
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 18 August 2022
Sec. Orthopedic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.962425
This article is part of the Research Topic Diagnostics and Treatment for Bone and Joint Infections View all 10 articles
A correction has been applied to this article in:
Corrigendum: Risk factors for tuberculous or nontuberculous spondylitis after percutaneous vertebroplasty or kyphoplasty in patients with osteoporotic vertebral compression fracture: A case-control study
 Bo-Wen Zheng1,2,3†
Bo-Wen Zheng1,2,3† Fu-Sheng Liu1,2,†
Fu-Sheng Liu1,2,† Bo-Yv Zheng4
Bo-Yv Zheng4 Hua-Qing Niu4
Hua-Qing Niu4 Jing Li2
Jing Li2 Guo-Hua Lv2
Guo-Hua Lv2 Ming-Xiang Zou1
Ming-Xiang Zou1 Zhun Xu1*
Zhun Xu1*
Objectives: The contributing factors for spondylitis after percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP) remain unclear. Here, we sought to investigate the factors affecting spondylitis occurrence after PVP/PKP. We also compared the clinical characteristics between patients with tuberculous spondylitis (TS) and nontuberculous spondylitis (NTS) following vertebral augmentation.
Methods: Literature searches (from January 1, 1982 to October 16, 2020) using MEDLINE, EMBASE, Google Scholar and Web of science databases were conducted to identify eligible studies according to predefined criteria. The local database was also retrospectively reviewed to include additional TS and NTS patients at our center.
Results: Thirty studies from the literature and 11 patients from our local institute were identified, yielding a total of 23 TS patients and 50 NTS patients for analysis. Compared with NTS group, patients in the TS group were more likely to have a history of trauma before PVP/PKP treatment. Univariate analyses of risk factors revealed pulmonary tuberculosis and diabetes were significant factors for TS after PVP/PKP. Analyzing NTS, we found obesity, a history of preoperative trauma, urinary tract infection, diabetes and multiple surgical segments (≥2) were significantly associated with its occurrence following PVP/PKP treatment. Multivariate logistic analyses showed a history of pulmonary tuberculosis and diabetes were independent risk factors for TS after PVP/PKP, while diabetes and the number of surgically treated segments independently influenced NTS development.
Conclusions: A history of pulmonary tuberculosis and diabetes were independent risk factors for TS. For NTS, diabetes and the number of surgically treated segments significantly influenced the occurrence of postoperative spinal infection. These data may be helpful for guiding risk stratification and preoperative prevention for patients, thereby reducing the incidence of vertebral osteomyelitis after PVP/PKP.
Percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP) is currently widely used for the treatment of osteoporotic vertebral compression fractures (OVCFs) (1). Although it is relatively safe and effective, PVP/PKP can still cause complications in some situations. Among them, bone cement leakage is most frequently encountered and may lead to neurological dysfunction or even pulmonary embolism. Generally, infection of the vertebral body treated with subsequent PVP/PKP is rare, with an incidence of less than 1% (2). The most common type of spondylitis is purulent infection caused by bacteria (3). In addition, cases of tuberculous spondylitis (TS) after bone cement infusion have also been documented in the literature (2, 3). TS, the most common and severe form of bone tuberculosis, accounts for 50% of extrapulmonary tuberculosis cases and its incidence is very low in developed Western countries (4), while in developing countries, probably due to the lack of medical equipment (e.g., imaging systems and examination laboratories) and inadequate levels of diagnosis and treatment, the mortality rate from tuberculosis is much higher than in developed Western countries (4).
Currently, the cause of spondylitis after PVP/PKP remains unclear. Studies have demonstrated that the pathogen may already exist in patients before PVP/PKP treatment, the process of bone cement injection and vertebral augmentation initiates the occurrence of subsequent spinal infections (2). For example, infections involving the visceral organs (such as urinary tract infection, cholecystitis, meningitis) or pathogen adhesion in the skin may contribute to nontuberculous spondylitis (NTS) after PVP/PKP (2, 5, 6). Regarding TS following PVP/PKP surgery, some studies have proven that a history of pulmonary tuberculosis is closely related to the occurrence of spondylitis (2, 7, 8).This may be due to the presence of tuberculosis bacteria in recovered pulmonary tuberculosis patients, and PVP/PKP may allow these quiescent tuberculosis bacteria to spread around the bone cement, leading to infection (2).
