- 1Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 2Faculty of Science and Marine Environment, Universiti Malaysia Terengganu, Kuala Nerus, Malaysia
- 3Department of Obstetrics and Gynaecology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
De novo hypertensive disorders of pregnancy (HDP) which consist of gestational hypertension and preeclampsia affect maternal and offspring morbidity and mortality, and potentially increase the risk of cardiovascular disease in the offspring. It is well known that de novo HDP causes various maternal complications, including cardiovascular diseases, placental abruption and liver and kidney failure. However, there are studies suggesting that offspring of pregnancies complicated by de novo HDP have an increased risk of long-term cardiovascular disease. The endothelium is an important regulator of vascular function, and its dysfunction is highly associated with the development of cardiovascular diseases. Hence, this review aimed to systematically identify articles related to the effect of de novo HDP on the endothelial function of the offspring. A computerized database search was conducted on PubMed, Scopus, and Medline from 1976 until 2022. A total of 685 articles were obtained. We identified another three additional articles through review articles and Google Scholar. Altogether, we used 13 articles for data extraction. All studies reported that endothelial function was impaired in the offspring of de novo HDP. This is most likely attributed to impaired vasodilation, subclinical atherosclerosis formation, inflammation, and dysregulated epigenetic regulation of endothelial functions.
Introduction
Hypertensive disorders of pregnancy (HDP) are the most common medical disorder during pregnancy. HDP shows an increasing incidence rate globally with a total increase of 10.92% from 1990 to 2019 (1). The International Society for the Study of Hypertension in Pregnancy (ISSHP) classified HDP as chronic (pre-dating pregnancy or diagnosed prior to 20 weeks of pregnancy) or de novo (gestational hypertension or preeclampsia) (2, 3). Among all, preeclampsia (PE) was the most complex disorder, deteriorating rapidly with more than 70,000 maternal deaths and 500,000 fetal and neonatal deaths per year (2).
A previous study revealed that women with HDP have a higher tendency to have neonatal adverse outcomes than normotensive women (4). Alongside the complications of HDP to the mother, HDP also has short- and long-term adverse effects on the offspring. In the short term, neonates of mothers with HDP have a high risk of developing fetal hypoxia, premature birth, placental abruption, low birth weight, small for gestational age, respiratory distress syndrome, and death in utero. In the long term, neonates develop hypertension, arterial thickening, increased left ventricular wall thickness and reduced left ventricular end-diastolic volume (5).
Studies also found that HDP increases the risk of hypertension and cardiovascular disease (CVD) not only to the mother, but also to the offspring (6–8). The relevance of HDP on the offspring's health extends into adulthood. The HUNT study showed that as young adults, the offspring of women with HDP have higher CVD risk in terms of higher blood pressure (BP), body mass index (BMI) and waist circumference compared to the offspring of women with normal pregnancies (6).
HDP is considered a maternal and fetal endothelial disorder. The endothelium is a functional organ formed by a single layer of squamous endothelial cells that line all blood vessels. Endothelial cells play a role in vascular homeostasis and angiogenesis in response to injury or hypoxia. Studies have demonstrated that in preeclampsia, endothelial dysfunction occurs in the vascular endothelium of both mothers (9) and offspring (10). Preeclampsia is also the most common HDP that is associated with endothelial dysfunction (11).
Preeclampsia starts when placental perfusion is reduced by abnormal cytotrophoblast invasion of the spiral arteries (12). The imbalance between pro- and anti-angiogenic factors in preeclampsia is proposed to trigger the abnormal placental vascularization and the onset of preeclampsia (13). The dysfunctional placentation causes oxidative stress and increased resistance to placental blood flow. Subsequently, hypoperfusion, chronic placental ischemia (5) and endothelial dysfunction ensue (14). Endothelial dysfunction alters the capacity of endothelial cells to maintain homeostasis and leads to the development of CVD (15). Additionally, the premature offspring of women with preeclampsia had changes in their endothelial function from early life and a high risk to develop hypertension later (10).
The impact of de novo HDP on the offspring's endothelial function has just started to be valued. Most reviews highlight the effect of de novo HDP on maternal endothelial function, while the review on de novo HDP's effect on the offspring is limited, particularly the ones involving clinical studies. Therefore, this systematic review is aimed to evaluate the current database related to the outcome of de novo HDP, which includes gestational hypertension and preeclampsia, on endothelial function of human offspring in utero, at birth and long term.
Method
Search strategy
We identified the relevant studies on the effect of de novo HDP on offspring endothelial function using three electronic databases; PubMed, Scopus and Medline which were assessed between 1976 and 2022. The search strategy involved a combination (“AND”) of the following three sets of keywords: (1) Hypertension in pregnancy OR pre-eclampsia OR maternal hypertension OR gestational hypertension; (2) endothelial OR endothelial function OR endothelial dysfunction OR endothelial cell; and (3) neonate OR neonatal OR offspring OR fetal OR children. During the search, an asterisk (*) was used in Scopus as a truncation sign to broaden the search to include various word endings. We also searched the list of references of the articles selected for relevant citations.
Inclusion and exclusion criteria
Studies that fulfill the following criteria were included: (i) studies that investigated the effects or association of de novo HDP (gestational hypertension or preeclampsia) on offspring endothelial function, (ii) human studies, (iii) studies published from 1976 to 2022, and (iv) articles published in English. The following studies were excluded: (i) studies that investigated the effects or association of chronic HDP on offspring endothelial function (ii) studies that associate CVD in neonates with congenital or other pathological changes unrelated to HDP (iii) review articles, meta-analyses, letters, newsletters, editorial, conference abstracts or case studies (iv) duplicated studies (v) animal studies and (iv) articles published in language other than English.
Screening of articles for eligibility and data extraction
Firstly, articles that did not match the inclusion criteria based solely on their titles were excluded. Then, the abstracts of the remaining articles were screened to exclude articles that did not match the inclusion criteria. Finally, the full text of the remaining articles was read and assessed completely. At least two reviewers assessed the articles. Discussion between reviewers was done to resolve any issues. The data were extracted using a data collection form. The following data were extracted from the selected studies: (1) study population, (2) gestational/offspring age, (3) parameters measured, (4) findings, and (5) conclusion.
