- Department of Cardiothoracic Surgery, The Third Affiliated Hospital of Soochow University, Changzhou, China
Background: As the population ages, there will be an increasing number of octogenarian patients with non-small cell lung cancer (NSCLC). In carefully selected elderly patients, surgery can improve long-term survival. To identify candidates who would benefit from surgery, we performed this study and built a predictive model.
Materials and methods: Data from NSCLC patients over 80 years old were obtained from the Surveillance, Epidemiology and End Results database. A 1:1 propensity score matching was performed to balance the clinicopathological features between the surgery and non-surgery groups. Kaplan-Meier analyses and log-rank tests were used to assess the significance of surgery to outcome, and Cox proportional-hazards regression and competing risk model were conducted to determine the independent prognostic factors for these patients. A nomogram was built using multivariable logistic analyses to predict candidates for surgery based on preoperative factors.
Results: The final study population of 31,462 patients were divided into surgery and non-surgery groups. The median cancer-specific survival time respectively was 53 vs. 13 months. The patients’ age, sex, race, Tumor, Node, Metastasis score, stage, chemotherapy use, tumor histology and nuclear grade were independent prognostic factors. Apart from race and chemotherapy, other variates were included in the predictive model to distinguish the optimal surgical octogenarian candidates with NSCLC. Internal and external validation confirmed the efficacy of this model.
Conclusion: Surgery improved the survival time of octogenarian NSCLC patients. A novel nomogram was built to help clinicians make the decision to perform surgery on elderly patients with NSCLC.
Introduction
Lung cancer is the leading cause of death in the global cancer population (1, 2). In 2020, lung cancer was the second most common cancer and the leading cause of cancer-related deaths worldwide (2). Non-small cell lung cancer (NSCLC) accounts for about 80% of lung cancers. The average age of patients diagnosed with lung cancer is about 70 years old, and the incidence of lung cancer increases with age (1). In Europe, patients younger than 50 years old account for about 6% of lung cancer patients, while 44% of patients diagnosed with lung cancer are over 70 years old (3). In 2018, there were 2.3 million new cancer cases diagnosed in patients over 80 years old worldwide, accounting for 13.3% of all new global cancer cases (4). With the increase in the elderly population base, the number of new cancer cases rises annually. An estimated 6.9 million new cancer cases are expected to be diagnosed in patients over 80 years old worldwide, representing 21.5% of global cases by 2050 (4). Thoracic surgeons will soon face an increasing number of elderly NSCLC patients eligible for surgical treatment.
The management of elderly NSCLC patients is complicated because of age-related physiological changes, comorbidities, frailty, and shorter relative life expectancies (5). At present, it is lack of evidence for elderly patients with NSCLC to guide appropriate treatment decisions. A higher operative mortality rate has been observed in octogenarians compared with patients 60 to 79 years old (6), however, surgical resection of tumors is justified and is an effective treatment for octogenarian and older patients with early-stage NSCLC (7, 8). Nevertheless, several studies have focused on the differences in outcome between octogenarian patients who have undergone surgery and those who have not. Ganti et al. demonstrated in a cohort of 1,338 elderly NSCLC patients that surgery is an independent factor in stage I and stage II NSCLC octogenarians (9). Surgical resection is also the primary treatment choice for a subset of elderly patients with advanced NSCLC (10). Since patients greater than 80 years old are seldom included in randomized clinical trials, prospective studies that are relevant to elderly NSCLC patients are lacking. However, retrospective analyses suggest that surgery should not be abandoned according to age alone in octogenarians with NSCLC (3, 9, 11). In addition, age is not completely representative of an individual's physiological or functional status and should not be used as the sole determining factor for clinical decision-making (12, 13). In the clinic, the choice of surgery for octogenarians depends more on the experience of clinicians from large medical centers, and is therefore not supported by scientific data.
In this study, we attempt to build a visual nomogram to distinguish the optimal surgical candidates among NSCLC patients 80 years old and over, which provides a reliable method for clinicians to make the decision whether to recommend surgery or not.
Materials and methods
Study population
The Surveillance, Epidemiology and End Results (SEER) database was used, and the study population was primarily NSCLC patients at least 80 years old between 2014 and 2018. These data were collected using the SEER*Stat Software (version 8.3.9). The matched variables including patient ID, gender, age at diagnosis, race, tumor site, histology, nuclear grade, tumor size, Tumor, Node, Metastasis (TNM) stage, surgical method, radiotherapy, chemotherapy, overall survival (OS), cancer-specific survival (CSS), and survival months, were acquired from this database. The TNM stage of patients was reclassified according to the American Joint Committee on Cancer eighth edition. All data were from the SEER database and anonymized. It is unnecessary to obtain the ethical approval and consent for participants.
