7. Daigeler A, Druecke D, Hakimi M, Duchna HW, Goertz O, Homann HH, et al. Reconstruction of the thoracic wall-long-term follow-up including pulmonary function tests. Langenbecks Arch Surg. (2009) 394(4):705–15. doi: 10.1007/s00423-008-0400-9
8. Friesenbichler J, Leithner A, Maurer-Ertl W, Szkandera J, Sadoghi P, Frings A, et al. Surgical therapy of primary malignant bone tumours and soft tissue sarcomas of the chest wall: a two-institutional experience. Int Orthop. (2014) 38(6):1235–40. doi: 10.1007/s00264-014-2304-3
9. Hanna WC, Ferri LE, McKendy KM, Turcotte R, Sirois C, Mulder DS. Reconstruction after major chest wall resection: can rigid fixation be avoided? Surgery. (2011) 150(4):590–7. doi: 10.1016/j.surg.2011.07.055
10. Hayashi T, Sakakura N, Ishimura D, Kozawa E, Yoshida M, Sakao Y, et al. Surgical complication and postoperative pulmonary function in patients undergoing tumor surgery with thoracic wall resection. Oncol Lett. (2019) 17(3):3446–56. doi: 10.3892/ol.2019.9997
11. Leuzzi G, Nachira D, Cesario A, Novellis P, Petracca Ciavarella L, Lococo F, et al. Chest wall tumors and prosthetic reconstruction: a comparative analysis on functional outcome. Thorac Cancer. (2015) 6(3):247–54. doi: 10.1111/1759-7714.12172
12. Mátrai Z, Tóth L, Szentirmay Z, Papp J, Antal I, Vadász P, et al. Mellkasfali és intrathoracalis desmoid tumorok sebészi kezelésének kihívásai [surgical challenges of chest wall and intra-thoracic desmoid tumors]. Orv Hetil. (2011) 152(1):3–13. Hungarian. doi: 10.1556/OH.2011.29009
13. Spicer JD, Shewale JB, Antonoff MB, Correa AM, Hofstetter WB, Rice DC, et al. The influence of reconstructive technique on perioperative pulmonary and infectious outcomes following chest wall resection. Ann Thorac Surg. (2016) 102(5):1653–9. doi: 10.1016/j.athoracsur.2016.05.072
14. Tsukushi S, Nishida Y, Sugiura H, Yamada Y, Kamei Y, Toriyama K, et al. Non-rigid reconstruction of chest wall defects after resection of musculoskeletal tumors. Surg Today. (2015) 45(2):150–5. doi: 10.1007/s00595-014-0871-y
15. van Geel AN, Wouters MW, Lans TE, Schmitz PI, Verhoef C. Chest wall resection for adult soft tissue sarcomas and chondrosarcomas: analysis of prognostic factors. World J Surg. (2011) 35(1):63–9. doi: 10.1007/s00268-010-0804-x
16. Wald O, Islam I, Amit K, Ehud R, Eldad E, Omer O, et al. 11-Year experience with chest wall resection and reconstruction for primary chest wall sarcomas. J Cardiothorac Surg. (2020) 15(1):29. doi: 10.1186/s13019-020-1064-y
17. Girotti P, Leo F, Bravi F, Tavecchio L, Spano A, Cortinovis U, et al. The “rib-like” technique for surgical treatment of sternal tumors: lessons learned from 101 consecutive cases. Ann Thorac Surg. (2011) 92(4):1208–15. doi: 10.1016/j.athoracsur.2011.05.016
18. Schroeder-Finckh A, Lopez-Pastorini A, Galetin T, Defosse J, Stoelben E, Koryllos A. Anterior chest wall reconstruction using polypropylene mesh: a retrospective study. Thorac Cardiovasc Surg. (2020) 68(4):341–51. doi: 10.1055/s-0039-1694033
19. Huang H, Kitano K, Nagayama K, Nitadori J, Anraku M, Murakawa T, et al. Results of bony chest wall reconstruction with expanded polytetrafluoroethylene soft tissue patch. Ann Thorac Cardiovasc Surg. (2015) 21(2):119–24. doi: 10.5761/atcs.oa.14-00195
20. Akiba T, Marushima H, Nogi H, Kamiya N, Kinoshita S, Takeyama H, et al. Chest wall reconstruction using Gore-Tex® dual mesh. Ann Thorac Cardiovasc Surg. (2012) 18(2):166–9. doi: 10.5761/atcs.cr.11.01718
21. Divisi D, Tosi D, Zaccagna G, De Vico A, Diotti C, Crisci R. Case report: a new tool for anterior chest wall reconstruction after sternal resection for primary or secondary tumors. Front Surg. (2021) 8:691945. doi: 10.3389/fsurg.2021.691945
22. Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg. (2011) 25(1):34–42. doi: 10.1055/s-0031-1275169.22294941
23. Weyant MJ, Bains MS, Venkatraman E, Downey RJ, Park BJ, Flores RM, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg. (2006) 81(1):279–85. doi: 10.1016/j.athoracsur.2005.07.001
24. Kilic D, Gungor A, Kavukcu S, Okten I, Ozdemir N, Akal M, et al. Comparison of mersilene mesh-methyl metacrylate sandwich and polytetrafluoroethylene grafts for chest wall reconstruction. J Invest Surg. (2006) 19(6):353–60. doi: 10.1080/08941930600985694
25. Heo CY, Kang B, Jeong JH, Kim K, Myung Y. Acellular dermal matrix and bone cement sandwich technique for chest wall reconstruction. Arch Plast Surg. (2022) 49(1):25–8. doi: 10.5999/aps.2021.01067
Keywords: chest wall tumors, resection, reconstruction, mesh, myoplastic
Citation: Colella S, Brandimarte A, Marra R, Marinari S, D’Incecco A, Di Genesio Pagliuca M, De Vico A, Crisci R and Divisi D (2022) Chest wall reconstruction in benign and malignant tumors with non-rigid materials: An overview. Front. Surg. 9:976463. doi: 10.3389/fsurg.2022.976463
Received: 23 June 2022; Accepted: 11 July 2022;
Published: 3 August 2022.
Edited by:
Federico Raveglia, ASST-Monza, ItalyReviewed by:
Riccardo Orlandi, University of Milan, ItalyFabrizio Minervini, University of Lucerne, Switzerland
Ugo Cioffi, University of Milan, Italy
© 2022 Colella, Brandimarte, Marra, Marinari, D'Incecco, Di Genesio Pagliuca, De Vico, Crisci and Divisi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duilio Divisi ZHVpbGlvLmRpdmlzaUBhc2x0ZXJhbW8uaXQ=; ZHVpbGlvLmRpdmlzaUB1bml2YXEuaXQ=
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Sara Colella
Sara Colella Alessandro Brandimarte1
Alessandro Brandimarte1 Milena Di Genesio Pagliuca
Milena Di Genesio Pagliuca Duilio Divisi
Duilio Divisi