Results
Studies selected
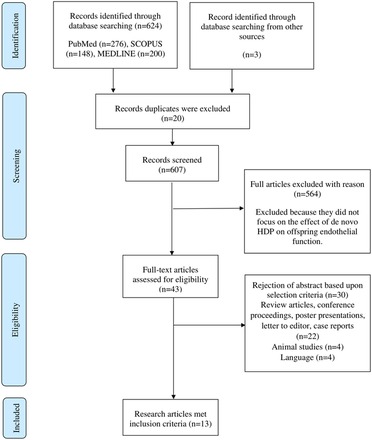
The initial literature search conducted in PubMed, Scopus and MEDLINE via EBSCOhost databases identified 685 potentially relevant articles. In the first phase, 643 articles were excluded as the articles were not related to the effect of de novo HDP on the offspring endothelial function based on the titles, abstracts, and keywords. Furthermore, 30 articles were excluded for several reasons: articles that are not original research, animal studies, articles not written in English, and duplicate articles. A hand-selected or snowball search was performed using Google Scholar, and we added another three relevant articles. After assessment by the reviewers, 13 articles were included in this review. The steps involved in the article selection process are shown in Figure 1. Based on the 13 included studies, the effects of de novo HDP on offspring endothelial function were classified into three major effects, namely, the impact on vasodilation and subclinical atherosclerosis (Table 1), inflammation (Table 2), and epigenetic regulation of endothelial functions (Table 3).
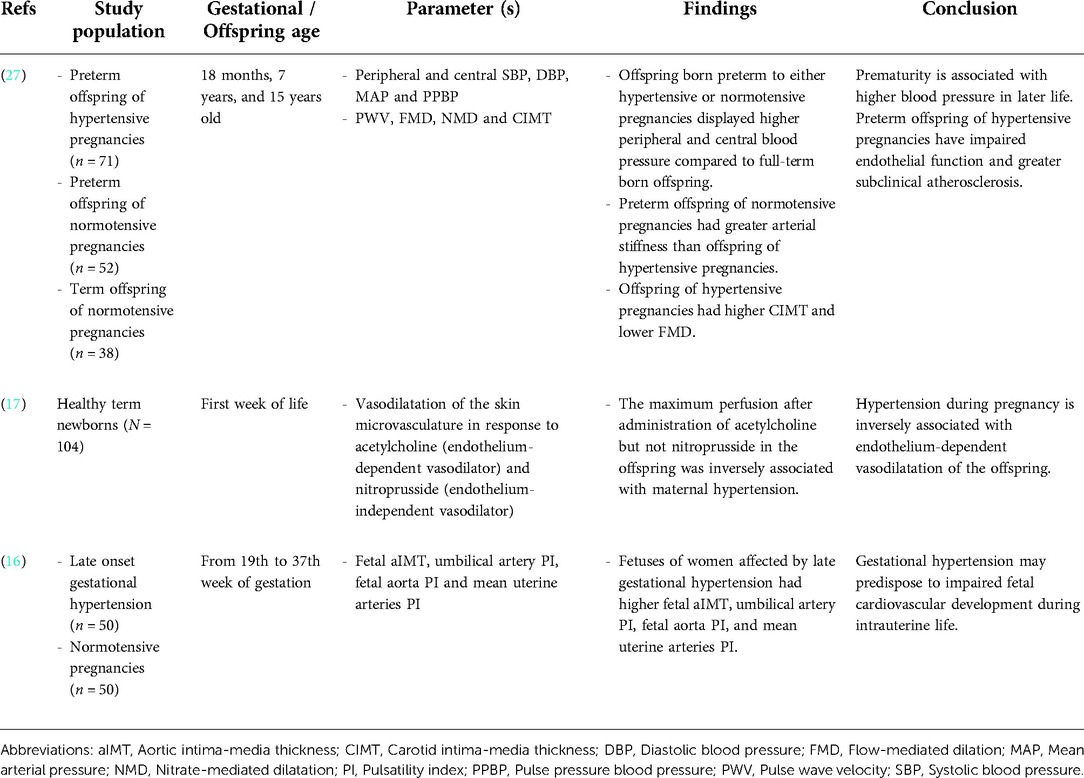
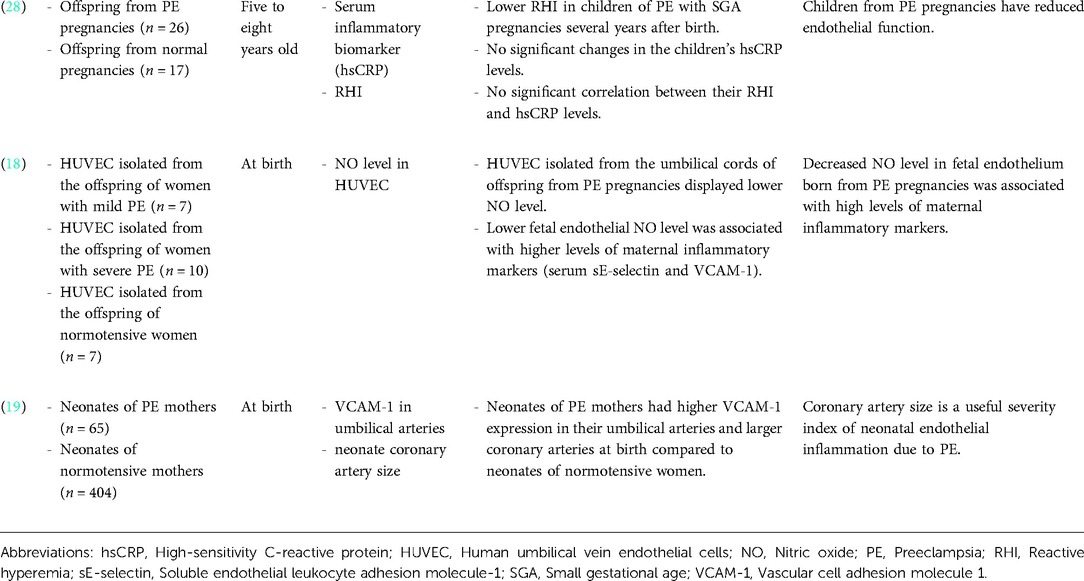
Table 1. Summary of the impact of de novo HDP on offspring vasodilation and subclinical atherosclerosis.