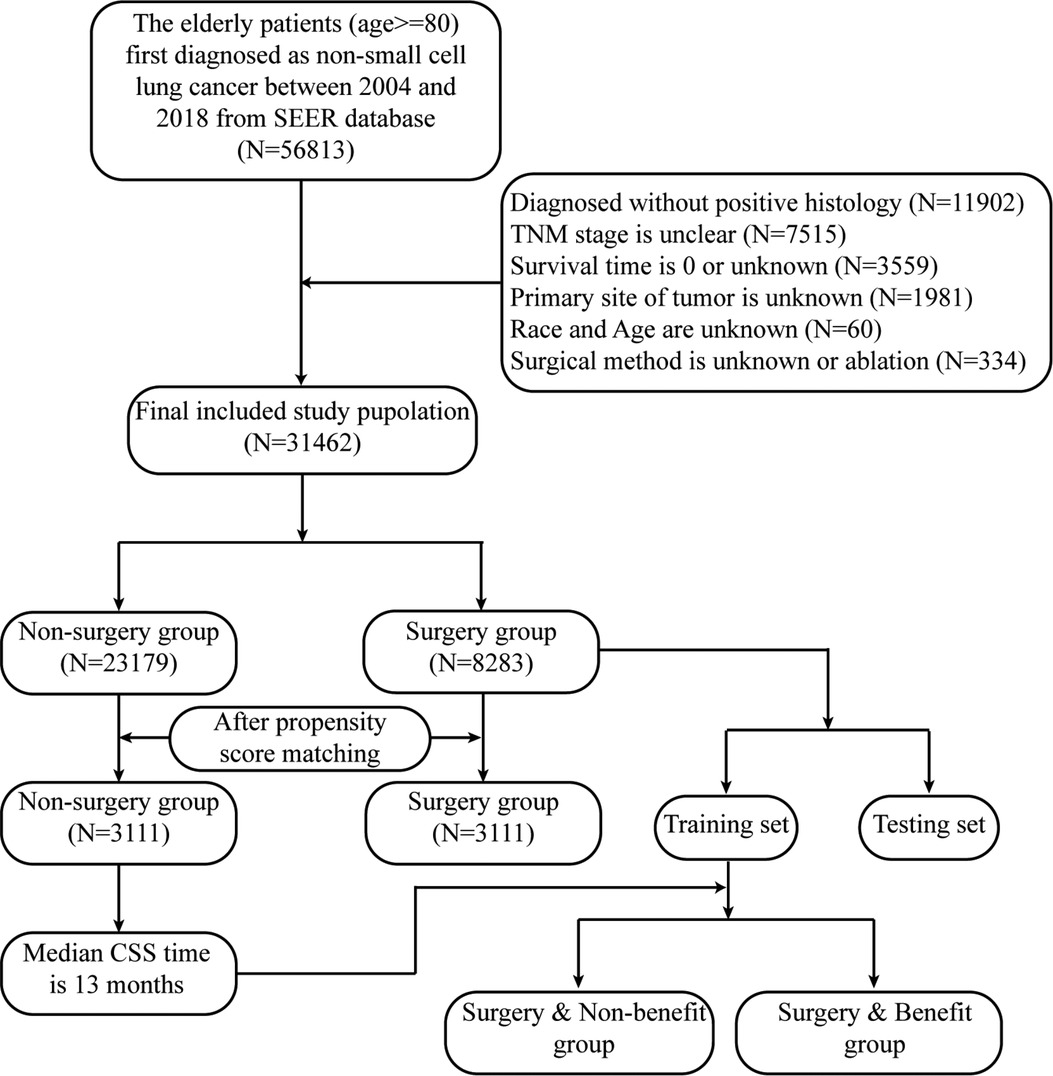
Based on the following exclusion and inclusion criteria, the final study population was confirmed. The inclusion criteria were as follows: initial diagnosis with primary NSCLC occurring between 2014 and 2018, at least 80 years old, a surgical code of 0, 21–22, and 30–70. The exclusion criteria were as follows: unknown race, age, and tumor site, unclear TNM stage and histology, unknown or null survival time. The detailed flowchart is shown in Figure 1.
Statistical analyses
A Pearson's chi-squared and Student's t-test were used to compare baseline data and clinical characteristics of patients between the surgery and non-surgery groups, respectively. The Kaplan-Meier method and log-rank test were used to confirm the surgical benefit for the NSCLC patients aged 80 years old and over. A 1:1 matched propensity score matching (PSM) was conducted to balance the variables between the surgery and non-surgery groups using the “MatchIt” R package. Variables including patient age, race, sex, tumor site, nuclear grade, histology type, T stage, N stage, M stage, radiotherapy, and chemotherapy were included in the logistical model to calculate the propensity score with a caliper of 0.01. The univariate and multivariate Cox proportional-hazards regression and competing risk model were performed to identify the independent factors related to the prognosis. R software (version 4.1.2) was used to conduct the statistical analyses with two-sided testing. A P < 0.05 was considered statistically significant.
Nomogram construction and validation
Compared with patients in the non-surgery group, patients undergoing surgery had a longer OS and CSS time. The median CSS of patients in the surgery group was 53 months, while one patient in the non-surgery group had a CSS of 13 months. We assumed that patients who underwent surgery and who had longer CSS times than patients who did not undergo surgery could benefit from a primary tumor resection. Based on this, patients undergoing surgery were randomly divided into a training and testing dataset using a ratio of 7:3.
The “rms” R package and R software (version 4.1.2) were used to complete the construction of the nomogram. Univariate and multivariable logistic analyses were performed to select the final variables included in the predictive model among the variables that had been identified as independent factors for prognosis. The nomogram was built using the multivariable logistic regression analyses based on variables including age, sex, histology type, nuclear grade, T stage, N stage, and M stage.
To test the discriminatory ability and accuracy of this nomogram, internal and external validations were conducted. The ROC curve, calibration plots, and decision curve analysis (DCA) plots were utilized in training and testing datasets, respectively. The predictive model was tested in the population after PSM. The Kaplan-Meier curve was drawn to compare the CSS time of the surgery with benefit group, the surgery without benefit group, and the non-surgery group.
Results
Study population pre- and post-PSM
Based on the exclusion and inclusion criteria, the final study population from SEER database included 31,462 patients at least 80 years old and diagnosed with a primary NSCLC. Only 8,283 (26.3%) of octogenarians received surgical treatment. Among them, more than half of patients had a TNM I stage with adenocarcinoma. Surgery was more likely in the white and female population. Patients who did not receive surgical treatment had more access to radiotherapy and chemotherapy. The number of octogenarians with NSCLC have gradually increased in the last decade. However, patients receiving surgery made up a relatively small proportion of this group (Supplementary Figure A1). After PSM, 6,222 patients were included into the matched population and the variables between the surgery and non-surgery groups were balanced (Table 1).