Noticeably, patients undergoing PVP/PKP therapy generally have an advanced age, and infectious spondylitis in this patient group tends to progress rapidly once it develops (9), which may pose a challenge for subsequent treatment (usually requiring traumatic debridement surgery and long-term use of antibacterial drugs with side effects (2, 10, 11), and it can even lead to catastrophic consequences. Therefore, it is necessary to summarize the influencing factors of secondary vertebral infection after PVP/PKP to guide prevention approaches to reduce postoperative spinal infections, thus improving the clinical outcome of patients. In this study, we aimed to investigate the factors affecting spondylitis occurrence after PVP/PKP. We also compared the clinical characteristics between patients with TS and NTS.
A literature search through the MEDLINE, EMBASE, Google Scholar and Web of science databases was conducted to identify eligible studies from January 1, 1982 to October 16, 2020. The keywords or combinations used for the search were (“spondylitis” or “spondylodiscitis” or “osteomyelitis” or “bacterial” or “fungal” or “pyogenic” or “tuberculosis” or “bacterial spondylitis” or “pyogenic spondylitis” or “tuberculous spondylitis” or “tubercular spondylitis” or “mycobacteria tuberculosis” or “TB” or “Pott’s” or “infection” or “infectious”) and (“spine” or “spinal” or “vertebral” or “cervical spine” or “thoracic spine “or “lumbar spine”) and (“VP” or “PVP” or “PKP” or “vertebroplasty” or “kyphoplasty” or “augmentation” or “percutaneous vertebroplasty” or “percutaneous kyphoplasty”). To obtain comprehensive results and to avoid omissions, no restrictions were applied for the above keywords. Moreover, we also manually reviewed the references of the included studies to find any potential documents that met the inclusion criteria. The detailed process for the literature search is shown in Figure 1. We included OVCF (It is directly described in the literature, and no specific inspection method is described) patients who developed a vertebral infection (including TS and NTS) after undergoing PVP/PKP surgery. The exclusion criteria of the study included: failing to offer any evidence of etiology or histopathology for diagnosis confirmation (for NTS, the diagnosis should be based on the pathogenic growth observed in the culture of infected tissues, while a diagnosis of TS requires detection of Mycobacterium tuberculosis in the tissue culture, or positive acid-fast staining or pathology findings showing caseous necrosis and/or granulomatous inflammation and/or multinucleated giant cells); patients with confirmed vertebral osteomyelitis before PVP/PKP treatment; patients not having a preoperative diagnosis of OVCF (including those with pathological fractures or others); patients diagnosed with malignant or benign tumors before surgery; and patients without any information eligible for analysis.
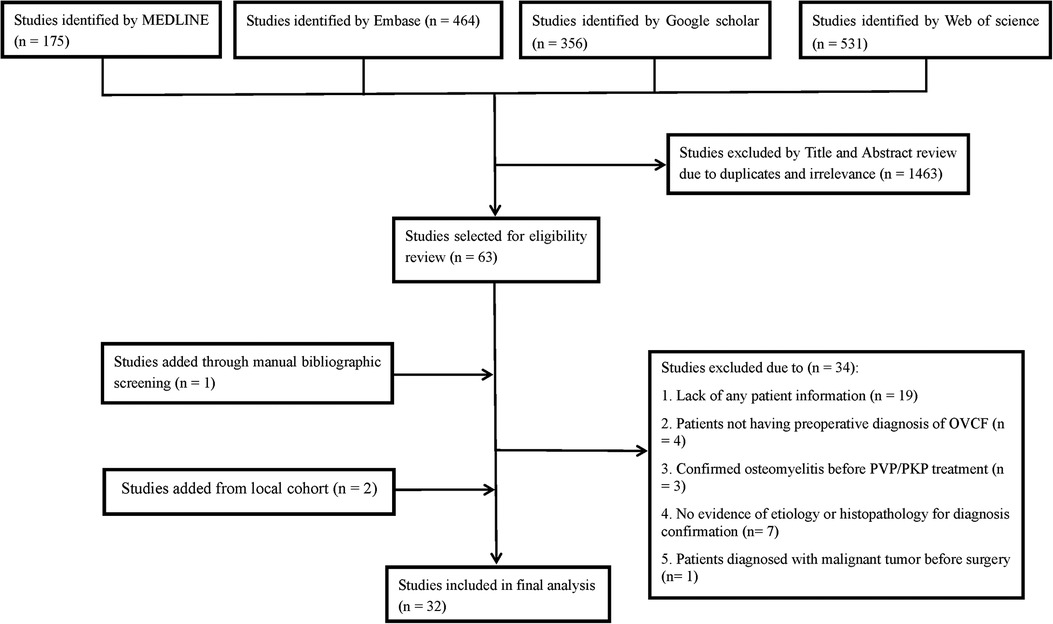
Figure 1. Flow diagram of literature search showing studies identified, included and excluded at each stage.
