- Department of General Surgery, Hangzhou TCM Hospital affiliated to Zhejiang Chinese Medical University, Hangzhou, China
Background: Cholecystoenteric fistula (CEF) is an uncommon complication of cholelithiasis. Here, we report our experience on diagnostic methods and surgical management of CEF patients with and without gallstone ileus (GI).
Methods: This is a retrospective cases series over an 11-year period (2011–2022). Data analyzed included preoperative characteristics, ultrasound, imaging features, operation findings and postoperative course.
Results: A total of 29 patients diagnosed with CEF were enrolled, 51.7% (15/29) of whom were female, with a median age of 66 years (range: 35–96 years). With regards to subtype distribution, seventeen patients had cholecystoduodenal fistula (CDF), six had cholecystoconlonic fistula (CCF), three exhibited cholecystogastric fistula (CGF), one CDF combination with CCF and two CDF combination with type I Mirizzi syndrome. Twelve patients presented with gallstone ileus, and received one stage procedure or simple Enterolithotomy. The median operation time and blood loss of 157 min (range: 65–360 min) and 40 ml (range: 10–450 ml), respectively. Surgical complications, evidenced by fistula recurrence, were recorded in three patients (3/22; 13.6%), while four (4/29; 13.8%) and one patient (1/29; 3.4%) presented with wound infection and residual stone in common bile duct, respectively. No deaths were reported in our study.
Conclusion: CEF is a rare complication of gallstone disease that is occasionally found during operation. To date, no consensus has been reached regarding efficacious treatment therapies for CEF patients. For a CEF patient with GI, one stage procedure should be selected prudently, while simple Enterolithotomy would be a mainstream choice for relieving bowel obstruction.
Introduction
Cholecystoenteric fistula (CEF), a relatively rare complication of gallstone disease, is defined as an autonomous communication between the inflamed gallbladder and the surrounding adherent gastrointestinal tract (1). Previous studies have shown that the incidence of CEF ranges between 3%–5% in patients with cholelithiasis and 0.15%–4.8% in all the biliary operations (2, 3). It usually occurs at the advanced age, and most frequently presents in the duodenum where it compromises 75%–80% of all the CEF, followed by colon and stomach (4, 5). In general, GI which means a gallstone impaction within the intestinal tract, is always accompanied by CEF. The condition, first described by Thomas Bartholin in 1,654, is responsible for 1%–4% of all the mechanical bowel obstruction (1, 6). The precise preoperative diagnosis can be quite difficult, owing to a lack of specific clinical manifestations and physical signs between CEF and the uncomplicated cholelithiasis. Fortunately, advancement of imaging-diagnosis coupled with development of endoscopic techniques, have markedly improved preoperative diagnostic accuracy (4).
In this study, we retrospectively analyzed all CEF patients with and without GI, who were treated at our institution. Particularly, we assessed their diagnosis, surgical management and treatment outcomes, with the aim of generating insights to help surgeons resolve the rare problem based on the experience of our therapy center.
Methods
The study protocol was approved by the ethnic committee of TCM Hospital affiliated to Zhejiang Chinese Medical University (No.2022KY070). We conducted an ICD-10 code retrieval (code K82.301, K82.302, K82.303, K82.304) in our clinical database from Jan 2010 to Dec 2021 to identify patients with CEF. Overall, we enrolled 29 patients with CEF (12 patients with GI), while those treated conservatively without surgical intervention were excluded. We collected and analyzed their demographic data, clinical manifestations, physical signs, laboratory test, medical examinations, operation dates, postoperative courses and complications.
Upon admission, all enrolled patients were routinely subjected to laboratory tests. Medical examinations included US, abdominal computed tomography (CT, plain scan or contrast-enhanced scan), magnetic resonance cholangiopancreatography (MRCP), upper gastrointestinal imaging and gastroscopy/colonoscopy were performed based on the patients' conditions. Patients combined with choledocholithiasis were subjected to an endoscopic retrograde cholangiopancreatography (ERCP) procedure selectively before cholecystectomy. Notably, 21 of the 29 patients accepted laparoscopic surgery with a standard four-port technique, and 8 conversion cases were found due to dense adhesion between gallbladder and adjacent gastrointestinal tract. The following key considerations were taken into account during surgical management: (a) For CEF patients without GI, cholecystectomy + Fistula repair and cholecystectomy + choledocholithotomy + T-tube insertion+ Fistula repair could be selected based on the patients' conditions; (b) Fistula repair was accomplished by way of simple interrupted sutures, applying Endo-GIA device or Billroth II (complete resection of fistula) for the CEF patients with and without GI; and (c) For the CEF patients with GI, surgical management included simple enterolithotomy, enterolithotomy + cholecystectomy + closure of cholecystoenteric fistula (one-stage procedure) or enterolithotomy + cholecystostomy. Surgical relief of bowel obstruction remains the mainstream of GI.
