
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 22 July 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.969418
This article is part of the Research Topic Robotic Surgery: Human Learning, Simulation and Training on Surgical Education View all 8 articles
Background: The robotic surgical system is being used in various bariatric procedures. However, only a few studies with very small sample size are present on robotic single-anastomosis duodenal–ileal bypass with sleeve gastrectomy (SADI-S). Moreover, to date, the learning curve of robotic SADI-S has been poorly evaluated yet.
Objective: This retrospective study aimed to estimate the learning curve of robotic SADI-S.
Methods: 102 consecutive patients who underwent robotic SADI-S between March 2020 and December 2021 were included. Textbook outcome standard was performed to comprehensively evaluate clinical outcome of robotic SADI-S. Based on the textbook outcome, we evaluated the learning curve of robotic SADI-S by the cumulative sum (CUSUM) method.
Results: The mean operative time was 186.13 ± 36.91 min. No conversion to laparotomy or deaths occurred during the study period. The rate of complications was 6.9% (n = 7), of which major complications were identified in 2.9% (n = 3), including 2 gastric leakages and 1 respiratory failure. A total of 60 patients reached the textbook outcome standard. The rate of textbook outcome was positive and was steadily increasing after the number of surgical cases accumulated to the 58th case. Taking the 58th case as the boundary, all the patients were divided into the learning stage group (the first 58 patients) and mastery stage group (the last 44 patients). The rate of complications, proportion of abdominal drainage tubes and postoperative hospital stay were significantly higher in the learning stage group compared with the mastery stage group (P < 0.05). No significant difference was observed between the two groups in terms of patient demographic data, operative times, reoperations and readmission.
Conclusion: Robotic SADI-S is a feasible and reproducible surgical technique with a learning curve of 58 cases.
Compared with conventional laparoscopy, the robotic surgical system offers several advantages (1–8), including 7 degrees of freedom, tremor filtration, three-dimensional high-definition visualization, and superior ergonomics, which might contribute to improve surgical outcomes. Since Cadiere reported the world's first robotic bariatric surgery in 1999, the robotic surgical system has been used in bariatric procedures such as sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB) and biliopancreatic diversion with duodenal switch (BPD/DS) (9–12). In recent years, the robot surgical system begins to be applied in single-anastomosis duodenal–ileal bypass with sleeve gastrectomy (SADI-S), but only a few studies with very small sample size are present (13–16). Moreover, to date, the learning curve of robotic SADI-S has been poorly evaluated yet.
102 consecutive patients who underwent robotic SADI-S between March 2020 and December 2021 were included in this study. Any revisional operations were excluded. All the surgeries were performed by the same surgeon. We recorded and analyzed the following factors: patient gender, age, preoperative weight, body mass index, waistline, standard live volume, American Society of Anesthesiology Physical Status Classification, operative time, the proportion of abdominal drainage tube, length of postoperative stay, complications, conversion to laparotomy, mortality, reoperation, and readmission. The Dindo–Clavien classification was used to classify the severity of complications, and major complications were defined as grade III or above (17, 18).
The Da Vinci Xi® model was used for robotic SADI-S. The patient undergoing surgery was in reverse Trendelenburg position with open legs and arms. The first trocar for the 30° camera (8 mm, robotic arm 3) was placed at the lower edge of the navel. Other trocars were inserted under visual inspection. The second trocar for the stapler insertion (12-mm, robotic arm 1) was placed at the junction of the right anterior axillary line and right end of the greater curvature of the stomach. The third trocar for liver retractor (8 mm, robotic arm 2) was placed at the junction of the right midclavicular line and costal margin. The fourth trocar (8-mm, robotic arm 4) was placed at the junction of the left midclavicular line and left end of the greater curvature of the stomach. All the trocars were spaced more than 8 cm, avoiding each other's interference of robotic arms. A 300-cm common channel was measured retrograde from the ileocecal valve and marked by sutures. Sleeve gastrectomy was performed about 4 cm from the pylorus over a 34 Fr bougie tube. Complete duodenum transection was performed about 2 cm from the pylorus. Finally, duodenal–ileal anastomosis was performed continuously by using an absorbable 3-0 barbed suture.