Two investigators independently screened the publications based on the inclusion criteria and extracted clinical data for each patient. Any dispute was resolved through consensus. Patient information obtained from the studies included the following: demographics (age and sex), clinical characteristics (including OVCF location, number of segments treated by PVP/PKP and preoperative neurological function, a history of trauma (the specific injury mechanism is not explained in detail, and the description only reflects the “trauma history”), the presence or absence of pulmonary tuberculosis, obesity, smoking, and other comorbidities [such as diabetes, rheumatoid arthritis, pneumonia, chronic obstructive pulmonary disease, urinary tract infection, and high blood pressure], radiological findings (the occurrence of paravertebral abscesses at first diagnosis of infection), microbiological results and laboratory tests (including the pathogens as well as WBC, ESR, CRP levels at the time of diagnosis), treatment (including revision surgery or not and the specific type of surgery), the time interval between PVP/PKP and the first diagnosis of spinal infection, follow-up time and clinical outcomes of the patients (recovery, limited mobility/assisted walking and death).
A total of 1935 OVCF patients who were treated with PVP/PKP in our institute from March 2003 to March 2020 were identified. This duration of study was determined as the similar period in which the included cases were reported in the literature to allow for comparability. The medical records of the patients were reviewed retrospectively to include eligible cases with postoperative spondylitis. Patients in the local cohort were diagnosed with osteoporosis by bone density scans; all 8 patients included in this institution fell from low. The diagnosis of postoperative NTS was confirmed by microbiological evidence showing pathogenic growth in tissue culture. Postoperative TS was determined by acid-fast staining and the histopathological results of the lesion tissues. In total, 6 NTS cases and 5 TS cases after PVP/PKP were identified in our hospital. The overall incidence of spinal infection following PVP/PKP surgery was 0.57%. Among the 11 cases with postoperative spondylitis, two TS cases were previously described in our study (9). Using the PS matching plug-in of SPSS, 114 patients who did not develop vertebral osteomyelitis after PVP/PKP treatment in our hospital during the same period were randomly selected as the control group, and there was no significant difference in age or sex between it and the infected groups (control vs. TS: t = 0.828, P = 0.645 for age and χ2 = 0.550, P = 0.458 for sex; control vs NTS: t = 0.003, P = 0.994 for age and χ2 = 2.253, P = 0.133 for sex). The included patients with PVP/PKP in our hospital had normal preoperative inflammatory blood parameters and would not have undergone surgery otherwise. In addition, all patients received prophylactic intravenous antibiotics, specifically cefuroxime 0.5 g, on the day before surgery, the day of surgery, and the day after surgery. None of the patients included in our institution had any other form of surgical site infection or prolonged wound healing time during their hospitalization. Postoperatively, all patients underwent regular clinical and imaging follow-up, and the final follow-up time was November 2020. Patient clinical data were directly obtained from medical records.
All statistical analyses were performed by using SPSS 26.0 (SPSS, Chicago, Illinois, USA). Continuous data are expressed as the mean ± standard deviation and were analyzed by Student's t-test, while categorical data are presented as the frequency or composition ratio and were analyzed by the chi-square test or Fisher's exact test. The multivariate logistic regression model was used to assess the independent risk factors for vertebral infection after PVP/PKP surgery, in which the factors that were found to be statistically significant (P < 0.1) in our univariate analysis, as well as important predictors reported in the literature, were included (2, 7, 8). All tests were two-sided, and P < 0.05 was considered to be statistically significant.
A total of 30 studies met the inclusion criteria (2, 3, 5, 7–10, 12–34). Among them, 10 discussed TS after PVP/PKP, 19 analyzed the occurrence of postoperative NTS, and 1 evaluated both TS and NTS. After review, 20 TS patients and 44 NTS patients were identified from these studies. With an additional 5 TS patients and 6 NTS patients from our local center, a total of 23 TS patients and 50 NTS patients were finally included in this study. The clinical data of the included patients are shown in Tables 1, 2.
In the TS group, the average time interval from index surgery to the diagnosis of spondylitis was 8.45 ± 11.68 months. All patients received anti-tuberculosis drug treatment after surgery. Among them, one was treated with triple drugs (isoniazid, rifampicin and ethambutol), 13 were treated with quadruple drugs (isoniazid, rifampicin, pyrazinamide and ethambutol), and the remaining 9 were treated with anti-tuberculosis regimens that were not described. Twenty patients underwent revision surgery, among which 2 patients underwent anterior debridement and bone graft fusion; 12 patients underwent combined anterior and posterior debridement, instrumentation, and bone graft fusion; and 6 patients underwent one-stage posterior debridement, fixation and bone graft fusion. The remaining 3 patients were treated by unreported types of surgery. Mycobacterium tuberculosis was detected by polymerase chain reaction of the infected tissues in 2 patients, while TS was confirmed by findings from both polymerase chain reaction and acid-fast staining of the infected lesions in 14 patients. In 6 patients, TS diagnosis was made based on histopathological examination of the infected tissues showing granulomatous inflammation and/or caseous necrosis and/or multinucleated giant cells. The remaining 1 case had an unknown method of diagnosis. The average follow-up time was 26.2 ± 25.5 months. At the last follow-up, 11 patients experienced a good recovery (“good” was defined as walking normally without the aid of a walking aid), 8 patients required walking assistance, and 3 patients died (one due to paraplegia, the other due to bacteremia and multiple organ failure, and the third patient did not specify the cause of death).