Statistical analyses were performed using an Excel software 15.30 (Microsoft Corporation, Redmond, WA, USA). Continuous variables were expressed as medians (range), whereas categorical ones were presented as frequencies (percentages).
Results
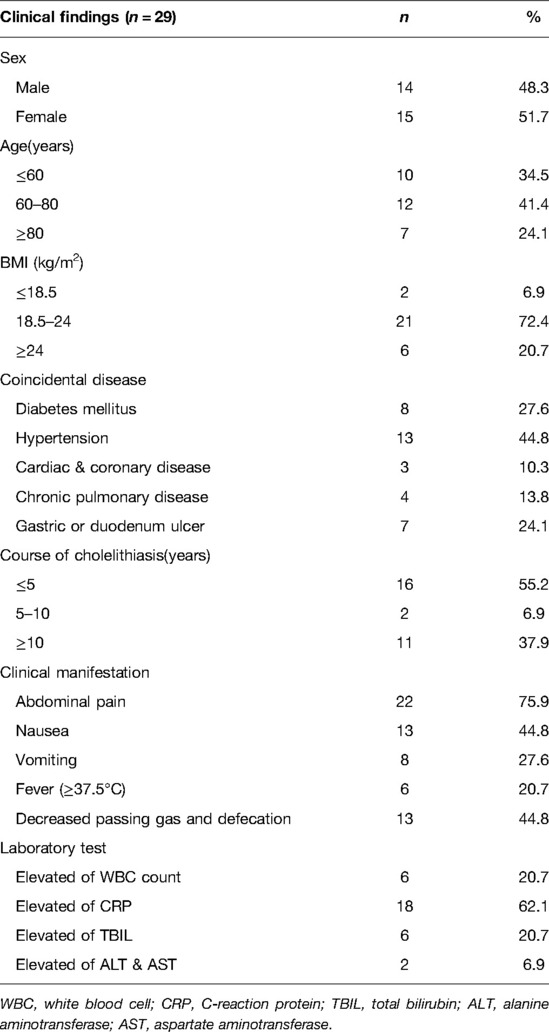
A total of 29 patients were included in our study over the 11-year period. Among them, 14 (48.3%) and 15 (51.7%) patients were male and female, respectively, with a median age 66 years (range: 35–95 years) (Table 1). Analysis of subtype distribution of CEF indicated that seventeen (58.6%) patients had CDF, six (20.7%) had CCF, three (10.3%) exhibited CGF, one (3.4%) patient presented with both CDF and CGF while two (6.8%) patients presented both Mirizzi syndrome and CDF (Table 3). With regards to other conditions, eight (27.6%) patients had diabetes mellitus (DM), thirteen (44.8%) had hypertension, three (10.3%) had cardiac and coronary disease, four (13.8%) had chronic pulmonary disease while seven (24.1%) exhibited gastric or duodenum ulcers. Thirteen (44.8%) patients presented with >5-year medical history of cholelithiasis. Notably, abdominal pain, especially the right upper quadrant abdominal pain, was the main complain, accounting for 75.9% of all the patients, followed by nausea (44.8%), vomiting (27.6%) and fever (20.7%). Decreased passing gas and defecation was observed in thirteen (44.8%) patients, which was typical presentation of bowel obstruction. Fluctuations among laboratory indices included elevated white blood cells (six patients, 20.7%), elevated levels of C-reaction protein (eighteen patients, 62.1%), increased levels of total bilirubin (six patients, 20.7%) and elevated levels of alanine aminotransferase and aspartate aminotransferase (two patients, 6.9%) (Table 1). Of all the 29 patients, seventeen (58.6%) patients achieved a preoperative diagnosis while the remaining 12 (41.4%) were diagnosed during the operation (Table 3).