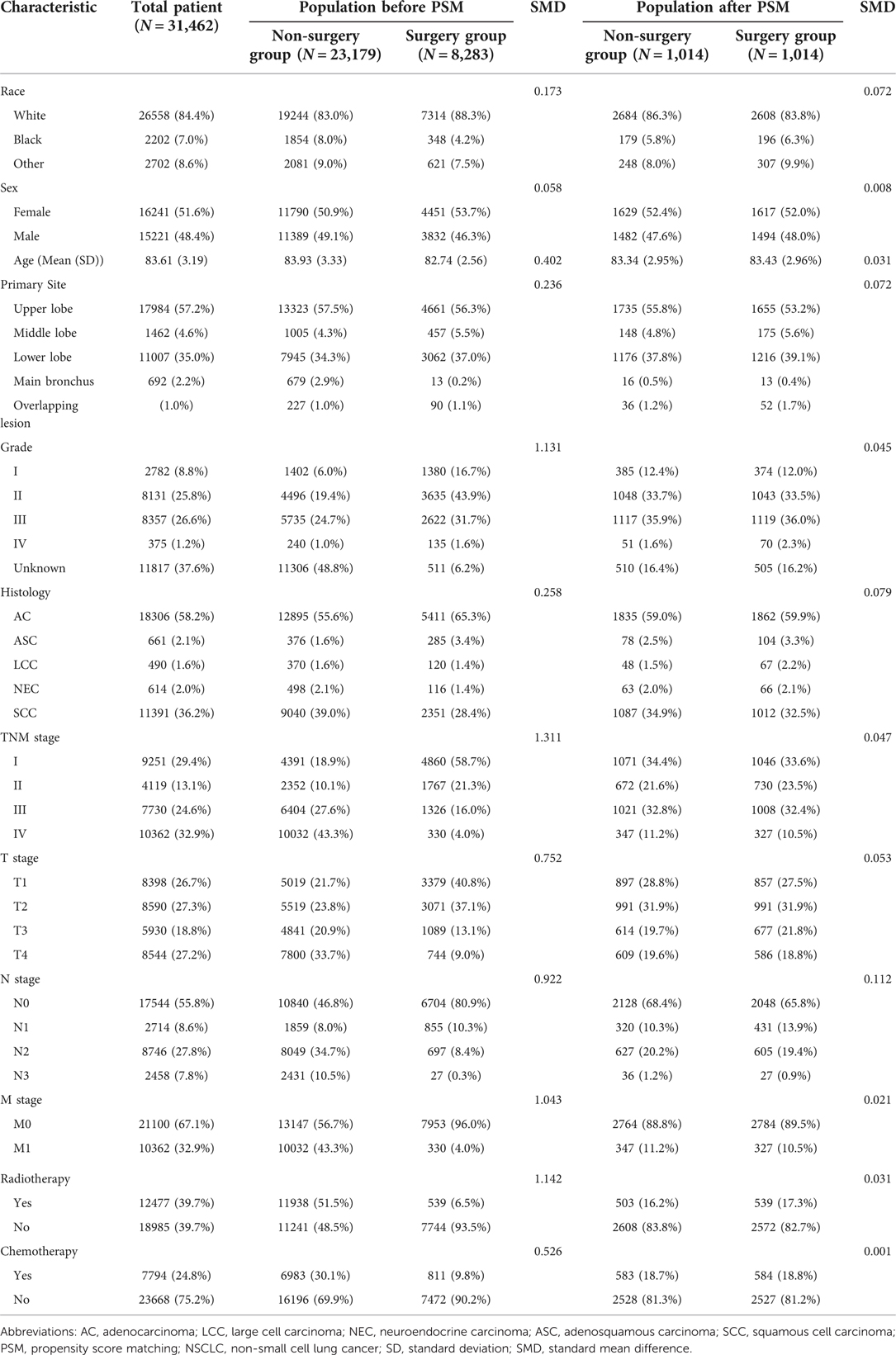
Table 1. The clinicopathologic baselines of patients between two groups in elderly patients with NSCLC before and after propensity score matching.
Surgical benefit for the NSCLC octogenarian group
As shown in the survival curve, patients who received surgical treatment had longer OS and CSS than patients who did not receive surgical treatment (Figures 2A,B). After PSM, the results of the survival analyses were largely unchanged (Figures 2C,D). The median OS and CSS of the surgery and non-surgery groups were 34 vs. 10 months, and 53 vs. 13 months, respectively. Moreover, the 1-year, 3-year, and 5-year OS and CSS rates are shown in Supplementary Table A1. In the Cumulative Incidence Function, the incidence rate of death from other causes was higher in the surgery group compared with the non-surgery group. However, early death induced by other causes was not significantly different between the surgery and non-surgery groups (Supplementary Figures A2A,B).
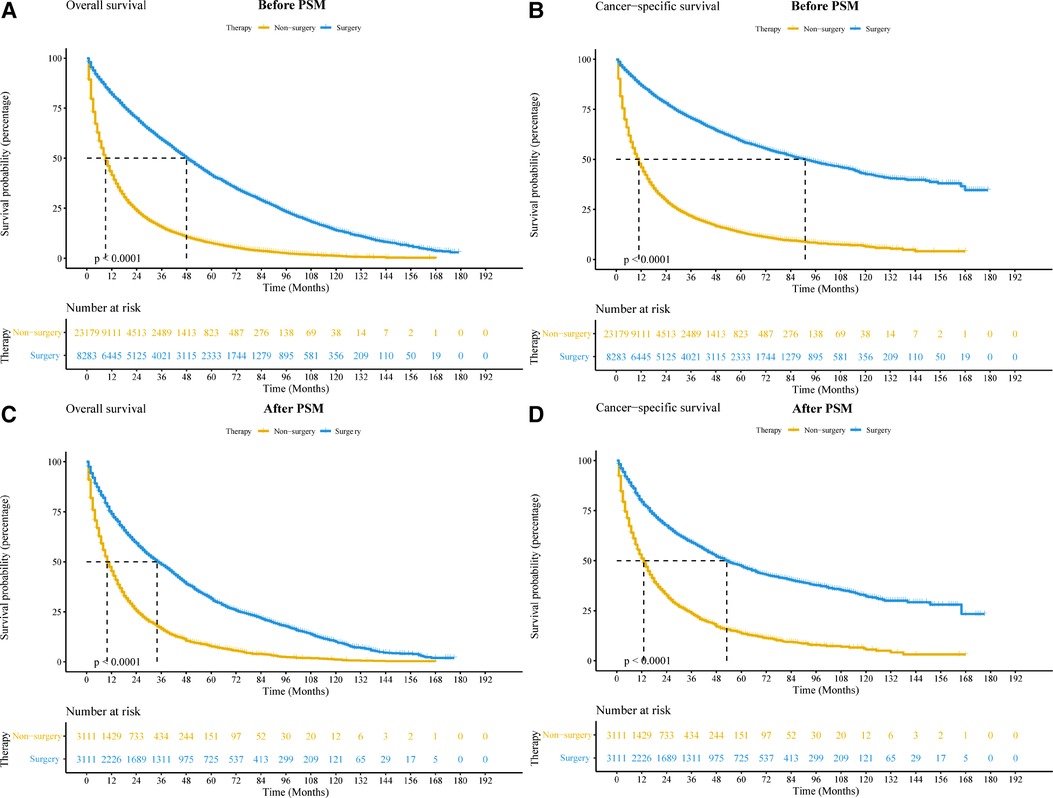
Figure 2. Kaplan-Meier curves of elderly patients with non-small cell lung cancer between surgery and non-surgery groups before and after PSM. (A,B) Kaplan-Meier curve comparing OS (A) and CSS (B) before PSM. (C,D): Kaplan-Meier curve comparing OS (C) and CSS (D) after PSM. Abbreviations: CSS, cancer-special survival; OS, overall survival; PSM, propensity score matching.