In the NTS group, the average time interval from the index surgery to the diagnosis of spinal infection was 6.36 ± 14.14 months. All patients received anti-infective treatment after surgery, and there were differences in the use of drugs across the studies. Forty-three patients underwent revision surgery, of whom 8 received anterior debridement and bone graft fusion, 25 received combined anterior and posterior debridement, fixation, and bone graft fusion, and 10 received one-stage posterior debridement, instrumentation and bone graft fusion. The remaining 2 cases were treated with an unknown type of surgery. The growth of pathogenic bacteria was detected in the tissue culture of the lesions for all patients. The average follow-up time was 16.7 ± 12.1 months. At the end of the follow-up, 30 patients had a good recovery, 8 patients required walking assistance, and 10 patients died.
The comparison results of the clinical characteristics of patients in the TS group and the NTS group are shown in Table 3. The analysis results showed that patients in the TS group were more likely to have a history of trauma before PVP/PKP treatment. However, due to the small number of TS groups providing trauma history data, this result may be biased. Analyzing the characteristics of infection after its occurrence, we found that the infection time of the TS group patients was longer than that of the NTS patients, while the ESR index of the NTS group patients was higher than that of the TS group patients, but these differences were not statistically significant. There were no significant differences between the TS group and the NTS group in other clinical data.
A comparison of the clinical data between the TS group and the control group is shown in Table 4. Our analysis revealed that TS patients were more likely to have pulmonary tuberculosis and diabetes before receiving PVP/PKP. No significant differences were observed for other clinical characteristics between the two groups.
Similarly, the results of a comparison of the clinical features between the NTS and control subgroups are shown in Table 5. These outcomes showed that diabetes and multiple surgical segments (≥2) were significant factors for NTS after PVP/PKP. In addition, obese patients seemed to be more likely to develop NTS after surgery. Moreover, our study also indicated that a history of preoperative trauma and urinary tract infection were closely related to the occurrence of NTS, although the results were not statistically significant. No significant differences were seen between the two groups in terms of other clinical characteristics.
A multivariate logistic regression model showed that a history of pulmonary tuberculosis and diabetes were independent risk factors for TS after PVP/PKP (Table 6).
Similarly, multivariate analysis found that diabetes and the number of surgical segments were independently associated with the occurrence of postoperative NTS, while urinary tract infection, obesity and a history of trauma did not affect NTS development (Table 7).
In this study, we summarized the influencing factors of spinal infection after PVP/PKP and analyzed the differences in clinical characteristics between TS and NTS patients. We found that a history of pulmonary tuberculosis and diabetes were closely related to the development of postoperative TS, while diabetes and the number of segments treated with surgery independently affected the occurrence of NTS after vertebral augmentation. Moreover, it appeared that patients with trauma were more likely to develop TS after surgery. These data provide a comprehensive understanding of the factors associated with spondylitis after PVP/PKP and may be helpful for guiding preoperative risk stratification and prevention to reduce or even avoid the occurrence of postoperative spondylitis following PVP/PKP treatment.
Currently, there is still a lack of reports on factors affecting spinal infection after PVP/PKP. Our study found that diabetes was an independent risk factor for TS and NTS after PVP/PKP, similar to previous reports showing that diabetes is an important factor for postoperative spinal infection (35–37), which can significantly increase the risk of a spinal infection caused by several specific bacteria (such as Staphylococcus aureus and Mycobacterium tuberculosis) (38, 39). The mechanism by which diabetes could increase the incidence of postoperative spinal infection remains unclear. Previous studies have revealed that elevated resistin levels in diabetic patients can impair the chemotaxis and phagocytosis of neutrophils by interfering with phosphatidylinositol-3-kinase-dependent downstream pathways (40, 41). In addition, studies have pointed out that high blood sugar levels can weaken the function of antigen-presenting cells, thereby damaging the adaptive immune response mediated by T cells (7, 42, 43). Furthermore, OVCF patients receiving PVP/PKP treatment are generally of an advanced age and have a relatively low immunity (44, 45). These findings may provide a theoretical explanation for how diabetes can promote the incidence of spinal infection after vertebral augmentation. These data also highlight the importance of insulin use during the perioperative period for diabetes patients. However, it should be noted that whether the use of insulin alone can effectively reduce the presence of postoperative spondylitis in diabetic patients after PVP/PKP deserves further investigation, considering that diabetes is linked to various metabolic disorders (such as dyslipidemia, high uric acid, and hypertension).