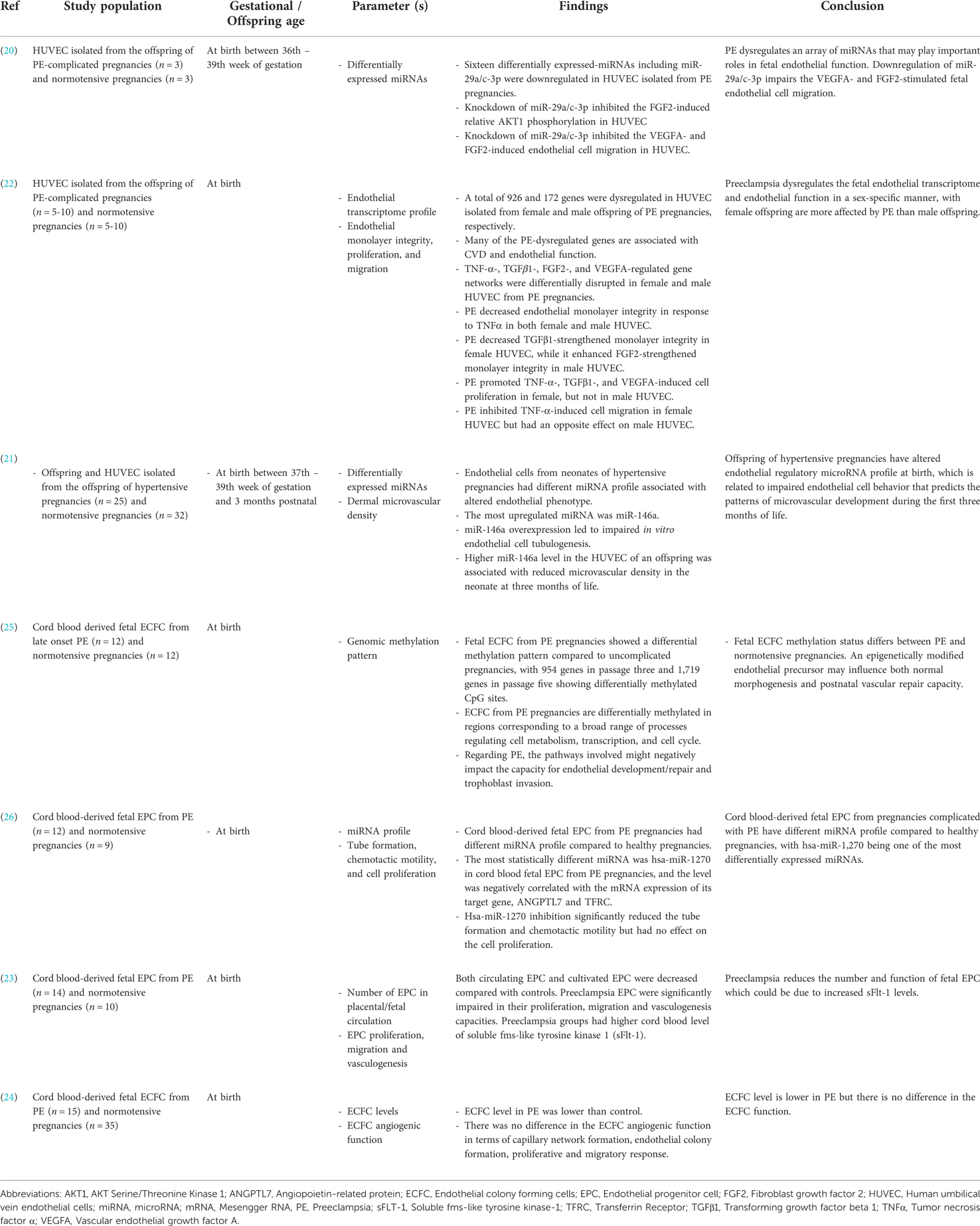
Table 3. Summary of the impact of de novo HDP on offspring epigenetic regulation of endothelial function.
Study characteristics
The study design, including the age of the offspring and the study population differed in several ways. The age of the offspring involved in the studies varied greatly. There were a few different life stages, namely prenatal age (19th-37th week of gestation) (16), neonates (aged less than four weeks old) (17–26), infants (aged 2–12 months old) (21), toddlers (aged 1–3 years old) (27), children (aged 5–12 years old) (27, 28) and adolescent (aged 12–19 years old) (27). The study population also varied. Nine of the studies involved the offspring of mothers with preeclampsia (18–20, 22–26, 28), while the remaining four studies involved the offspring of mothers with gestational hypertension (16, 17, 21, 27).
Impact of de novo HDP on offspring vasodilatation and subclinical atherosclerosis
A study by Touwslager et al. measured vasodilatation and perfusion of the offspring's skin microvasculature in response to acetylcholine (endothelium-dependent vasodilator) and nitroprusside (endothelium-independent vasodilator) using laser-Doppler device and iontophoresis. The maximum perfusion in the offspring's vasculature following acetylcholine administration, but not nitroprusside, was inversely associated with maternal HDP (17). In a 20-year follow-up study, offspring born preterm to either hypertensive or normotensive pregnancy had higher peripheral and central blood pressure than full-term born offspring (27). However, there were differences in their underlying vascular phenotype. Flow-mediated dilatation (FMD), which is the gold standard measurement for endothelial function, was lower in the preterm offspring of hypertensive pregnancies. The offspring also displayed a greater carotid intima-media thickness (IMT), a marker of subclinical atherosclerosis (27). In contrast, the preterm offspring of normotensive pregnancies had greater arterial stiffness than the offspring of hypertensive pregnancies (27). In another study, the fetus of mothers diagnosed with late gestational hypertension showed higher aortic IMT, umbilical artery pulsatility index (PI), fetal aorta PI and mean uterine arteries PI, which suggested subclinical atherosclerosis (16). In summary, the offspring from de novo HDP showed impaired vasodilatation and signs of subclinical atherosclerosis.