Identification of independent prognostic factors of OS and CSS
A univariate and multivariate Cox proportional-hazards regression and competing risk model were used to identify independent prognostic factors for OS and CSS. The results showed that surgery significantly improved the prognosis of elderly NSCLC patients (OS: HR = 0.37, 95% CI: 0.35–0.39, P < 0.001; CSS: HR = 0.40, 95% CI: 0.38–0.43, P < 0.001). Age, race, sex, histology type, nuclear grade, T stage, N stage, M stage, and chemotherapy were also independent prognostic factors (Table 2). Although radiotherapy was not a prognostic factor in the multivariate Cox and competing risk model, it was still included in the multivariate analyses to take into account the widespread usage of radiotherapy in elderly NSCLC patients. Radiotherapy could be an independent factor for overall survival. Interestingly, chemotherapy was a protective factor in the multivariate competing risk model, but an unfavorable factor in the univariate competing risk model. In the subgroup analysis, surgery may not improve long-term survival in patients with main bronchus tumor (OS: HR = 0.42, 95% CI: 0.17–1.01, P = 0.051; CSS: HR = 0.60, 95% CI: 0.29–1.23, P = 0.163). Likely, octogenarians who received radiotherapy did not have a longer cancer-specific survival undergoing surgery (CSS: HR = 0.94, 95% CI: 0.82–1.08, P = 0.388). In addition to these, surgery could improve long-term survival in other subgroups (Supplementary Figures A3, A4).
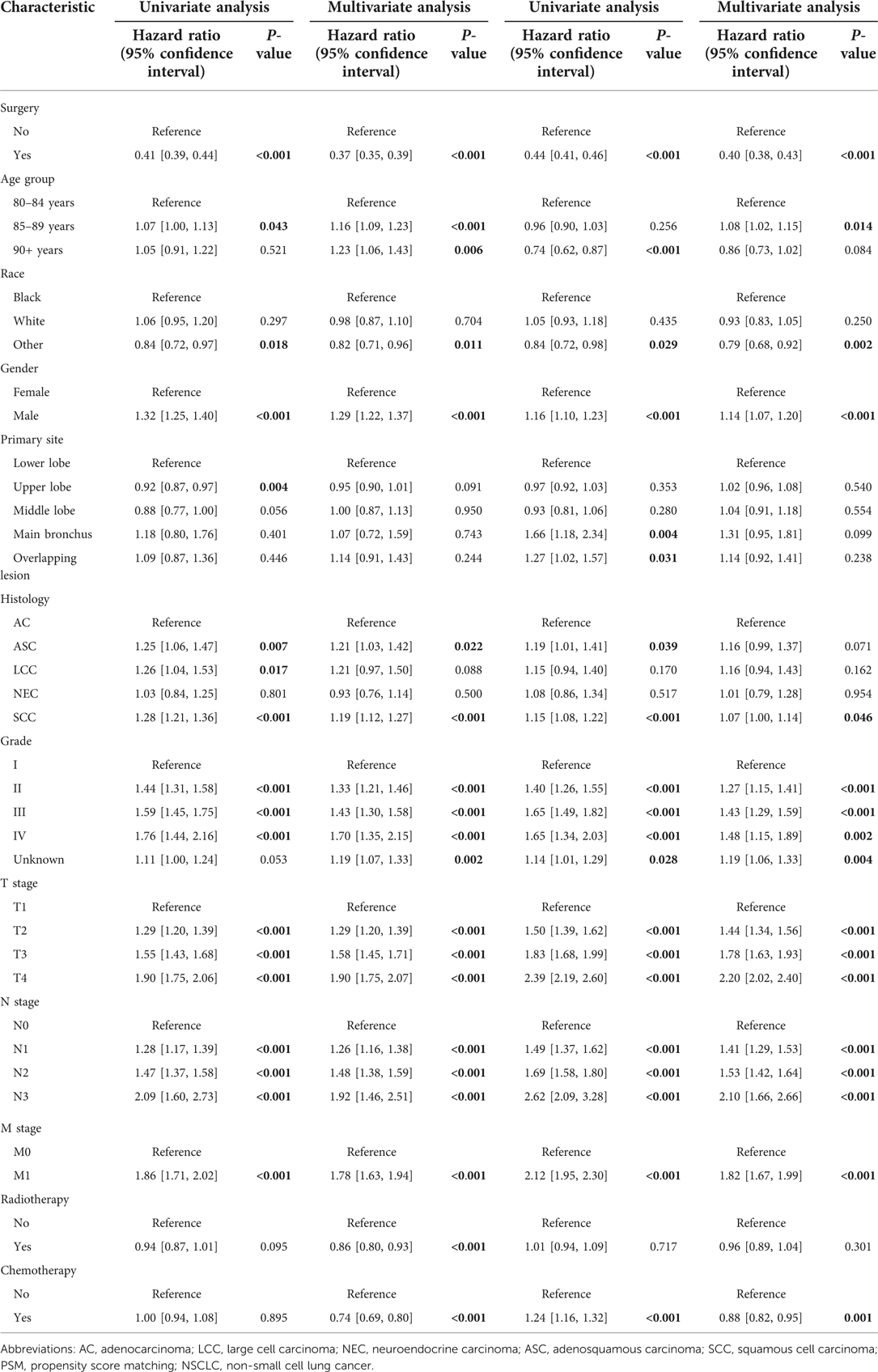
Table 2. Univariate and multivariate Cox proportional Hazard analyses and competing risk model for the overall survival and cancer-specific survival in patients with NSCLC after propensity score matching.
Building the nomogram to identify surgical candidates
We divided the patients in the surgery group into two groups based on their CSS time: surgery with benefit (CSS time >13 months) and surgery without benefit (CSS time ≤13 months). Patients who were alive or died of other cause but did not have more than 13 months of follow-up were excluded. In the end, 7,303 patients were included in the dataset, and 6,216 patients were included in the surgery with benefit group. The whole dataset population was randomly divided into a training and testing dataset using a ratio of 7:3. In the training dataset, univariate and multivariate logistic regression analyses were conducted to identify risk factors of surgical benefit. Variables including age, sex, histology type, nuclear grade, and TNM stage were included in this logistic model. Based on the logistic model, a novel nomogram was drawn using the “rms” R package (Figure 3). In this nomogram, every variable corresponds to a score point.