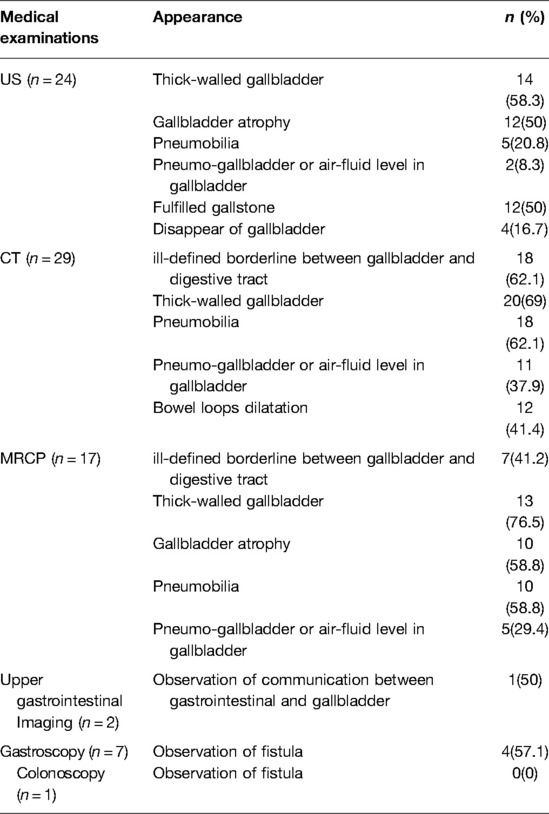
As a convenient and cost-effective examination, US was applied to twenty-four patients as a routine test. Consequently, US provide significant clues including thick-walled gallbladder (58.3%), Gallbladder atrophy (50%), Pneumobilia (20.8%) and Pneumo-gallbladder or air-fluid level in gallbladder (8.3%). All the 29 patients accepted abdominal CT, either plain scan or contrast-enhanced scan. Typical signs observed in the abdominal CT included ill-defined borderline between gallbladder and digestive tract, thick-walled gallbladder, pneumobilia, pneumo-gallbladder or air-fluid level in gallbladder, as well as bowel loop dilatation (Figures 1A–D, 2A,B). MRCP was applied to seventeen patients. Notably, similar signs to those observed in abdominal CT were evident, except bowel loops dilatation (Figures 2C–E). Two patients accepted upper gastrointestinal imaging, one of whom was diagnosed CDF by observation communication between gastrointestinal and gallbladder. Moreover, seven patients accepted gastroscopy, three were diagnosed with CDF while one was diagnosed with both CDF and CGF through observation of fistula (Table 2).
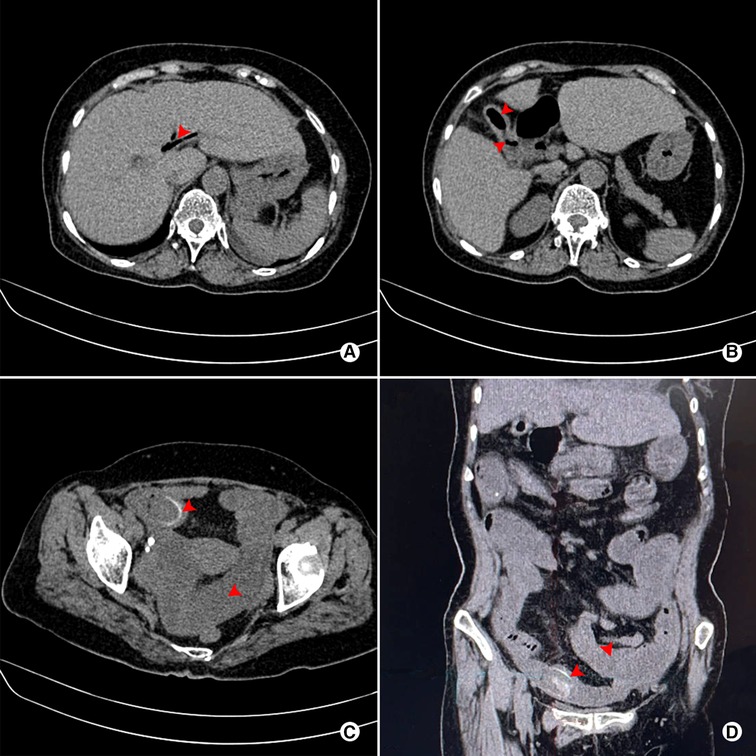
Figure 1. Abdominal plain CT scan of a 71-year-old female patient who was preoperatively diagnosed with CDF combined with GI. (A) Pneumobilia (red arrow); (B) Pneumo-gallbladder and ill-defined borderline between gallbladder and digestive tract (red arrow); (C) Rim-calcified gallstone impacted in the small bowel, combined with dilatation of proximal small bowel (red arrow); (D) Coronal view of impacted rim-calcified gallstone and dilatation of proximal small bowel (red arrow).