The moving average method is a simple smoothing forecasting technique. According to time series, item by item, sequential time averages containing a certain number of items are sequentially calculated to reflect the long-term trend (19). We applied the method to the operative time of robotic SADI-S with a moving average order of 20 cases by the following formulate:
Textbook outcome (TO) was performed to comprehensively evaluate clinical outcome of robotic SADI-S. According to a review of existing “Textbook Outcome” metrics in the literature (20–24), the definition of TO was subsequently expanded for robotic SADI-S-specific outcomes selected based on clinician consensus among a team of bariatric surgeons at our institution. The final definition of TO in robotic SADI-S included the following 5 key parameters: the operative time less than or equal to the 75th percentile of overall operation time (210 min); the postoperative hospital stay less than or equal to the 75th percentile of overall postoperative hospital stay (6 days); complication grade lower than Dindo–Clavien grade II; no conversion to laparotomy, and no rehospitalization or death after robotic SADI-S. TO was recorded when all of the aforementioned parameters were observed.
According to the order of operation date, all patients are numbered 1–102. Based on the TO standard, the clinical outcomes of all patients are classified and quantified, that is, the clinical outcome is represented by 1 when it meets the TO standard; otherwise, it is represented by –1. We calculated the rate of TO by the cumulative sum analysis (CUSUM) method. The curve was drawn by case number as x-axis and CUSUM (TO rate) as y-axis so as to understand the learning curve of robotic SADI-S.
SPSS 22.0 was used for statistical analysis. Measurement data were expressed as mean ± standard deviation and were analyzed by the independent-sample Student's t-test (Normality data) or Mann–Whitney U test (skewed data), as appropriate. The calculated data were analyzed by the χ2 test. A P-value of <0.05 was considered statistically significant.
102 consecutive patients who underwent robotic SADI-S were included in this study and the overall follow-up rate was 100%. Among the 102 patients, 57 were women and 45 were men with a mean age of 34 years (range, 17–61 years). The patient demographic data are summarized in Table 1.
Overall, mortality and conversion to laparotomy did not occur in this study. Seven patients (6.9%) suffered through complications, including two with gastric leakage (2.0%), one with duodenal-ileal anastomotic leakage (1.0%), one with respiratory failure (1.0%), one with postoperative abdominal bleeding (1.0%), one with seroperitoneum (1.0%), and one with delayed gastric emptying (1.0%). Among the seven patients with complication, major complications were identified in 2.9% (n = 3), including 2 gastric leakages and 1 respiratory failure. The rates of reoperation and readmission were 2.0% and 2.9%, respectively. Four patients suffered through grade II complications classified on the basis of the Dindo–Clavien classification (one with seroperitoneum, one with postoperative abdominal bleeding, one with delayed gastric emptying, and one with duodenal-ileal anastomotic leakage), and all of them were cured successfully by the conservative treatment. Two patients suffered from gastric leakage (grade IIIb) and required reoperation. One patient was transferred to the intensive care unit because of respiratory failure (grade IV) and was eventually cured.
The raw data of operative time were plotted as blue solid points in chronological case order (Figure 1). Along with the increase of the number of surgical cases, an overall downward trend for operative time was observed. The moving average method showed that in the initial period of developing robotic SADI-S, the slope of operative time went down most sharply, then tended towards stable (The green line in Figure 1).