Published data suggest that pulmonary tuberculosis is closely associated with the occurrence of TS after PVP/PKP. Although the precise mechanism is unknown, researchers consider that TS can occur in the case of active pulmonary tuberculosis by direct hematogenous dissemination of Mycobacterium tuberculosis or indirect spread of this pathogen through proximal para-aortic lymph nodes to the surgical site (8, 9). In contrast, some studies have shown that vertebral augmentation may cause tuberculosis infection by reactivating static Mycobacterium tuberculosis or inducing the release of this mycobacterium from infected macrophages to the surgically treated area under the condition of inactive pulmonary tuberculosis (7–9). In addition, for patients with diabetes or any other immunosuppressive disorders, the impaired adaptive immune response may also reactivate Mycobacterium tuberculosis or aggravate any existing tuberculosis (7, 42, 43). In this study, we found that pulmonary tuberculosis was a significant predictor for TS after PVP/PKP. This result provides the first statistical evidence to support the above speculations (7–9). Additionally, this finding also emphasizes the importance of monitoring patients with preoperative pulmonary tuberculosis after PVP/PKP, given that the risk of postoperative TS is high in these patients and that TS may progress rapidly in this situation (9).
In addition, this study also showed that the number of segments treated by PVP/PKP was a significant factor associated with NTS after surgery. This is not difficult to understand because more surgical levels are usually correlated with a longer operation time, which is generally considered to increase the risk of infection after spinal surgery (36). Another possible explanation may be the fact that more surgical segments commonly reflect severe preoperative trauma, which can likely reduce the specific adaptive immunity of T cells (46–48), thus leading to postoperative spondylitis. Another finding of this study was that obesity might increase the incidence of postoperative spondylitis after PVP/PKP, consistent with the findings of previous observations (49). A possible reason is that the abnormal regulation of hormones and adipokines in obese patients likely compromises T-cell function, thereby weakening the adaptive immune response to infection in this population (50).
Interestingly, our analysis found that preexisting infection in other regions of the body did not contribute to NTS occurrence after PVP/PKP, which contradicts previous reports in the literature (2, 5, 6). Prior data have indicated that vertebral augmentation as an invasive operation may lead to the relative susceptibility of the surgical area (the principle of locus minoris resistentia) (2), which then creates a microenvironment suitable for pathogens to invade the surgical site, thereby resulting in subsequent infection. Moreover, during the process of bone cement infusion, repeated C-arm fluoroscopy and frequent personnel movements during the surgery may also increase the risk of postoperative spinal infection. These factors offer a possible route for the development of spondylitis caused by external pathogens after PVP/PKP therapy, although this idea requires further confirmation.
In addition, frailty is one of the most serious global public health challenges we face right now. Rapidly aging populations have brought about an increase in the number of frailty older adults, which in turn has put increasing pressure on healthcare systems worldwide (51, 52). When a stressful event (e.g., acute illness, trauma) occurs, the functional capacity of frailty individuals deteriorates rapidly, but the patients we included did not have a complete frailty evaluation, so “frailty” was not included in this study, but its It is still a very meaningful variable that deserves further exploration in the future (53).
Most of the included studies failed to provide complete clinical data of the patients, which may introduce bias into the results. However, to minimize the heterogeneity among studies and to make the analysis results more reliable and the statistical analysis feasible, we simplified the grouping criteria for most variables in the data processing.