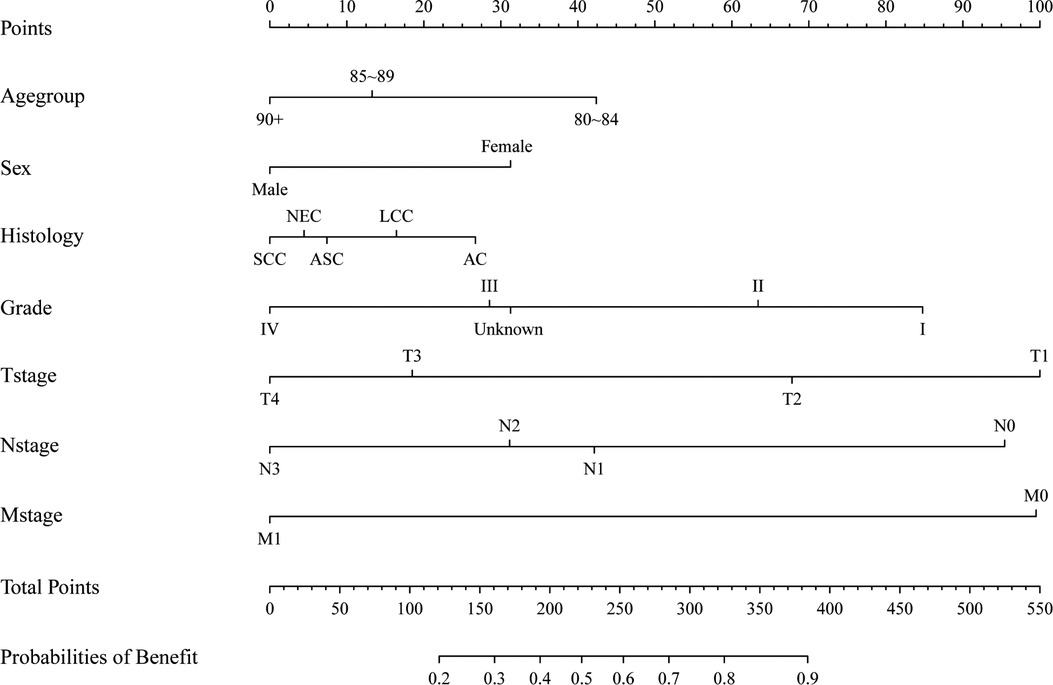
Figure 3. A nomogram to determining surgical beneficial population in elderly patients with non-small cell lung cancer.
Internal and external nomogram validation
First, we generated an ROC curve, calibration plots, and a DCA curve to measure the discrimination and accuracy of the nomogram in the training set. Subsequently, the same method was used in the testing set. The ROC curves showed that the nomogram had an evident classification effect in the training dataset (AUC: 0.754 (95% CI: 0.736–0.773), Figure 4A) and testing dataset (AUC: 0.751 (95% CI: 0.723–0.780), Figure 4B). The nomogram fit well in the training and testing datasets (Hosmer-lemeshow test: P = 0.303 and P = 0.367, respectively). The predictive probability of the nomogram was well correlated with the actual probability in the calibration plots (Figures 4C,D). The DCA curves suggested that the nomogram could be applied in clinical decision-making to identify surgical candidates (Figures 4E,F).
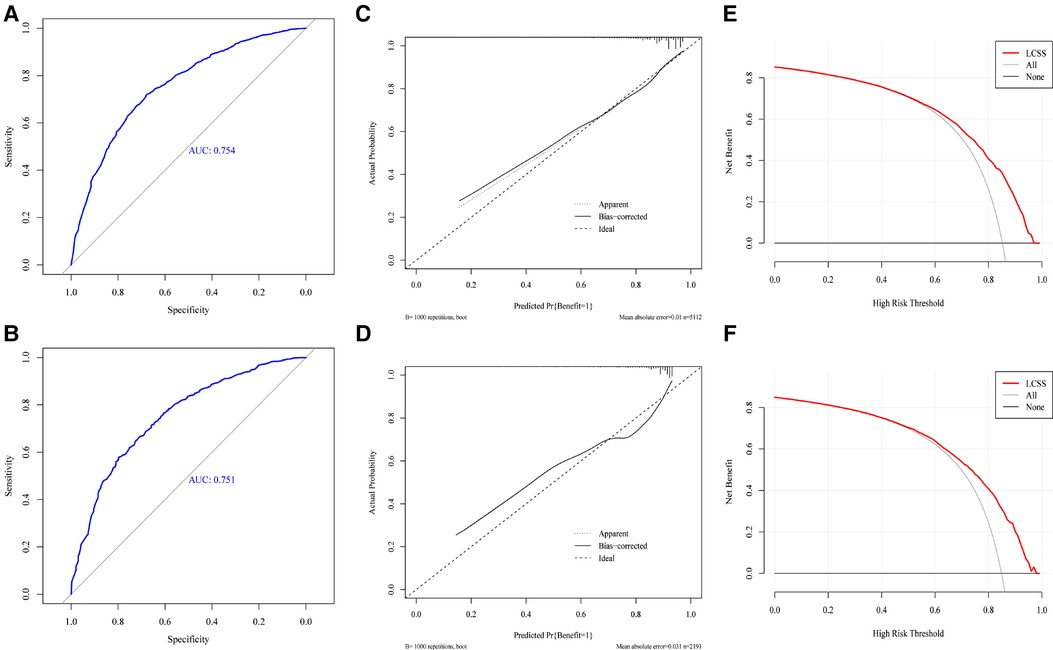
Figure 4. The ROC curve (A,B), calibration plots (C,D), decision curve analysis curve (E,F) of nomogram in training and testing set.
We then redivided the population after PSM to test the classification effect of this nomogram. Patients in the surgery with benefit group had a longer CSS time than the surgery without benefit group (HR = 3.08, 95% CI: 2.64–3.60, P < 0.001) and the non-surgery group (HR = 3.06, 95% CI: 2.85–3.28, P < 0.001). However, there was no significant difference between the surgery without benefit group and the non-surgery group (HR = 0.99, 95% CI: 0.85–1.15, P = 0.923) (Figure 5).