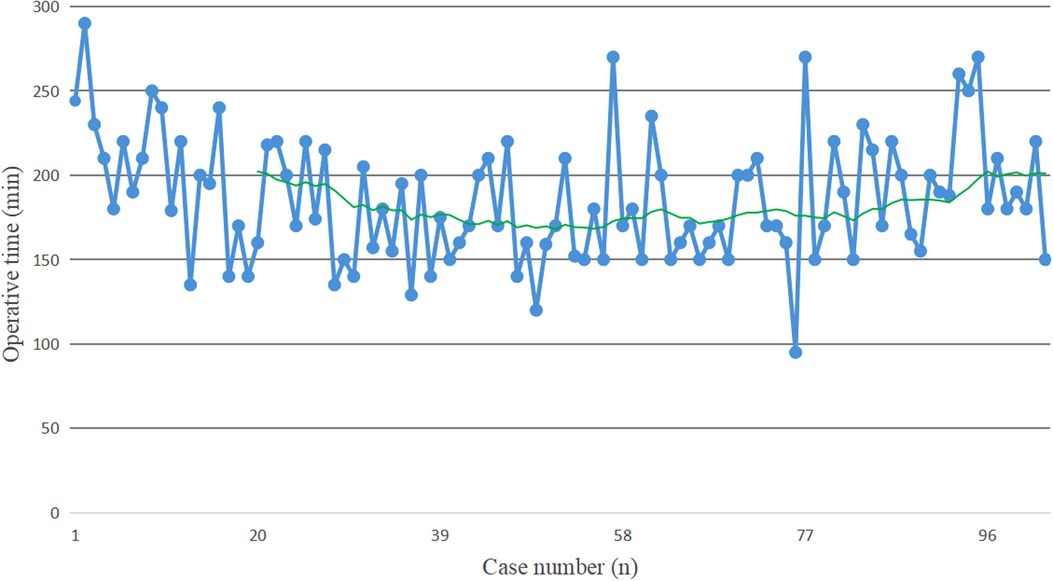
Figure 1. The blue solid points indicate the operative time for each patient in chronological case order. The green line represents the moving average of operative time, which is used to help determine the trend of operative time. In the initial period of developing robotic SADI-S, the slope of operative time goes down most sharply, then tends towards stable.
The CUSUM plot indicated that the rate of textbook outcome, as previously outlined, was positive and was steadily increasing after the number of surgical cases accumulated to the 58th case (Figure 2). This suggests that the learning curve of totally robotic SADI-S was 58 cases. Subsequently, all the patients were classified into the learning stage group (the first 58 patients) and mastery stage group (the last 44 patients). No significant difference was observed between the learning stage group and mastery stage group in terms of patient's demographic data (Table 2). Except for the rates of complication, proportion of abdominal drainage tubes and lengths of postoperative hospital stay, no significant difference was observed between the learning stage group and mastery stage group in terms of operation-related outcomes (Table 3).
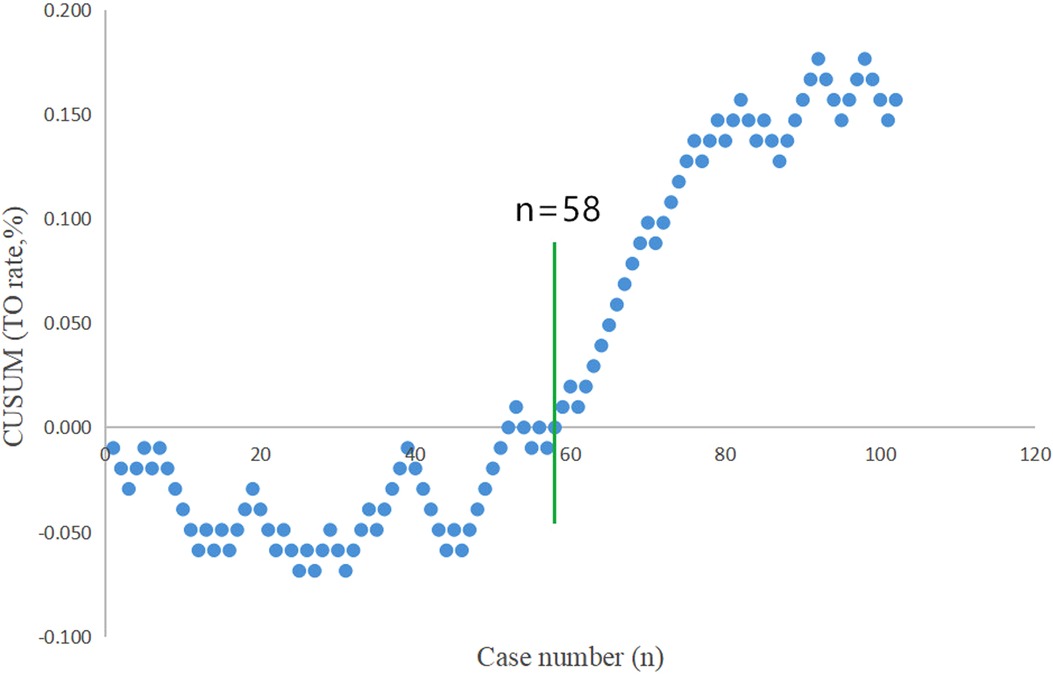
Figure 2. “Textbook outcome (TO)” for robotic SADI-S was defined by all of the following 5 parameters: operative time ≤210 min, the length of postoperative stay ≤6 days, postoperative morbid event <Clavien grade II, no conversion to laparotomy, and no rehospitalization or death after totally robotic SADI-S. TO was recorded when all of the aforementioned parameters were observed. The rate of TO for robotic SADI-S is positive and is steadily increasing after the number of surgical cases accumulated to the 58th case.