The present study performed a comprehensive summary of the risk factors for vertebral infection after PVP/PKP. We found that a history of pulmonary tuberculosis and diabetes were independent risk factors for TS. For NTS, our analysis revealed that diabetes and the number of surgically treated segments significantly influenced the occurrence of postoperative spinal infection. These data may be helpful for guiding risk stratification and preoperative prevention for patients, thereby reducing the incidence of vertebral osteomyelitis after PVP/PKP.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board at The First Affiliated Hospital, University of South China, Hunan, P.R. China. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors participated in data acquisition. BWZ, JL, HQN and MXZ contributed to the conception and design of the study. BWZ, GHL and MXZ did the data analysis and interpretation. ZX, FSL, BYZ and MXZ contributed to drafting and revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81871821 to JL and 82002364 to MXZ), Natural Science Foundation of Hunan Province (2022JJ40403 to ZX), and Project for Clinical Research of Hunan Provincial Health Commission (20201956 to MXZ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Muijs SP, van Erkel AR, Dijkstra PD. Treatment of painful osteoporotic vertebral compression fractures: a brief review of the evidence for percutaneous vertebroplasty. J Bone Joint Surg Br. (2011) 93(9):1149–53. doi: 10.1302/0301-620X.93B9.26152
2. Liao JC, Lai PL, Chen LH, Niu CC. Surgical outcomes of infectious spondylitis after vertebroplasty, and comparisons between pyogenic and tuberculosis. BMC Infect Dis. (2018) 18(1):555. doi: 10.1186/s12879-018-3486-x
3. Lai PJ, Liao JC, Chen LH, Lai PL. Tuberculous spondylitis after percutaneous vertebroplasty: a case series of 9 cases. Biomed J. (2019) 42(4):285–92. doi: 10.1016/j.bj.2019.04.002
4. Alothman A, Memish ZA, Awada A, Al-Mahmood S, Al-Sadoon S, Rahman MM, et al. Tuberculous spondylitis: analysis of 69 cases from Saudi Arabia. Spine (Phila Pa 1976). (2001) 26(24):E565–70. doi: 10.1097/00007632-200112150-00020
5. Walker DH, Mummaneni P, Rodts GE Jr. Infected vertebroplasty. Report of two cases and review of the literature. Neurosurg Focus. (2004) 17(6):E6. doi: 10.3171/foc.2004.17.6.6
6. Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine (Phila Pa 1976). (2009) 34(11):1228–32. doi: 10.1097/BRS.0b013e3181a3c742
7. Kang JH, Kim HS, Kim SW. Tuberculous spondylitis after percutaneous vertebroplasty: misdiagnosis or complication? Korean J Spine. (2013) 10(2):97–100. doi: 10.14245/kjs.2013.10.2.97
8. Ivo R, Sobottke R, Seifert H, Ortmann M, Eysel P. Tuberculous spondylitis and paravertebral abscess formation after kyphoplasty: a case report. Spine (Phila Pa 1976). (2010) 35(12):E559–63. doi: 10.1097/BRS.0b013e3181ce1aab
9. Zou MX, Wang XB, Li J, Lv GH, Deng YW. Spinal tuberculosis of the lumbar spine after percutaneous vertebral augmentation (vertebroplasty or kyphoplasty). Spine J. (2015) 15(6):e1–e6. doi: 10.1016/j.spinee.2015.02.032
10. Park JW, Park SM, Lee HJ, Lee CK, Chang BS, Kim H. Infection following percutaneous vertebral augmentation with polymethylmethacrylate. Arch Osteoporos. (2018) 13(1):47. doi: 10.1007/s11657-018-0468-y
11. Zhang Y, Wu S, Xia Y, Wang N, Zhou L, Wang J, et al. Adverse events associated with treatment of multidrug-resistant Tuberculosis in China: an ambispective cohort study. Med Sci Monit. (2017) 23:2348–56. doi: 10.12659/MSM.904682
12. Bouvresse S, Chiras J, Bricaire F, Bossi P. Pott's disease occurring after percutaneous vertebroplasty: an unusual illustration of the principle of locus minoris resistentiae. J Infect. (2006) 53(6):e251–3. doi: 10.1016/j.jinf.2006.02.014
13. Kim HJ, Shin DA, Cho KG, Chung SS. Late onset tuberculous spondylitis following kyphoplasty: a case report and review of the literature. Korean J Spine. (2012) 9(1):28–31. doi: 10.14245/kjs.2012.9.1.28
14. Ge CY, He LM, Zheng YH, Liu TJ, Guo H, He BR, et al. Tuberculous spondylitis following kyphoplasty: a case report and review of the literature. Medicine (Baltimore. (2016) 95(11):e2940. doi: 10.1097/MD.0000000000002940