Impact of de novo HDP on offspring endothelial inflammation and function
Children of women with a history of severe preeclampsia and small for gestational age pregnancies had impaired endothelial function as evidenced by reduced reactive hyperemia index (RHI) measured five to eight years after birth. However, there was no significant change in their circulating inflammatory biomarker level (high-sensitivity C-reactive protein; hsCRP), and there was no significant correlation between their RHI and hsCRP level (28). Meanwhile, human umbilical vein endothelial cells (HUVEC) isolated from the offspring of mothers with preeclampsia showed lower nitric oxide (NO) levels. NO is an important vasodilator and a marker of endothelial function. This reduction in fetal endothelial NO level was associated with higher levels of maternal inflammatory markers (serum soluble endothelial leukocyte adhesion molecule-1 (sE-selectin) and vascular cell adhesion molecule 1 (VCAM-1)) (18). Furthermore, offspring coronary artery size is a useful parameter to assess the severity of neonate's endothelial inflammation and dysfunction due to maternal preeclampsia. It was demonstrated that the neonates of mothers with preeclampsia had higher VCAM-1 expression in their umbilical arteries and larger coronary arteries, suggesting that coronary artery size is an indicator of neonatal endothelial inflammation (19). Taken together, the studies suggested that de novo HDP promotes inflammation which contributes to offspring's endothelial dysfunction.
Impact of de novo HDP on offspring epigenetic regulation of endothelial function
Investigations on the epigenetic modifications of preeclampsia on offspring endothelial cells and endothelial progenitor cells (EPC) revealed an array of differentially expressed microRNAs (miRNAs or miRs), including hsa-miR-1270 (26), miR-29a/c-3p (20) and miR-146a (21). EPC are circulating cells that play an essential role in maintaining vascular function. Cord blood-derived fetal EPC from preeclampsia pregnancies had a different miRNA profile compared to healthy pregnancies. RNA sequencing analysis showed significant downregulation of hsa-miR-1270 in the EPC (26). The level of hsa-miR-1270 was negatively correlated with the mRNA expression of its target gene, angiopoietin-related protein 7 (ANGPTL7) and transferrin receptor (TFRC). ANGPTL7 and TFRC are important proteins involved in angiogenesis and cellular iron uptake, respectively. Furthermore, inhibition of hsa-miR-1270 decreased tube formation capacity and chemotactic motility of the EPC (26). Taken together, the results suggested that preeclampsia caused overexpression of hsa-miR-1270, which led to impaired angiogenic function of the offspring EPC. This corresponds with another study that showed preeclampsia reduced the number and function of fetal EPC, which could be due to an increase in the anti-angiogenic factor; soluble fms-like tyrosine kinase 1 (sFlt-1) (23).
Meanwhile, downregulation of miR-29a/c-3p was observed in HUVEC isolated from the offspring of preeclampsia pregnancies. MiR-29a/c-3p inhibition impaired vascular endothelial growth factor A (VEGFA) and fibroblast growth factor 2 (FGF-2)-induced endothelial cell migration (20). Additionally, upregulation of miR-146a was observed in HUVEC isolated from the offspring of hypertensive pregnancies. Overexpression of miR-146a led to impaired endothelial tubulogenesis. A higher miR-146a expression was also associated with reduced microvascular density in the offspring at three months of life, which might increase the risk of developing hypertension later (21). In short, the findings indicate that preeclampsia altered the expression of an array of miRNAs, which caused significant dysregulation of the offspring endothelial function.
Endothelial colony forming cells (ECFC), which is a proliferative subtype of EPC, was found to be reduced in the cord blood of offspring from preeclampsia pregnancies (24). Genomic methylation pattern of fetal ECFC from preeclampsia pregnancies also differed from normal pregnancies, with 954 genes in passage three and 1,719 genes in passage five showing differentially methylated CpG sites (25). This difference in genomic methylation pattern of ECFC in preeclampsia might negatively impact the capacity of offspring endothelial development and repair functions (25). In another study, endothelial transcriptome profiling revealed dysregulation of 926 and 172 genes in HUVEC isolated from the male and female neonate of preeclampsia pregnancies, respectively. Many of the dysregulated gene networks are associated with CVD and endothelial function, such as tumor necrosis factor α (TNF-α), transforming growth factor beta-1 (TGFβ1), FGF-2 and VEGFA (22). Further functional analysis showed a weakening of the endothelial monolayer integrity in HUVEC from female offspring in response to TNF-α. In the meantime, exposure to FGF-2 strengthened cell monolayer integrity in both male and female offspring endothelial cells. Preeclampsia also promoted TNF-α-, TGFβ1-, and VEGFA-induced proliferation of HUVEC from female offspring, but not in HUVEC from male offspring. In addition, preeclampsia inhibited TNF-α-induced migration of HUVEC of female offspring, with an opposite effect on HUVEC of male offspring (22). In summary, preeclampsia dysregulates the fetal endothelial transcriptome and endothelial function in a sex-specific manner, with female offspring more severely affected by preeclampsia than male offspring.
Discussion
De novo HDP disrupted offspring endothelial function by causing impaired vasodilatation, subclinical atherosclerosis formation, inflammation, and epigenetic dysregulation of endothelial functions (Figure 2).