SADI-S is being used as a surgical procedure in the treatment of morbid obesity for 14 years since it was first proposed in 2007 by Torres et al. as a simplified procedure of BPD/DS (25). SADI-S is preferred over BPD/DS because of the reduced operative risk by eliminating one anastomosis and similar weight loss and remission of metabolic diseases. Nonetheless, there are still some technical challenges for surgeons to perform SADI-S by laparoscopy because of patient's large waistline, large liver, thick abdominal walls, and substantial visceral fat. Several studies have proved that the advantages of robotic surgical system can contribute to reduce the complication rates and increase the safety of surgery compared with conventional laparoscopy (26–30). Similarly, the robotic surgical system might be helpful in performing SADI-S.
To our knowledge, this study is the first to estimate the learning curve of robotic SADI-S by multifactorial analysis. According to our results, the learning curve of totally robotic SADI-S is 58 cases and can be divided into Phase 1 and Phase 2. Phase 1 includes the first 58 patients and represents the initial learning stage. Phase 2 represents the mastery stage, with a significant reduction in the rate of complications, proportion of abdominal drainage tube and postoperative hospital stay. The learning curve of other bariatric procedures has been reported previously. Previous studies (10, 31) have shown that the learning curve of robotic SG is 20–25 cases. The learning curve for robotic RYGB ranged from 14 to 84 cases (11, 32–35). Sudan et al. (12) reported that the learning curve for robot-assisted BPD/DS was 50 cases.
This study showed that robotic SADI-S is a feasible and safe surgical approach for morbid obesity. A total of 7 patients developed complications (6.9%). In addition to two patients with gastric leakage (grade IIIb) requiring reoperation, the other 5 patients with the complications were cured by the conservative treatment. In general, surgeons are concerned about a higher complication rate during the initial learning phase of the learning curve, wherein they plan to develop a new procedure. In this study, although statistical difference was observed in terms of morbidity between the first 58 patients (learning stage) and the last 44 patients (mastery stage) (12.1% vs. 0%; P = 0.018), robotic SADI-S for patients with obesity is relatively safe during the initial phase of the learning curve.
The mean total operative time in this study was 186 min, which is within the range of those reported previously for robotic SADI-S (145–204 min) (13–16). An increase in surgeon's proficiency with the increase in surgical experience was reflected in the operative time required; of note, the slope was more pronounced in the initial phase. However, in the late period of developing robotic SADI-S, we observed an abnormal increase in operative time in some patients, resulting in a significant elevation of the moving average curve. This can be explained by the fact that we measured the length of small intestine twice using two different methods during operation. In this study, the results of Pearson correlation analysis indicated that operative time was negatively correlated with assistant's experience (r = −0.214, P = 0.031). However, our study showed that mean operative time didn't decrease in mastery stage group compared with learning stage group. The reason for this difference is associated with the use of new assistants during the mastery stage. Our results showed that postoperative hospital stay was more than five days in both groups, which didn't display the principles of ERAS. The reason for this prolonged admission is that SADI-S is a restrictive and malabsorptive bariatric operation. It is quite possible that patients have trouble taking in adequate liquids after SADI-S, resulting in dehydration. For this, in our center, all patients need to take extra several days for intravenous hydration. Of course, we also timely deal with symptoms caused by food intolerance during this period.