15. Sun JJ, Sun ZY, Qian ZL, Yang HL, Zhu XY. Tuberculous spondylitis after vertebral augmentation: a case report with a literature review. J Int Med Res. (2018) 46(2):916–24. doi: 10.1177/0300060517728008
16. Santbergen B, Vriens PH, de Lange WC, Van Kasteren ME. Combined infection of vertebroplasty and aortic graft after intravesical BCG treatment. BMJ Case Rep. (2013) 2013:bcr2012008161. doi: 10.1136/bcr-2012-008161
17. Ha KY, Kim KW, Kim YH, Oh IS, Park SW. Revision surgery after vertebroplasty or kyphoplasty. Clin Orthop Surg. (2010) 2(4):203–8. doi: 10.4055/cios.2010.2.4.203
18. Lee CB, Kim HS, Kim YJ. Pyogenic spondylitis after vertebroplasty - a report of two cases. Asian Spine J. (2007) 1(2):106–9. doi: 10.4184/asj.2007.1.2.106
19. Chen LH, Yang SC, Niu CC, Lai PL, Chen WJ. Percutaneous drainage followed by antibiotic-impregnated cement vertebroplasty for pyogenic vertebral osteomyelitis: a case report. J Trauma. (2008) 64(1):E8–E11. doi: 10.1097/TA.0b013e318160f72b
20. Gaye M, Fuentes S, Pech-Gourg G, Benhima Y, Dufour H. [Spondylitis following vertebroplasty. Case report and review of the literature]. Neurochirurgie. (2008) 54(4):551–5. doi: 10.1016/j.neuchi.2008.02.063
21. Kim KY, Hur J, Jo W, Hong J, Cho OH, Kang DH, et al. Infectious spondylitis with bacteremia caused by Roseomonas mucosa in an immunocompetent patient. Infect Chemother. (2015) 47(3):194–6. doi: 10.3947/ic.2015.47.3.194
22. Lee JS, Choi SM, Kim KW. Triparesis caused by gas-containing extensive epidural abscess secondary to Aeromonas hydrophila infection of a thoracic vertebroplasty: a case report. Spine J. (2013) 13(10):e9–e14. doi: 10.1016/j.spinee.2013.03.045
23. Lin WC, Lee CH, Chen SH, Lui CC. Unusual presentation of infected vertebroplasty with delayed cement dislodgment in an immunocompromised patient: case report and review of literature. Cardiovasc Intervent Radiol. (2008) 31(Suppl 2):S231–5. doi: 10.1007/s00270-007-9234-z
24. Schofer MD, Lakemeier S, Peterlein CD, Heyse TJ, Quante M. Primary pyogenic spondylitis following kyphoplasty: a case report. J Med Case Rep. (2011) 5:101. doi: 10.1186/1752-1947-5-101
25. Syed MI, Avutu B, Shaikh A, Sparks H, Mohammed MI, Morar K. Vertebral osteomyelitis following vertebroplasty: is acne a potential contraindication and are prophylactic antibiotics mandatory prior to vertebroplasty? Pain Physician. (2009) 12(4):E285–90. doi: 10.36076/ppj.2009/12/E285
26. Alfonso Olmos M, Silva Gonzalez A, Duart Clemente J, Villas Tome C. Infected vertebroplasty due to uncommon bacteria solved surgically: a rare and threatening life complication of a common procedure: report of a case and a review of the literature. Spine (Phila Pa 1976). (2006) 31(20):E770–3. doi: 10.1097/01.brs.0000240202.91336.99
27. Soyuncu Y, Ozdemir H, Soyuncu S, Bigat Z, Gur S. Posterior spinal epidural abscess: an unusual complication of vertebroplasty. Joint Bone Spine. (2006) 73(6):753–5. doi: 10.1016/j.jbspin.2006.01.015
28. Youn MS, Shin JK, Goh TS, Lee JS. Minimally invasive percutaneous endoscopic treatment for acute pyogenic spondylodiscitis following vertebroplasty. Eur Spine J. (2018) 27(Suppl 3):458–64. doi: 10.1007/s00586-018-5478-3
29. Mummaneni PV, Walker DH, Mizuno J, Rodts GE. Infected vertebroplasty requiring 360 degrees spinal reconstruction: long-term follow-up review. Report of two cases. J Neurosurg Spine. (2006) 5(1):86–9. doi: 10.3171/spi.2006.5.1.86
30. Vats HS, McKiernan FE. Infected vertebroplasty: case report and review of literature. Spine (Phila Pa 1976). (2006) 31(22):E859–62. doi: 10.1097/01.brs.0000240665.56414.88
31. Shin JH, Ha KY, Kim KW, Lee JS, Joo MW. Surgical treatment for delayed pyogenic spondylitis after percutaneous vertebroplasty and kyphoplasty. Report of 4 cases. J Neurosurg Spine. (2008) 9(3):265–72. doi: 10.3171/SPI/2008/9/9/265
32. Buttermann GR, Mullin WJ. Percutaneous vertebral body cement augmentation for back pain related to occult osteomyelitis/diskitis. Orthopedics. (2011) 34(11):e788–92. doi: 10.3928/01477447-20110922-31
33. Abdelrahman H, Siam AE, Shawky A, Ezzati A, Boehm H. Infection after vertebroplasty or kyphoplasty. A series of nine cases and review of literature. Spine J. (2013) 13(12):1809–17. doi: 10.1016/j.spinee.2013.05.053