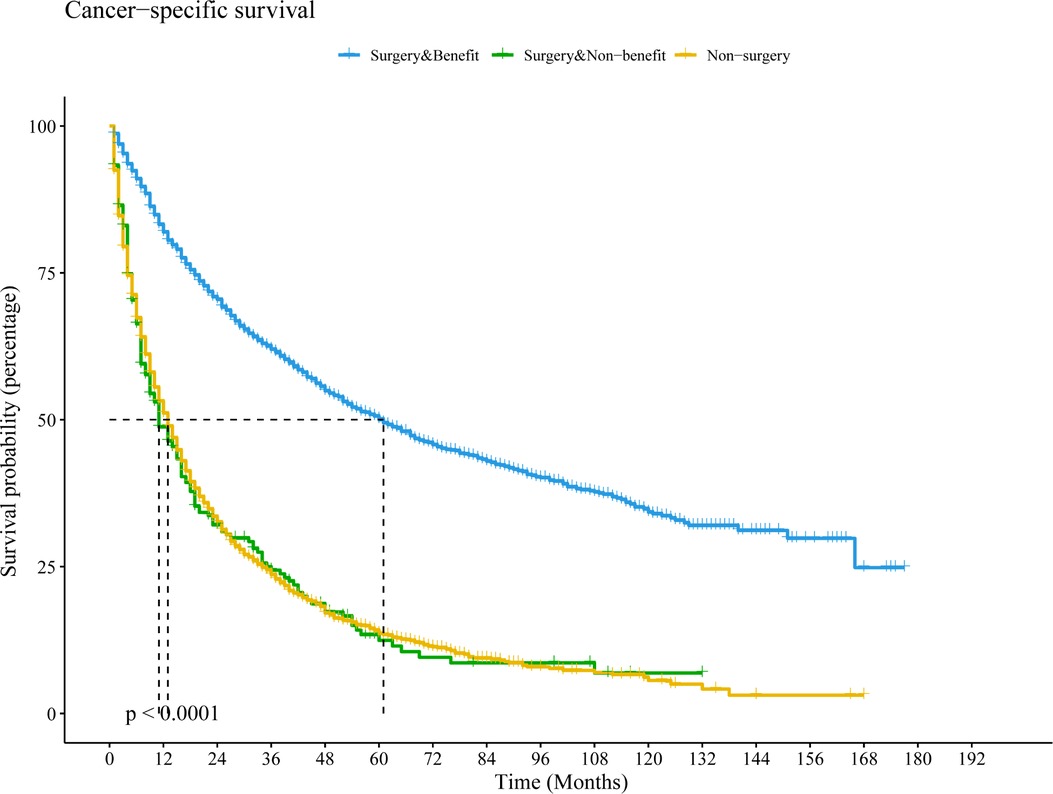
Figure 5. Kaplan–Meier curve to compare differential beneficial groups in the population after PSM based on this nomogram. Abbreviations: PSM, propensity score matching; LCSS, lung cancer-special survival.
Discussion
As the global population ages, the proportion of octogenarians with NSCLC is increasing annually. Although the health of elderly patients has seen increased attention in the last decade, there is a lack of evidence-based medical treatment approaches to apply to NSCLC patients greater than 80 years old. In recent studies, an increasing number of clinicians have expressed that age is not a limiting factor for surgical treatment for elderly NSCLC patients (5, 14). In this study, we demonstrated that octogenarians with NSCLC could benefit from primary tumor resection. Herein, a novel nomogram was developed to help clinicians identify potential surgical candidates from elderly NSCLC patients. We have shown that this model is effective and stable through ROC curve analyses, calibration plots, DCA curve, and application to the whole cohort.
Previously, thoracic surgeons seldomly performed surgery on octogenarians with NSCLC because of higher postoperative cardiac and pulmonary complications (15). With the development and widespread usage of minimally invasive technology and robotic surgery, the quality of life among octogenarians after surgery remains similar to younger patients (16, 17). Age may not influence the development of complications (18, 19). In our study, age was still an independent factor for prognosis, and surgery did not increase the rate of early death from other causes for octogenarians with NSCLC. Therefore, age only is an important factor for surgery. Elderly males had a poor prognosis, which could be associated with smoking and postoperative complications (20). Lung squamous cell carcinoma was identified as a poor prognostic factor with the univariate and multivariate analysis, which was consistent with previous studies (21). However, nuclear grade, TNM stage, and chemotherapy were also independent prognostic factors and T stage and N stage could potentially be positively associated with prognosis in octogenarians with NSCLC. It is necessary for octogenarians to complete a preoperative examination, especially regarding pulmonary puncture. For OS, radiotherapy was a protective factor. However, the multivariable competing risk model did not support this. It is possible that radiotherapy resulted in more deaths from other causes in elderly patients. A prospective study including multiple centers would be needed to verify this.
In our predictive model, tumor characteristics including differentiation and TNM stage were the factors most related to surgical benefit. The actual TNM stage and pathological information were critical factors for octogenarians with NSCLC. Moreover, younger female octogenarians, or patients with lung adenocarcinoma, could benefit from surgical section. These data suggest that certain patient characteristics could decrease postoperative complications.
There is presently a lack of high-level randomized controlled clinical trials for elderly patients with NSCLC. Octogenarians are usually excluded from randomized controlled clinical trials. In the literature, it has been shown that surgical treatment is feasible for octogenarians with NSCLC, and there are comparable survival rates between octogenarians and younger age groups who underwent surgical resection (8, 9, 17, 22, 23). In addition, minimally invasive surgical resection can improve long-term survival compared with traditional thoracotomy and stereotactic body radiotherapy in early-stage NSCLC (14, 24). Lymph node dissection could limitedly improve long-term survival, but this approach is conducive to subsequent true nodal staging and adjuvant treatment (14, 25, 26). Therefore, wedge or partial resections could be an alternative treatment for octogenarians (27). For advanced NSCLC octogenarians, our model suggests that specific patients may also benefit from surgical resection. However, retrospective and prospective studies in the literature have only focused on the efficiency of chemotherapy and targeted therapy for octogenarians (28–30). In the management of elderly patients (≥65 years old) with NSCLC, surgery is still the main choice for elderly patients (5). In general, treatment-related toxicity increases according with age (10). Patients over 70 years old have similar survival rates compared with patients between 55 and 69 years old with locally advanced NSCLC (31). Therefore, treatment of elderly advanced NSCLC patients should include chemotherapy, radiotherapy, and surgery in select cases (10). In recent studies, stereotactic ablative radiotherapy has emerged as a treatment alternative instead of surgery for early stage NSCLC (32, 33). However, lobectomy offers better survival than stereotactic ablative radiotherapy for patients aged 80 years or more with stage I NSCLC (14). Meanwhile, immunotherapy and targeted therapy are the vital treatment methods for patients with advanced NSCLC. Compared with younger patients, Geriatric patients are more susceptible to developing more serious adverse reactions (34). Even so, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) may play an important role for octogenarian patients with NSCLC in the initial treatment modalities (35). And, osimertinib, targeting EGFR T790M-mutated, has been approved in patients with resistance of EGFR-TKI and showed a good therapeutic effect for octogenarian patients with NSCLC (29). Although targeted therapies are expected to be a first-line treatment for advanced NSCLC, it is not ignored to reduce the local tumor burden by surgery. Maybe, comprehensive personalized treatment is the best methods for management of octogenarian patients with NSCLC. Surgery should be performed in carefully selected octogenarian patients with advanced NSCLC.