The main limitation of robotic surgery is the perceived higher cost compared with that of laparoscopy. Most previous studies reported that the use of a robotic surgical system increases the cost of the procedure (36–38). However, Hagen et al. (39) reported that the overall cost of robotic RYGB is less compared with laparoscopy. We did not analyze the cost in our study; however, more safety may equalize higher cost. Moreover, the cost of robotic surgery is not necessarily higher than conventional laparoscopy (39).
This study is the first to evaluate the learning curve of robotic SADI-S by multifactorial analysis. However, the study has some limitations. On the one hand, it is a retrospective study. One the other hand, there is no control group in this study. Randomized controlled trials using large sample sizes are required for further study.
Robotic SADI-S is a feasible and reproducible surgical technique with a learning curve of 58 cases.
The original contributions presented in the study are included in the article/Suplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by China-Japan Union Hospital of Jilin University.
LW: Conceptualization, Data Curation, Formal Analysis, Writing-Original Draft; YY, JW, SL: Data Curation, Formal Analysis; TJ: Conceptualization, Supervision, Validation, Writing-Review and Editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Park YS, Oo AM, Son SY, Shin DJ, Jung DH, Ahn SH, et al. Is a robotic system really better than the three-dimensional laparoscopic system in terms of suturing performance?: comparison among operators with different levels of experience. Surg Endosc. (2016) 30(4):1485–90. doi: 10.1007/s00464-015-4357-9
2. Chandra V, Nehra D, Parent R, Woo R, Reyes R, Hernandez-Boussard T, et al. A comparison of laparoscopic and robotic assisted suturing performance by experts and novices. Surgery. (2010) 147(6):830–9. doi: 10.1016/j.surg.2009.11.002
3. Moorthy K, Munz Y, Dosis A, Hernandez J, Martin S, Bello F, et al. Dexterity enhancement with robotic surgery. Surg Endosc. (2004) 18(5):790–5. doi: 10.1007/s00464-003-8922-2
4. Jourdan IC, Dutson E, Garcia A, Vleugels T, Leroy J, Mutter D, et al. Stereoscopic vision provides a significant advantage for precision robotic laparoscopy. Br J Surg. (2004) 91(7):879–85. doi: 10.1002/bjs.4549
5. Zwimpfer TA, Wismer C, Fellmann-Fischer B, Geiger J, Schotzau A, Heinzelmann-Schwarz V. Comparison of 2D 4 K vs. 3D HD laparoscopic imaging systems using a pelvitrainer model: a randomized controlled study. Updates Surg. (2022) 74(3):1137–47. doi: 10.1007/s13304-021-01195-0
6. Zihni AM, Ohu I, Cavallo JA, Cho S, Awad MM. Ergonomic analysis of robot-assisted and traditional laparoscopic procedures. Surg Endosc. (2014) 28(12):3379–84. doi: 10.1007/s00464-014-3604-9
7. Elhage O, Challacombe B, Shortland A, Dasgupta P. An assessment of the physical impact of complex surgical tasks on surgeon errors and discomfort: a comparison between robot-assisted, laparoscopic and open approaches. BJU Int. (2015) 115(2):274–81. doi: 10.1111/bju.12680
8. Zarate Rodriguez JG, Zihni AM, Ohu I, Cavallo JA, Ray S, Cho S, et al. Ergonomic analysis of laparoscopic and robotic surgical task performance at various experience levels. Surg Endosc. (2019) 33(6):1938–43. doi: 10.1007/s00464-018-6478-4
9. Cadiere GB, Himpens J, Vertruyen M, Favretti F. The world’s First obesity surgery performed by a surgeon at a distance. Obes Surg. (1999) 9(2):206–9. doi: 10.1381/096089299765553539
10. Vilallonga R, Fort JM, Gonzalez O, Caubet E, Boleko A, Neff KJ, et al. The initial learning curve for robot-assisted sleeve gastrectomy: a surgeon’s experience while introducing the robotic technology in a bariatric surgery department. Minim Invasive Surg. (2012) 2012:347131. doi: 10.1155/2012/347131