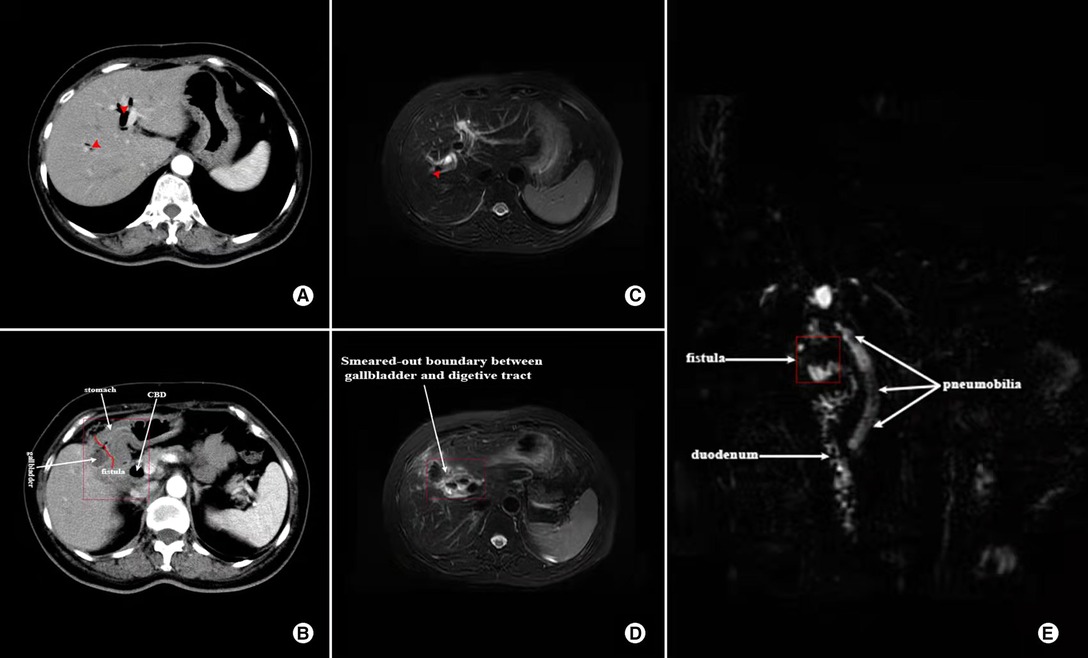
Figure 2. Contrast-enhanced CT and MRCP images showing the characteristics of a 57-year-old female who was diagnosed with both CDF and CGF; (A) CT image of Pneumobilia (red arrow); (B) CT image showing ill-defined borderline between gallbladder and digestive tract (marked in the red box); (C) MRCP image showing Pneumobilia (red arrow); (D) MRCP image of ill-defined borderline between gallbladder and digestive tract (marked in the red box); (E) Reconstruction of MRCP showing Pneumobilia and fistula (white arrow).
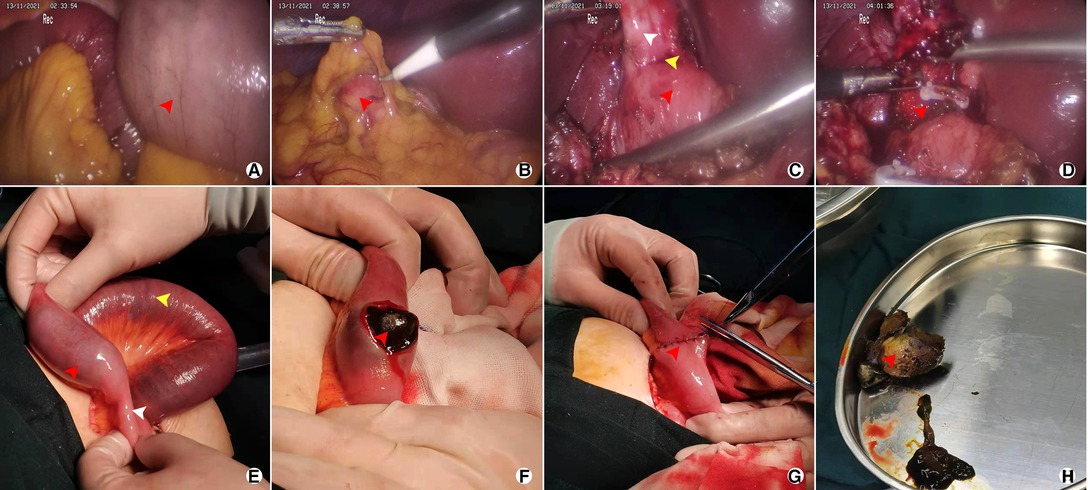
Figure 3. Representative images after operation of the 71-year-old female patient who was diagnosed CDF with GI; (A) Dilatation of small bowel (red arrow); (B) Atrophic gallbladder covered by great omentum; (C) CDF from a laparoscopic view, including atrophic gallbladder (white arrow), ill-defined borderline (yellow arrow), and duodenum (red arrow); (D) Partial cholecystectomy and fistula (red arrow); (E) Impacted gallstone (red arrow), dilatation of small bowel (yellow arrow) and emptiness of distal intestinal (white arrow); (F) Enterolithotomy (longitudinal incision, red arrow); (G) Closure of the intestinal (red arrow); (H) gallstone extracted from small bowel (red arrow).