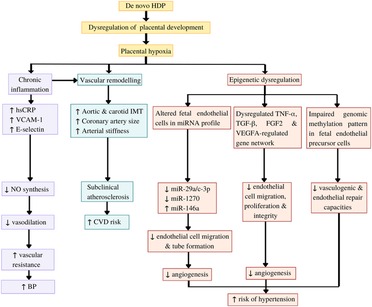
Figure 2. Potential mechanisms underlying the impact of de novo hypertensive disorders of pregnancy (HDP) on offspring endothelial function. HDP leads to dysregulation of placental development and placental hypoxia. Hypoxia induces chronic inflammation, which inhibits nitric oxide (NO) synthesis, resulting in impaired vasodilatation and increased blood pressure (BP). Both hypoxia and chronic inflammation cause vascular remodelling, which can be detected as subclinical atherosclerotic changes. Furthermore, hypoxia leads epigenetic dysregulation of the offspring endothelial function by causing alteration in the offspring endothelial cells and endothelial precursor cells microRNA (miRNA) profile, dysregulation of several gene networks related to endothelial functions, and impaired genomic methylation pattern in the endothelial precursor cells. These epigenetic dysregulations eventually caused impaired angiogenesis and endothelial repair capacities, which increase the risk of the offspring to develop hypertension (HPT) in later life. CVD: Cardiovascular diseases, FGF2: Fibroblast growth factor 2, hsCRP: high-sensitivity C-reactive protein, IMT: intima-media thickness, TGFβ1: Transforming growth factor beta-1, TNFα: tumour necrosis factor α, VCAM-1: vascular cell adhesion molecule 1, VEGFA: vascular endothelial growth factor A.
HDP impairs vasodilatation and promotes subclinical atherosclerosis in the offspring
The endothelium is a thin membrane that lines the vascular network and plays an important role in vascular homeostasis. Maintaining the endothelium's structural and functional integrity is essential, particularly in balancing vasodilatation and vasoconstriction. Evidence suggests that offspring born to de novo HDP had impaired endothelium-dependent vasodilatation as measured by FMD (27), This result was in accordance with other studies done specifically in preeclampsia pregnancies (29, 30), indicating the possible increase in total peripheral resistance and blood pressure in the offspring. FMD is the gold standard method to measure endothelial function clinically. It is an ultrasound-based, non-invasive technique that measures endothelium-dependent vasodilatation in response to shear stress (31). However, further mechanistic studies are needed to fully understand the underlying mechanisms that contribute to the impaired vasodilatation in the offspring.
Microvascular structural changes can be seen in the offspring of mothers with HDP after birth due to heritable predisposition from the mothers or a reflection of intrauterine endothelial dysfunction (32). The aortic and carotid IMT are the markers of subclinical atherosclerosis, whereby IMT refers to the distance between the leading edge of the intimal interface and the leading edge of the media-adventitia interface at the outer part of the vessel (33). IMT is a well-established independent predictor of future CVD risk and previous studies have demonstrated a higher carotid IMT in children and adults with cardiovascular risk factors such as hypercholesterolemia (34), smoking (35) and family history of CVD (36). Fetuses of mothers with late gestational hypertension have higher aortic IMT detected at 29th -32nd weeks of gestation (16). This indicates vascular remodelling in utero (16). However, whether the increase in carotid IMT in HDP offspring is persistent or temporary is a matter of debate. A 20-year follow up study showed higher carotid IMT in the offspring of HDP (27). However, another study showed that the increase in offspring carotid IMT due to preeclampsia was attenuated at 18 months of life (37). Further studies are needed to address the underlying factors contributing to the difference. In short, de novo HDP leads to vascular dysfunction in the offspring by impairing the endothelium-dependent vasodilatation and inducing vascular remodeling such as the subclinical atherosclerosis formation.
HDP promotes endothelial inflammation and dysfunction
In preeclampsia, chronic immune system activation leads to the release of proteins and factors by the placenta. This is part of the inflammatory response that promotes hypertension and proteinuria (38). The factors released include pro-inflammatory cytokines such as hsCRP and TNF-α, which have been reported to be elevated in mothers with preeclampsia (39). The human placenta secretes TNF-α under hypoxia-reoxygenation conditions in vitro (40), mimicking the fluctuation of oxygen levels observed in preeclampsia. TNF-α induces the expression of inflammatory cytokines such as interleukin (IL)-6, IL-8 and monocyte chemotactic protein (MCP-1) and the cellular adhesion molecules including VCAM-1, intercellular adhesion molecule 1 (ICAM-1), E-selectin and epithelial-cadherin (E-cadherin) (41). Furthermore, preeclampsia dysregulates the inducible nitric oxide synthase (iNOS) signaling, thus implicating higher inflammatory responses which potentially lead to severely impaired endothelial functions (22).
High maternal serum VCAM-1 and E-selectin levels in preeclampsia pregnancies was associated with 60%–70% decrease in NO level in the fetal endothelium (18). NO is a marker of endothelial function that acts as a vasodilator, thus reducing vascular resistance and blood pressure. Studies have shown that inhibition of NO synthesis results in reduced blood flow in human (18, 42, 43), vasoconstriction and increased blood pressure in animal offspring (44). This is further supported by another study that showed the RHI of preeclampsia offspring was reduced even at five to eight years after birth (28). RHI is a non-invasive method to measure endothelial function in which the response is partially mediated by endothelium-derived NO. The findings suggest that inflammatory response in preeclampsia inhibits NO synthesis, thus causing endothelial dysfunction and increased risk of hypertension.
HDP causes epigenetic dysregulation of endothelial functions
Epigenetic plays an important role in the developmental origins of health and diseases, where its modifications would be potential mechanisms of the altered environment to be translated into disease development. MiRNAs are a class of noncoding RNAs that regulate essential cellular functions (45), including endothelial cell proliferation, migration, apoptosis, and angiogenesis (46). Previous studies demonstrated that stress conditions including in utero stress exposure and inflammation could alter endothelial miRNAs expression (20, 21, 47).
The altered miRNAs expression in de novo HDP impacts fetal endothelial functions through gene dysregulation of many signaling pathways. These include the estrogen signaling pathway (48), TGFβ signaling pathway (49), focal adhesion kinase pathway (50), phosphoinositide-3-kinase-protein kinase B (PI3K-Akt) signaling pathway (51), and also through impairment of VEGFA and FGF2 -stimulated angiogenesis (20). Moreover, the alteration of endothelial miRNAs in preeclampsia is not only associated with impaired endothelial cell function and behavior, but it also disrupts angiogenesis and microvascular development in infants as early as the first three months of life (20, 21). It was postulated that impaired angiogenesis in utero and early in life predisposes to hypertension development. However, on the brighter side, it is possible that microRNA modification and manipulation could restore the impaired angiogenesis, hence reducing the risk of the offspring to develop hypertension in later life (21).