34. Trojani MC, Lamy B, Ruimy R, Amoretti N, Risso K, Roux C. An unusual Staphylococcus saccharolyticus spondylodiscitis post kyphoplasty: a case report. BMC Infect Dis. (2020) 20(1):539. doi: 10.1186/s12879-020-05263-5
35. Liu JM, Deng HL, Chen XY, Zhou Y, Yang D, Duan MS, et al. Risk factors for surgical site infection after posterior lumbar spinal surgery. Spine (Phila Pa 1976). (2018) 43(10):732–7. doi: 10.1097/BRS.0000000000002419
36. Peng XQ, Sun CG, Fei ZG, Zhou QJ. Risk factors for surgical site infection after spinal surgery: a systematic review and meta-analysis based on twenty-seven studies. World Neurosurg. (2019) 123:e318–29. doi: 10.1016/j.wneu.2018.11.158
37. Yao R, Zhou H, Choma TJ, Kwon BK, Street J. Surgical site infection in spine surgery: who is at risk? Global Spine J. (2018) 8(4 Suppl):5S–30S. doi: 10.1177/2192568218799056
38. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. (1999) 341(25):1906–12. doi: 10.1056/NEJM199912163412507
39. Knapp S. Diabetes and infection: is there a link?–A mini-review. Gerontology. (2013) 59(2):99–104. doi: 10.1159/000345107
40. Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. (1997) 14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V
41. Cohen G, Ilic D, Raupachova J, Horl WH. Resistin inhibits essential functions of polymorphonuclear leukocytes. J Immunol. (2008) 181(6):3761–8. doi: 10.4049/jimmunol.181.6.3761
42. Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. (2010) 184(11):6275–82. doi: 10.4049/jimmunol.1000304
43. Ayelign B, Negash M, Genetu M, Wondmagegn T, Shibabaw T. Immunological impacts of diabetes on the susceptibility of Mycobacterium tuberculosis. J Immunol Res. (2019) 2019:6196532. doi: 10.1155/2019/6196532
44. Leng SX, Margolick JB. Aging, sex, inflammation, frailty, and CMV and HIV infections. Cell Immunol. (2020) 348:104024. doi: 10.1016/j.cellimm.2019.104024
45. Sadighi Akha AA. Aging and the immune system: an overview. J Immunol Methods. (2018) 463:21–6. doi: 10.1016/j.jim.2018.08.005
46. Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Exp Med. (2011) 208(13):2581–90. doi: 10.1084/jem.20111354
47. Keel M, Trentz O. Pathophysiology of polytrauma. Injury. (2005) 36(6):691–709. doi: 10.1016/j.injury.2004.12.037
48. Cole E, Davenport R, Willett K, Brohi K. The burden of infection in severely injured trauma patients and the relationship with admission shock severity. J Trauma Acute Care Surg. (2014) 76(3):730–5. doi: 10.1097/TA.0b013e31829fdbd7
49. Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. (2012) 71(2):298–306. doi: 10.1017/S0029665112000158
50. Green WD, Beck MA. Obesity altered T cell metabolism and the response to infection. Curr Opin Immunol. (2017) 46:1–7. doi: 10.1016/j.coi.2017.03.008
51. Mousa A, Savva GM, Mitnitski A, Rockwood K, Jagger C, Brayne C, et al. Is frailty a stable predictor of mortality across time? Evidence from the cognitive function and ageing studies. Age Ageing. (2018) 47(5):721–7. doi: 10.1093/ageing/afy077
52. Ilinca S, Calciolari S. The patterns of health care utilization by elderly Europeans: frailty and its implications for health systems. Health Serv Res. (2015) 50(1):305–20. doi: 10.1111/1475-6773.12211
Keywords: percutaneous vertebroplasty, percutaneous kyphoplasty, tuberculous spondylitis, nontuberculous spondylitis, pyogenic spondylitis, risk factors
Citation: Zheng B, Liu F, Zheng B, Niu H, Li J, Lv G, Zou M and Xu Z (2022) Risk factors for tuberculous or nontuberculous spondylitis after percutaneous vertebroplasty or kyphoplasty in patients with osteoporotic vertebral compression fracture: A case-control study. Front. Surg. 9:962425. doi: 10.3389/fsurg.2022.962425
Received: 6 June 2022; Accepted: 4 August 2022;
Published: 18 August 2022.
Edited by:
Markus Rupp, University Medical Center Regensburg, GermanyReviewed by:
Siegmund Lang, University Medical Center Regensburg, Germany© 2022 Zheng, Liu, Zheng, Niu, Li, Lv, Zou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhun Xu eHV6aHVuMjAxMkAxNjMuY29t
†These authors have contributed equally to this work.
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.