11. Buchs NC, Pugin F, Bucher P, Hagen ME, Chassot G, Koutny-Fong P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc. (2012) 26(4):1116–21. doi: 10.1007/s00464-011-2008-3
12. Sudan R, Bennett KM, Jacobs DO, Sudan DL. Multifactorial analysis of the learning curve for robot-assisted laparoscopic biliopancreatic diversion with duodenal switch. Ann Surg. (2012) 255(5):940–5. doi: 10.1097/SLA.0b013e31824c1d06
13. Tarasco Palomares J, Caballero Boza A, Sanchez Haro E, Herrero Vicente C, Moreno Santabarbara P. Really totally robotic SADI-S in a patient with extreme morbid obesity and non-reducible umbilical hernia: case report. Obes Surg. (2020) 30(10):4171–3. doi: 10.1007/s11695-020-04802-y
14. Tat C, Del Gobbo GD, Klingler M, Corcelles R. How I do it: robotic single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADIS). J Gastrointest Surg. (2020) 24(12):2893–5. doi: 10.1007/s11605-020-04789-y
15. Laviano Martinez E, Lammers L, Goergen M, Azagra Soria JS. Full robotic single anastomosis duodeno-ileal bypass (SADI). Cir Esp (Engl Ed). (2019) 97(9):535. doi: 10.1016/j.ciresp.2019.05.013
16. Vilallonga R, Fort JM, Caubet E, Gonzalez O, Balibrea JM, Ciudin A, et al. Robotically assisted single anastomosis duodenoileal bypass after previous sleeve gastrectomy implementing high valuable technology for complex procedures. J Obes. (2015) 2015:586419. doi: 10.1155/2015/586419
17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
18. Schlegel A, van Reeven M, Croome K, Parente A, Dolcet A, Widmer J, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J Hepatol. (2022) 76(2):371–82. doi: 10.1016/j.jhep.2021.10.004
19. Kim CW, Kim WR, Kim HY, Kang J, Hur H, Min BS, et al. Learning curve for single-incision laparoscopic anterior resection for sigmoid colon cancer. J Am Coll Surg. (2015) 221(2):397–403. doi: 10.1016/j.jamcollsurg.2015.02.016
20. Halpern SE, Moris D, Gloria JN, Shaw BI, Haney JC, Klapper JA, et al. Textbook outcome: definition and analysis of a novel quality measure in lung transplantation. Ann Surg. (2021) 9. doi: 10.1097/SLA.0000000000004916. [Epub ahead of print]
21. Busweiler LA, Schouwenburg MG, van Berge Henegouwen MI, Kolfschoten NE, de Jong PC, Rozema T, et al. Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg. (2017) 104(6):742–50. doi: 10.1002/bjs.10486
22. Ten Berge MG, Beck N, Steup WH, Verhagen AFTM, van Brakel TJ, Schreurs WH, et al. Textbook outcome as a composite outcome measure in non-small-cell lung cancer surgery. Eur J Cardiothorac Surg. (2021) 59(1):92–9. doi: 10.1093/ejcts/ezaa265
23. Merath K, Chen Q, Bagante F, Alexandrescu S, Marques HP, Aldrighetti L, et al. A multi-institutional international analysis of textbook outcomes among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma. JAMA Surg. (2019) 154(6):e190571. doi: 10.1001/jamasurg.2019.0571
24. Moris D, Shaw BI, Gloria J, Kesseli SJ, Samoylova ML, Schmitz R, et al. Textbook outcomes in liver transplantation. World J Surg. (2020) 44(10):3470–7. doi: 10.1007/s00268-020-05625-9
25. Sanchez-Pernaute A, Rubio Herrera MA, Perez-Aguirre E, García Pérez JC, Cabrerizo L, Díez Valladares L, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. (2007) 17(12):1614–8. doi: 10.1007/s11695-007-9287-8
26. Snyder BE, Wilson T, Scarborough T, Yu S, Wilson EB. Lowering gastrointestinal leak rates: a comparative analysis of robotic and laparoscopic gastric bypass. J Robot Surg. (2008) 2(3):159–63. doi: 10.1007/s11701-008-0104-8