An epigenetically modified endothelial precursor cell may influence both normal morphogenesis of endothelial cells in utero and postnatal vascular repair capacity, hence contributing to CVD risk in the offspring (25). EPC and ECFC are endothelial precursor cells originating from the bone marrow stem cells and human umbilical cord blood. Upon maturation, they become mature endothelial cells and release pro-angiogenic factors like VEGF and placental growth factor (PlGF), which enhance vasculogenesis and endothelial repair (52). Studies found that the numbers of EPC and ECFC in the offspring of preeclampsia mothers were reduced (23, 24), and the EPC were also found to be more senescent, consequently reducing their functional ability (53, 54). Altered number and function of fetal EPC in preeclampsia were associated with increased arterial stiffness (52), which is a risk factor for CVD. Besides, preeclampsia resulted in a different methylation pattern in fetal ECFC, with several differentially methylated regions identified in vascular-related genes (25). DNA methylation plays a pivotal role in regulating biological processes underlying CVD such as atherosclerosis, inflammation and hypertension (55–57). This suggests that epigenetic modifications in HDP may increase the risk of transgenerational vascular disease (58). Besides, these findings open the opportunity to introduce novel epigenetic targets for further experimental study.
Strengths and limitations of the study
To the best of our knowledge, this is the first article that systematically reviewed current evidence related to the effect of de novo HDP on offspring endothelial function. The systematic literature search ensures all relevant articles were identified. Studies involving offspring from the prenatal period until adulthood were included, which enable us to understand the effects of de novo HDP on offspring endothelial function at different stages of life. However, the current review is not without its limitations. Only one of the studies involved the offspring of mothers with late onset gestational hypertension, while the rest of the studies did not specify the disease stage (i.e., early, or late). Therefore, comparison on the effect of early onset vs. late onset HDP on the offspring endothelial function could not be made. Comparing the effect of different stages of HDP on the offspring endothelial function is an interesting area to be explored. Furthermore, this review was only focused on human studies, while animal studies were excluded. Since animal studies are important tools for investigating how diseases in pregnancy can affect the offspring, further reviews that include animal models of hypertension in pregnancy are needed in the future.
Conclusion
De novo HDP has a deleterious impact on offspring endothelial function. This is most likely attributed to impaired vasodilation, subclinical atherosclerosis formation, inflammation, and dysregulated epigenetic modification of endothelial functions. Endothelial dysfunction in the offspring of de novo HDP may contribute to their risk of developing CVD in later life. A cohort study involving this group of individuals is beneficial to establish the link between endothelial dysfunction in the offspring of HDP with CVD occurrence in adulthood for prevention and early intervention in the future.
Author contributions
AAH and AU contributed to the framework and design of the manuscript. AU, AA, NM, SZA and AAH drafted the manuscript. AS and SJA contributed to the preparation of tables, figures, and figure legends. AU, AA, SZA, and AAH critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Ministry of Higher Education Malaysia Fundamental Research Grant Scheme (FRGS/1/2019/SKK08/UKM/03/2) and Faculty of Medicine, Universiti Kebangsaan Malaysia (Grant code: FF-2020-147).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC Pregnancy Childbirth. (2021) 21(1):1–10. doi: 10.1186/s12884-021-03809-2
2. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. (2018) 13:291–310. doi: 10.1016/j.preghy.2018.05.004
3. ACOG. Gestational hypertension and preeclampsia. Obstet Gynecol. (2019) 133(1):1. doi: 10.1097/AOG.0000000000003018
4. Esch JJAV, Heijst AFV, Haan AFJD, Heijden OWHVD. Early-onset preeclampsia is associated with perinatal mortality and severe neonatal morbidity. J Mater Fetal Neonatal Med. (2017) 30(23):2789–94. doi: 10.1080/14767058.2016.1263295
5. Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med. (2019) 8(10):1625. doi: 10.3390/jcm8101625
6. Alsnes IV, Vatten LJ, Fraser A, Bjørngaard JH, Rich-Edwards J, Romundstad PR, et al. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT study (nord-trøndelag health study) in Norway. Hypertension. (2017) 69(4):591–98. doi: 10.1161/HYPERTENSIONAHA.116.08414
7. Jansen MAC, Pluymen LPM, Dalmeijer GW, Groenhof TKJ, Uiterwaal CSPM, Smit HA, et al. Hypertensive disorders of pregnancy and cardiometabolic outcomes in childhood: a systematic review. Eur J Prev Cardiol. (2019) 26(16):1718–47. doi: 10.1177/2047487319852716
8. Cruz-Lemini M, Crispi F, Valenzuela-Alcaraz B, Figueras F, Gómez O, Sitges M, et al. A fetal cardiovascular score to predict infant hypertension and arterial remodeling in intrauterine growth restriction. Am J Obstet Gynecol. (2014) 210(6):552.e1–552.e22. doi: 10.1016/j.ajog.2013.12.031
9. Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M, Pandey D, Health G. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. (2014) 5:372. doi: 10.3389/fphys.2014.00372
10. Frost AL, Suriano K, Aye CYL, Leeson P, Lewandowski AJ. The immediate and long-term impact of preeclampsia on offspring vascular and cardiac physiology in the preterm infant. Front Pediatr. (2021) 9:1–6. doi: 10.3389/fped.2021.625726