There are several limitations to our study. First, performance status, comorbidities, and pulmonary function were unknown because of the lack of relevant data in the SEER database. These variables could result in surgical intolerance, serious postoperative complications, and even early death. Secondly, some cancer biomarkers were unknown, such as neuron-specific enolase, carbohydrate antigen 125 and squamous cell carcinoma antigen. Moreover, it has been reported that the C-reactive protein to albumin ratio is related to the prognosis of elderly patients (≥80 years old) (36). However, these serum biomarkers were not collected in the SEER database. Thirdly, neoadjuvant chemoradiotherapy use, the sequence of surgery and chemotherapy, and the method and doses of chemoradiotherapy were unknown in the SEER database.
Conclusions
Primary tumor resection could improve the long-term survival of octogenarians with NSCLC, and detailed TNM stage and pathological information are necessary prior to treatment. We built a novel nomogram with high specificity and stability to identify octogenarians with NSCLC who could benefit from surgery.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CC Conceptualization, Formal analysis, Software, Data curation, visualization, Writing-original draft. DD Formal analysis, Software, Data curation, visualization. MW Formal analysis. YL Formal analysis. BW Conceptualization, Formal analysis, Writing-review & editing. YQ Writing-review & editing. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by Young Talent Development Plan of Changzhou Health Commission (CZQM2020004, CZQM2020034), Basic Research Project of Changzhou science and Technology Bureau (CJ20200104), Social Development Projects of Changzhou science and Technology Bureau (CE20205039), Science and Technology Project of Changzhou Health Commission (WZ202106), and Young talents Science and technology project of Changzhou Health Commission (QN201913).
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.972014/full#supplementary-material.
Supplementary Figure 1
The trend of surgery over time in elderly patients with non-small cell lung cancer between 2004 and 2018 year.
Supplementary Figure 2
The cumulative incidence rate plots of elderly patients with non-small cell lung cancer before and after PSM.
Supplementary Figure 3
The forest plots comparing the effect of surgery on overall survival based on different subgroup variables.
Supplementary Figure 4
The forest plots comparing the effect of surgery on cancer-special survival based on different subgroup variables.
References
1. Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. (2020) 41:1–24. doi: 10.1016/j.ccm.2019.10.001
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Saftic I, Bille A, Asemota N, Berjon de la Vega L, Routledge T, King J, et al. Risks and rewards of the surgical treatment of lung cancer in octogenarians. Interact Cardiovasc Thorac Surg. (2021) 33:905–12. doi: 10.1093/icvts/ivab194
4. Pilleron S, Soto-Perez-de-Celis E, Vignat J, Ferlay J, Soerjomataram I, Bray F, et al. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int J Cancer. (2021) 148:601–8. doi: 10.1002/ijc.33232
5. Blanco R, Maestu I, de la Torre MG, Cassinello A, Nunez I. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. (2015) 26:451–63. doi: 10.1093/annonc/mdu268
6. Detillon D, Veen EJ. Postoperative outcome after pulmonary surgery for non-small cell lung cancer in elderly patients. Ann Thorac Surg. (2018) 105:287–93. doi: 10.1016/j.athoracsur.2017.07.032
7. Vazirani J, Moraes J, Barnett S, Johnson DF, Knight S, Miller A, et al. Outcomes following resection of non-small cell lung cancer in octogenarians. ANZ J Surg. (2018) 88:1322–7. doi: 10.1111/ans.14861
8. Tutic-Horn M, Gambazzi F, Rocco G, Mosimann M, Schneiter D, Opitz I, et al. Curative resection for lung cancer in octogenarians is justified. J Thorac Dis. (2017) 9:296–302. doi: 10.21037/jtd.2017.02.22
9. Ganti AK, Shostrom V, Alorabi M, Zhen WK, Marr AS, Trujillo K, et al. Early stage non-small-cell lung cancer in octogenarian and older patients: a SEER database analysis. Clin Lung Cancer. (2016) 17:285–91. doi: 10.1016/j.cllc.2015.11.014
10. Bonanno L, Attili I, Pavan A, Sepulcri M, Pasello G, Rea F, et al. Treatment strategies for locally advanced non-small cell lung cancer in elderly patients: translating scientific evidence into clinical practice. Crit Rev Oncol Hematol. (2021) 163:103378. doi: 10.1016/j.critrevonc.2021.103378
11. Chen C, Kolbe J, Christmas T. Surgical treatment of non-small-cell lung cancer in octogenarians: a single-centre retrospective study. Intern Med J. (2021) 51:596–9. doi: 10.1111/imj.15268
12. Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: aSCO guideline for geriatric oncology. J Clin Oncol. (2018) 36:2326–47. doi: 10.1200/JCO.2018.78.8687
13. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. (2018) 19:e305–16. doi: 10.1016/S1470-2045(18)30348-6
14. Razi SS, Kodia K, Alnajar A, Block MI, Tarrazzi F, Nguyen D, et al. Lobectomy versus stereotactic body radiotherapy in healthy octogenarians with stage I lung cancer. Ann Thorac Surg. (2021) 111:1659–65. doi: 10.1016/j.athoracsur.2020.06.097
15. Osaki T, Shirakusa T, Kodate M, Nakanishi R, Mitsudomi T, Ueda H. Surgical treatment of lung cancer in the octogenarian. Ann Thorac Surg. (1994) 57:188–92; discussion 192–183. doi: 10.1016/0003-4975(94)90392-1
16. Asemota N, Saftic I, Tsitsias T, King J, Pilling J, Bille A. Quality of life in octogenarians after lung resection compared to younger patients. Clin Lung Cancer. (2022) 23:e118–30. doi: 10.1016/j.cllc.2021.05.008