27. Feng Q, Ma H, Qiu J, Du Y, Zhang G, Li P, et al. Comparison of long-term and perioperative outcomes of robotic versus conventional laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis of PSM and RCT studies. Front Oncol. (2021) 11:759509. doi: 10.3389/fonc.2021.759509
28. Chiu CC, Hsu WT, Choi JJ, Galm B, Lee MG, Chang CN, et al. Comparison of outcome and cost between the open, laparoscopic, and robotic surgical treatments for colon cancer: a propensity score-matched analysis using nationwide hospital record database. Surg Endosc. (2019) 33(11):3757–65. doi: 10.1007/s00464-019-06672-7
29. Lo BD, Leeds IL, Sundel MH, Gearhart S, Nisly GRC, Safar B, et al. Frailer patients undergoing robotic colectomies for colon cancer experience increased complication rates compared with open or laparoscopic approaches. Dis Colon Rectum. (2020) 63(5):588–97. doi: 10.1097/DCR.0000000000001598
30. Ng KT, Tsia AKV, Chong VYL. Robotic versus conventional laparoscopic surgery for colorectal cancer: a systematic review and meta-analysis with trial sequential analysis. World J Surg. (2019) 43(4):1146–61. doi: 10.1007/s00268-018-04896-7
31. Romero RJ, Kosanovic R, Rabaza JR, Seetharamaiah R, Donkor C, Gallas M, et al. Robotic sleeve gastrectomy: experience of 134 cases and comparison with a systematic review of the laparoscopic approach. Obes Surg. (2013) 23(11):1743–52. doi: 10.1007/s11695-013-1004-1
32. Ayloo SM, Addeo P, Buchs NC, Shah G, Giulianotti PC. Robot-assisted versus laparoscopic Roux-en-Y gastric bypass: is there a difference in outcomes? World J Surg. (2011) 35(3):637–42. doi: 10.1007/s00268-010-0938-x
33. Renaud M, Reibel N, Zarnegar R, Germain A, Quilliot D, Ayav A, et al. Multifactorial analysis of the learning curve for totally robotic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. (2013) 23(11):1753–60. doi: 10.1007/s11695-013-1020-1
34. Tieu K, Allison N, Snyder B, Wilson T, Toder M, Wilson E. Robotic-assisted Roux-en-Y gastric bypass: update from 2 high-volume centers. Surg Obes Relat Dis. (2013) 9(2):284–8. doi: 10.1016/j.soard.2011.11.022
35. Yu SC, Clapp BL, Lee MJ, Albrecht WC, Scarborough TK, Wilson EB. Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux-en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg. (2006) 192(6):746–9. doi: 10.1016/j.amjsurg.2006.08.038
36. Hubens G, Balliu L, Ruppert M, Gypen B, Van Tu T, Vaneerdeweg W. Roux-en-Y gastric bypass procedure performed with the da Vinci robot system: is it worth it? Surg Endosc. (2008) 22(7):1690–6. doi: 10.1007/s00464-007-9698-6
37. Curet MJ, Curet M, Solomon H, Lui G, Morton JM. Comparison of hospital charges between robotic, laparoscopic stapled, and laparoscopic handsewn Roux-en-Y gastric bypass. J Robot Surg. (2009) 3(2):75–8. doi: 10.1007/s11701-009-0143-9
38. Scozzari G, Rebecchi F, Millo P, Rocchietto S, Allieta R, Morino M. Robot-assisted gastrojejunal anastomosis does not improve the results of the laparoscopic Roux-en-Y gastric bypass. Surg Endosc. (2011) 25(2):597–603. doi: 10.1007/s00464-010-1229-1
Keywords: learning curve, robotic, single-anastomosis duodenal-ileal bypass with sleeve gastrectomy, SADI-S, textbook outcome
Citation: Wang L, Yu Y, Wang J, Li S and Jiang T (2022) Evaluation of the learning curve for robotic single-anastomosis duodenal–ileal bypass with sleeve gastrectomy. Front. Surg. 9:969418. doi: 10.3389/fsurg.2022.969418
Received: 15 June 2022; Accepted: 6 July 2022;
Published: 22 July 2022.
Edited by:
Ka-Chun Siu, University of Nebraska Medical Center, United StatesReviewed by:
Mohammad Kermansaravi, Iran University of Medical Sciences, Iran© 2022 Wang, Yu, Wang, Li and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Jiang amlhbmd0YW85OUBqbHUuZWR1LmNu
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.