11. Ziganshina MM, Yarotskaya EL, Bovin NV, Sukhikh GT. Endothelial dysfunction as a consequence of endothelial glycocalyx damage: a role in the pathogenesis of preeclampsia. endothelial dysfunction - old concepts and new challenges [Internet]. IntechOpen. (2018). doi: 10.5772/intechopen.75043. [cited on 2022 Oct 13]
12. Hariharan N, Shoemaker A, Wagner S. Pathophysiology of hypertension in preeclampsia. Clin Pract. (2016) 13(2):33–7. doi: 10.4172/clinical-practice.100091
13. Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. (2019) 15(5):275–89. doi: 10.1038/s41581-019-0119-6
14. Kornacki J, Wirstlein P, Wender-Ozegowska E. Markers of endothelial injury and dysfunction in early- and late-onset preeclampsia. Life. (2020) 10(10):1–10. doi: 10.3390/life10100239
15. Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis. (2014) 25(8):713–24. doi: 10.1097/MCA.0000000000000178
16. Visentin S, Londero AP, Bellamio B, Giunta G, Cosma C, Faggian D, et al. Fetal endothelial remodeling in late-onset gestational hypertension. Am J Hypertens. (2016) 29(2):273–79. doi: 10.1093/ajh/hpv103
17. Touwslager RNH, Houben AJHM, Gielen M, Zeegers MP, Stehouwer CDA, Zimmermann LJ, et al. Endothelial vasodilatation in newborns is related to body size and maternal hypertension. J Hypertens. (2012) 30(1):124–31. doi: 10.1097/HJH.0b013e32834d75c6
18. Veas CJ, Aguilera VC, Muñoz IJ, Gallardo VI, Miguel PL, González MA, et al. Fetal endothelium dysfunction is associated with circulating maternal levels of SE-selectin, SVCAM1, and SFlt-1 during Pre-eclampsia. J Mater-Fetal Neonatal Med. (2011) 24(11):1371–77. doi: 10.3109/14767058.2011.556204
19. Lin IC, Hsu TY, Tain YL, Tsai CC, Huang HC, Lai YJ, et al. Coronary dilatation and endothelial inflammation in neonates born to mothers with preeclampsia. J Pediatr. (2021) 228:58–65.e3. doi: 10.1016/j.jpeds.2020.07.059
20. Zhou C, Zou QY, Li H, Wang RF, Liu AX, Magness RR, et al. Preeclampsia downregulates MicroRNAs in fetal endothelial cells: roles of MiR-29a/c-3p in endothelial function. J Clin Endocrinol Metab. (2017) 102(9):3470–79. doi: 10.1210/jc.2017-00849
21. Yu GZ, Reilly S, Lewandowski AJ, Aye CYL, Simpson LJ, Newton LD, et al. Neonatal microRNA profile determines endothelial function in offspring of hypertensive pregnancies. Hypertension. (2018) 72(4):937–45. doi: 10.1161/HYPERTENSIONAHA.118.11343
22. Zhou C, Yan Q, Zou QY, Zhong XQ, Tyler CT, Magness RR, et al. Sexual dimorphisms of preeclampsia-dysregulated transcriptomic profiles and cell function in fetal endothelial cells. Hypertension. (2019) 74(1):154–63. doi: 10.1161/HYPERTENSIONAHA.118.12569
23. Xia L, Zhou XP, Zhu JH, Xie XD, Zhang H, Xing X, et al. Decrease and dysfunction of endothelial progenitor cells in umbilical cord blood with maternal Pre-eclampsia. J Obstet Gynaecol Res. (2007) 33(4):465–74. doi: 10.1111/j.1447-0756.2007.00555
24. Muñoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernández JM, Dominguez-Simeon MJ, et al. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. (2014) 64(1):165–71. doi: 10.1161/HYPERTENSIONAHA.113.03058/-/DC1
25. Brodowski L, Zindler T, Hardenberg SV, Schröder-Heurich B, Kaisenberg CSV, Frieling H, et al. Preeclampsia-associated alteration of DNA methylation in fetal endothelial progenitor cells. Front Cell Dev Biol. (2019) 7:1–14. doi: 10.3389/fcell.2019.00032
26. Brodowski L, Schröder-Heurich B, Hardenberg SV, Richter K, Kaisenberg CSV, Dittrich-Breiholz O, et al. MicroRNA profiles of maternal and neonatal endothelial progenitor cells in preeclampsia. Int J Mol Sci. (2021) 22(10):5320. doi: 10.3390/ijms22105320
27. Lazdam M, Horra ADL, Pitcher A, Mannie Z, Diesch J, Trevitt C, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. (2010) 56(1):159–65. doi: 10.1161/HYPERTENSIONAHA.110.150235
28. Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension. (2011) 58(1):63–9. doi: 10.1161/HYPERTENSIONAHA.111.172387
29. Henriques A, Brandão F, Cabral MA, Leite HV, Carlos A, Cabral V. Endothelial function, uterine perfusion and central flow in pregnancies complicated by preeclampsia. Arq Bras Cardiol. (2012) 99(4):931–35. doi: 10.1590/S0066-782X2012005000087
30. Porto LB, Brandão AHF, Leite HV, Cabral ACV. Longitudinal evaluation of uterine perfusion. Endothelial Function and Central Blood Flow in Early Onset Pre-Eclampsia.” Pregnancy Hypertens. (2017) 10(8):161–64. doi: 10.1016/j.preghy.2017.08.005
31. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-Mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. (2013) 168(1):344–51. doi: 10.1016/j.ijcard.2012.09.047
32. Stark MJ, Clifton VL, Wright IMR. Neonates born to mothers with preeclampsia exhibit sex-specific alterations in microvascular function. Pediatr Res. (2009) 65(3):291–95. doi: 10.1203/PDR.0b013e318193edf1
33. Gomez-Roig MD, Mazarico E, Valladares E, Guirado L, Fernandez-Arias M, Vela A. Aortic intima-Media thickness and aortic diameter in small for gestational age and growth restricted fetuses. PLoS ONE. (2015) 10(5):e0126842. doi: 10.1371/journal.pone.0126842
34. Pauciullo P, Iannuzzi A, Sartorio R, Irace C, Covetti G, Costanzo AD, et al. Increased intima-Media thickness of the common carotid artery in hypercholesterolemic children. Arterioscler Thromb Vasc Biol. (1994) 14(7):1075–79. doi: 10.1161/01.ATV.14.7.1075
35. Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-Media thickness in adulthood: the cardiovascular risk in young Finns study. J Am Med Assoc. (2003) 290(17):2277–83. doi: 10.1001/jama.290.17.2277