17. Bongiolatti S, Gonfiotti A, Borgianni S, Crisci R, Curcio C, Voltolini L, et al. Post-operative outcomes and quality of life assessment after thoracoscopic lobectomy for non-small-cell lung cancer in octogenarians: analysis from a national database. Surg Oncol. (2021) 37:101530. doi: 10.1016/j.suronc.2021.101530
18. Stamenovic D, Messerschmidt A, Schneider T. Surgery for lung tumors in the elderly: a retrospective cohort study on the influence of advanced age (over 80 years) on the development of complications by using a multivariate risk model. Int J Surg. (2018) 52:141–8. doi: 10.1016/j.ijsu.2018.02.008
19. Saji H, Ueno T, Nakamura H, Okumura N, Tsuchida M, Sonobe M, et al. A proposal for a comprehensive risk scoring system for predicting postoperative complications in octogenarian patients with medically operable lung cancer: jACS1303. Eur J Cardiothorac Surg. (2018) 53:835–41. doi: 10.1093/ejcts/ezx415
20. Hino H, Karasaki T, Yoshida Y, Fukami T, Sano A, Tanaka M, et al. Risk factors for postoperative complications and long-term survival in lung cancer patients older than 80 years. Eur J Cardiothorac Surg. (2018) 53:980–6. doi: 10.1093/ejcts/ezx437
21. Iijima Y, Iwai S, Yamagata A, Motono N, Usuda K, Yamagishi S, et al. Is lung resection appropriate for late octogenarians? Surgical outcomes of patients aged ≥80 years with lung cancer. Clin Transl Oncol. (2021) 23:1585–92. doi: 10.1007/s12094-021-02554-4
22. Couderc AL, Tomasini P, Rey D, Nouguerede E, Correard F, Barlesi F, et al. Octogenarians treated for thoracic and lung cancers: impact of comprehensive geriatric assessment. J Geriatr Oncol. (2021) 12:402–9. doi: 10.1016/j.jgo.2020.10.005
23. Dillman RO, Zusman DR, McClure SE. Surgical resection and long-term survival for octogenarians who undergo surgery for non-small-cell lung cancer. Clin Lung Cancer. (2009) 10:130–4. doi: 10.3816/CLC.2009.n.017
24. Pages PB, Mariet AS, Madelaine L, Cottenet J, Hanna HA, Quantin C, et al. Impact of video-assisted thoracic surgery approach on postoperative mortality after lobectomy in octogenarians. J Thorac Cardiovasc Surg. (2019) 157:1660–7. doi: 10.1016/j.jtcvs.2018.11.098
25. Thomas PA, Couderc AL, Boulate D, Greillier L, Charvet A, Brioude G, et al. Early-stage non-small cell lung cancer beyond life expectancy: still not too old for surgery? Lung Cancer. (2021) 152:86–93. doi: 10.1016/j.lungcan.2020.12.009
26. Nakao M, Saji H, Mun M, Nakamura H, Okumura N, Tsuchida M, et al. Prognostic impact of mediastinal lymph node dissection in octogenarians with lung cancer: JACS1303. Clin Lung Cancer. (2021) 23:e176–e84. doi: 10.1016/j.cllc.2021.09.007
27. Mimae T, Miyata Y, Tsutani Y, et al. Wedge resection as an alternative treatment for octogenarian and older patients with early-stage non-small-cell lung cancer. Jpn J Clin Oncol. (2020) 50:1051–7. doi: 10.1093/jjco/hyaa085
28. Hesketh PJ, Lilenbaum RC, Chansky K, Dowlati A, Graham P, Chapman RA, et al. Chemotherapy in patients > or=80 with advanced non-small cell lung cancer: combined results from SWOG 0027 and LUN 6. J Thorac Oncol. (2007) 2:494–8. doi: 10.1097/JTO.0b013e318060097e
29. Auliac JB, Saboundji K, Andre M, Madelaine J, Quere G, Masson P, et al. Real-life efficacy of osimertinib in pretreated octogenarian patients with T790M-mutated advanced non-small cell lung cancer. Target Oncol. (2019) 14:307–14. doi: 10.1007/s11523-019-00646-4
30. Assie JB, Corre R, Levra MG, Calvet CY, Gaudin AF, Grumberg V, et al. Nivolumab treatment in advanced non-small cell lung cancer: real-world long-term outcomes within overall and special populations (the UNIVOC study). Ther Adv Med Oncol. (2020) 12:1758835920967237. doi: 10.1177/1758835920967237
31. Zhang L. Short- and long-term outcomes in elderly patients with locally advanced non-small-cell lung cancer treated using video-assisted thoracic surgery lobectomy. Ther Clin Risk Manag. (2018) 14:2213–20. doi: 10.2147/TCRM.S175846
32. Zheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. (2014) 90:603–11. doi: 10.1016/j.ijrobp.2014.05.055
33. Scotti V, Bruni A, Francolini G, Perna M, Vasilyeva P, Loi M, et al. Stereotactic ablative radiotherapy as an alternative to lobectomy in patients with medically operable stage I NSCLC: a retrospective, multicenter analysis. Clin Lung Cancer. (2019) 20:e53–61. doi: 10.1016/j.cllc.2018.09.003
34. Pozo Vico A, Gonzales Montejo N, Pagola Lorz I, Castillo Andueza A, Ortega Molina LY, Alonso Renedo FJ. [Pembrolizumab-associated autoimmune encephalitis in an elderly patient with advanced non-small cell lung cancer. A clinical case]. An Sist Sanit Navar. (2021) 44:291–7. doi: 10.23938/ASSN.0948
35. Chen KY, Chen JH, Shih JY, Yang CH, Yu CJ, Yang PC. Octogenarians with advanced non-small cell lung cancer: treatment modalities, survival, and prognostic factors. J Thorac Oncol. (2010) 5:82–9. doi: 10.1097/JTO.0b013e3181c09b28
Keywords: Octogenarians, NSCLC, surgery, SEER, nomogram
Citation: Chao C, Di D, Wang M, Liu Y, Wang B and Qian Y (2022) Identifying octogenarians with non-small cell lung cancer who could benefit from surgery: A population-based predictive model. Front. Surg. 9:972014. doi: 10.3389/fsurg.2022.972014
Received: 20 June 2022; Accepted: 12 July 2022;
Published: 28 July 2022.
Edited by:
Marco Scarci, Hammersmith Hospital, United KingdomReviewed by:
Leonidas Papastavrou, Athens Medical Center, GreeceSavvas Lampridis, Guy's and St Thomas’ NHS Foundation Trust, United Kingdom
© 2022 Chao, Di, Wang, Liu, Wang and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Wang Y29saW5faXZlcnNvbkAxNjMuY29t; Yongxiang Qian cXl4NjcxMDEyQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Ce Chao
Ce Chao Dongmei Di†
Dongmei Di† Min Wang
Min Wang Bin Wang
Bin Wang