Among the seventeen patients without GI, eight had CDF, six CCF, two exhibited CGF, while one patient had both CDF and CGF. Laparoscopy procedure was performed on sixteen patients. Among them, ten successfully underwent laparoscopy procedure while the other six underwent conversion to open surgery due to dense adhesion around the Calot's triangle, bleeding and intolerant operation time. Total or subtotal cholecystectomy was performed in all the seventeen patients and five patients underwent choledocholithotmy and T-tube insertion due to choledocholithiasis. One patient had both CDF and CGF, with fistula sizes of 2 and 1 cm, respectively. The Billroth II procedure was performed for the exceptional case (Table 3).
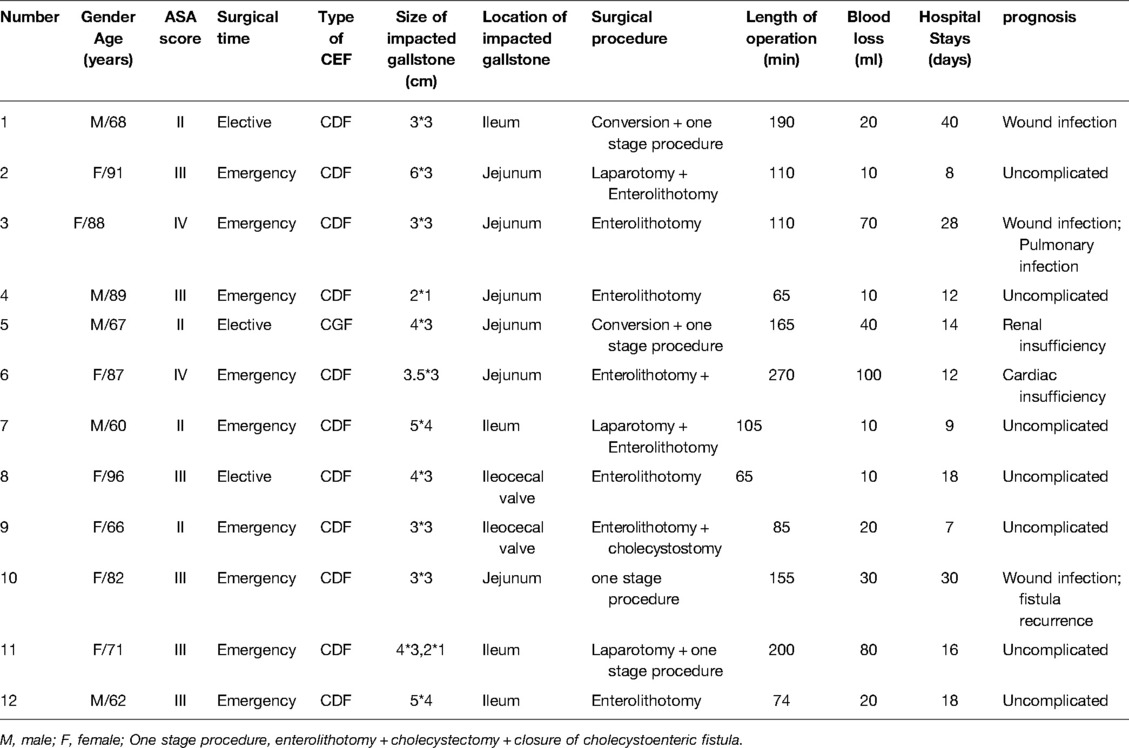
Eleven of the twelve patients with GI were diagnosed with CDF, with the remaining one exhibiting CGF. A majority of the cases were accomplished in an emergency manner (9 patients, 75%). Furthermore, laparoscopy procedure was tried in five patients with the remaining seven subjected to open surgery. Consequently, three and two patients successfully underwent laparoscopy procedure and conversion to open surgery, respectively. Seven patients underwent simple Enterolithotomy. Four patients underwent a one stage procedure which indicated finishing Enterolithotomy, cholecystectomy and closure of cholecystoenteric fistula. Distribution of location of impacted gallstone were as follows: jejunum (6 patients, 50%), ileum (4 patients, 33.3%) and ileocecal (2 patients, 16.7%). The median maximum diameter of the impacted gallstone was 3.8 cm (range: 2–6 cm) (Table 4).