36. Barra S, Gaeta G, Cuomo S, Guarini P, Foglia MC, Capozzi G, et al. Early increase of carotid intima-Media thickness in children with parental history of premature myocardial infarction. Heart. (2009) 95(8):642–45. doi: 10.1136/hrt.2008.142836
37. Yuan LJ, Xue D, Duan YY, Cao TS, Yang HG, Zhou N. Carotid arterial intima-Media thickness and arterial stiffness in Pre-eclampsia: analysis with a radiofrequency ultrasound technique. Ultrasound Obstet Gynecol. (2013) 42(6):644–52. doi: 10.1002/uog.12409
38. Geldenhuys J, Rossouw TM, Lombaard HA, Ehlers MM, Kock MM. Disruption in the regulation of immune responses in the placental subtype of preeclampsia. Front Immunol. Frontiers Media S.A. (2018) 9:1659. doi: 10.3389/fimmu.2018.01659
39. Lee DK, Nevo O. Tumor necrosis factor alpha expression is increased in maternal microvascular endothelial cells in preeclampsia. Hypertens Pregnancy. (2021) 00(00):1–9. doi: 10.1080/10641955.2021.1921794
40. Hung THD, Charnock-Jones S, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-α from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. (2004) 164(3):1049–61. doi: 10.1016/S0002-9440(10)63192-6
41. Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen Species and antioxidants. Antioxid Redox Signal. Mary Ann Liebert Inc. (2011) 15:1607. doi: 10.1089/ars.2010.3522
42. Haynes WG, Noon JP, Walker BR, Webb DJ. Inhibition of nitric oxide synthesis increases blood pressure in healthy humans. J Hypertens. (1993) 11(12):1375–80. doi: 10.1097/00004872-199312000-00009
43. Lepori M, Sartori C, Trueb L, Owlya R, Nicod P, Scherrer U. Haemodynamic and sympathetic effects of inhibition of nitric oxide synthase by systemic infusion of N(G)-monomethyl-L-arginine into humans are dose dependent. J Hypertens. (1998) 16(4):519–23. doi: 10.1097/00004872-199816040-00013
44. Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J Vasc Res. (2009) 46(3):229–39. doi: 10.1159/000166390
45. Prieto DMM, Markert UR. MicroRNAs in pregnancy. J Reprod Immunol. (2011) 88(2):106–11. doi: 10.1016/j.jri.2011.01.004
46. Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun. (2009) 386(4):549–53. doi: 10.1016/j.bbrc.2009.06.075
47. Suárez Y, Wang C, Manes TD, Pobe JS. TNF-induced MiRNAs regulate TNF-induced expression of E- selectin and ICAM-1 on human endothelial cells: feedback control of inflammation. J Immunol. (2010) 184(1):21–5. doi: 10.4049/jimmunol.0902369
48. Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension. (2013) 61(2):480–87. doi: 10.1161/HYPERTENSIONAHA.111.201624
49. Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. MiR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther. (2014) 22(5):974–85. doi: 10.1038/mt.2014.25
50. Quadri SK, Bhattacharjee M, Parthasarathi K, Tanita T, Bhattacharya J. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem. (2003) 278(15):13342–49. doi: 10.1074/jbc.M209922200
51. Wang K, Jiang YZ, Chen DB, Zheng J. Hypoxia enhances FGF2- and VEGF-stimulated human placental artery endothelial cell proliferation: roles of MEK1/2/ERK1/2 and PI3K/AKT1 pathways. Placenta. (2009) 30(12):1045–51. doi: 10.1016/j.placenta.2009.10.007
52. Berezin AE. The endothelial progenitor cell dysfunction in hypertension: the diagnostic and predictive values. Vessel Plus. (2018) 2:22. doi: 10.20517/2574-1209.2018.23
53. Hookham MB, Ali IHA, O’Neill CL, Hackett E, Lambe MH, Schmidt T, et al. Hypoxia-Induced responses by endothelial colony-forming cells are modulated by placental growth factor. Stem Cell Res Ther. (2016) 7(1):1–12. doi: 10.1186/s13287-016-0430-0
54. Hwang HS, Maeng YS, Park YW, Koos BJ, Kwon YG, Kim YH. Increased senescence and reduced functional ability of fetal endothelial progenitor cells in pregnancies complicated by preeclampsia without intrauterine growth restriction. Am J Obstet Gynecol. (2008) 199(3):259.e1–e7. doi: 10.1016/j.ajog.2008.06.060
55. Baccarelli A, Rienstra M, Benjamin EJ. Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ: Cardiovasc Genet. (2010) 3(6):567–73. doi: 10.1161/CIRCGENETICS.110.958744
56. Turunen MP, Aavik E, Ylä-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. (2009) 1790(9):886–91. doi: 10.1016/j.bbagen.2009.02.008
57. Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V, et al. Epigenetic control of 11 Beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. (2008) 199(2):323–27. doi: 10.1016/j.atherosclerosis.2007.11.029
Keywords: hypertensive disorders of pregnancy, preeclampsia, endothelial function, offspring, cardiovascular disease
Citation: Abdull Sukor AN, Ankasha SJ, Ugusman A, Aminuddin A, Mokhtar NM, Zainal Abidin S, Ahmad MF and Hamid A (2022) Impact of offspring endothelial function from de novo hypertensive disorders during pregnancy: An evidence-based review. Front. Surg. 9:967785. doi: 10.3389/fsurg.2022.967785
Received: 13 June 2022; Accepted: 29 September 2022;
Published: 7 November 2022.
Edited by:
Hamizah Ismail, International Islamic University Malaysia, MalaysiaReviewed by:
Natasha De Alwis, The University of Melbourne, AustraliaChoy Ker Woon, Universiti Teknologi MARA, Malaysia
© 2022 Abdull Sukor, Ankasha, Ugusman, Aminuddin, Mokhtar, Zainal Abidin, Ahmad and Hamid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adila A Hamid YWRpbGFoYW1pZEBwcHVrbS51a20uZWR1Lm15 Azizah Ugusman ZHIuYXppemFoQHBwdWttLnVrbS5lZHUubXk=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
 Aslah Nabilah Abdull Sukor
Aslah Nabilah Abdull Sukor Sheril June Ankasha1
Sheril June Ankasha1 Azizah Ugusman
Azizah Ugusman Amilia Aminuddin
Amilia Aminuddin Norfilza Mohd Mokhtar
Norfilza Mohd Mokhtar Shahidee Zainal Abidin
Shahidee Zainal Abidin Mohd Faizal Ahmad
Mohd Faizal Ahmad