A total of twenty patients underwent primary closure of fistula, by means of simple interrupted sutures (18 patients, 90%), application of the Endo-GIA device (1 patient, 5%) and Billroth II procedure (1 patient, 5%). The median operation time and blood loss were 157 min (range: 65–360 min) and 59 ml (range: 10–450 ml), respectively. Four patients (13.8%) were transferred to the intensive care unit due to unstable hemodynamics or sepsis secondary to GI or cholangitis. The median length of hospital stay was 17 days (5–40). Post-operative histopathologic finding revealed gallbladder cancer in one patient.
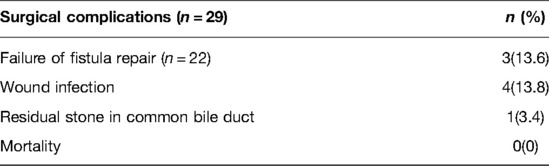
Overall, the post-operative complication rate was 36.4%, including wound infection in four patients (18.2%), failure of fistula repair in three patients (13.6%) and residual stone in common bile duct in one patient (3.4%). Most of the complications required additional surgery, including negative pressure drainage for wound infection, and ERCP combined with sphincterotomy for residual stone in common bile duct. With regards to fistula recurrence, two patients (CCF) received ultrasound-guided catheter drainage while one patient (CGF) recovered after conservative treatment. No deaths were reported in our study (Table 5).
Discussion
CEF, a condition first described by Thomas Bartholin in 1,654 and which allows gallstones to migrate into the digestive tract, is a rare complication of gallstone disease (7). The condition tends to predominantly occur in the female geriatric population which is partially confirmed in the present study (19/29 patients, 65.5%, over 60 years; 15/29 patients, 51.8%, female). These dates are slightly below international literature reported (80% female) (2, 8, 9). CEF most frequently presents in the duodenum (75%–80%), followed by colon and stomach. The detail dates recorded in the present study were 58.6% in CDF, 20.7% in CCF, 10.3% in CGF, respectively which were consistent with those reported in previous studies (4, 5, 9). The mechanism underlying of CEF is currently clear. Briefly, large gallstones (especially fulfilled gallstones) compress the gallbladder wall thereby causing wall ischemia, necrosis, erosion and eventually fistula formation with the adjacent viscus (Figure 4) (3, 9). According to Gonzalez-Urquijo et al. (9) and Glenn et al. (10), frequent acute cholecystitis and biliary calculus impaction cause dense adhesion, which ultimately leads to erosion between the gallbladder and the adjacent viscus. These consequently lead to fistula development.

Figure 4. Schematic diagram of the formation of CEF with GI. (A) gallstones; (B) Dense adhesion between gallbladder and duodenum; (C) Compression of fulfilled gallstones cause tissue ischemia, necrosis and erosion; (D) Fistula formation and gallstones migrate into digestive tract causing gallstone ileus.
Gallstones may migrate into the digestive tract (stomach, duodenum or colon), thereby resulting in bowel obstruction, a condition known as gallstone ileus. This condition most commonly affects the terminal ileum or ileocecal valve, and is less common in the jejunum, colon and stomach (Bouveret's syndrome) (1, 11–14). Most of the impacted gallstones are bigger than 2.5 cm in size (Figure 3F,H), normally ranging from 2 to 5 cm (1). Results from the present study indicated that all the impaction site of GI occurred in small intestinal, including jejunum (6 patients, 50%), ileum (4 patients, 33.3%) and ileocecal (2 patients, 16.7%), with a median size of 3.8 cm (range: 2–6 cm). The minute difference of the impaction site may be attributed to the large size of the gallstones that couldn't pass through the jejunum.
Clinical presentation of CEF is atypical and indistinguishable relative to uncomplicated cholelithiasis (2, 9). Results from the present study indicated that most patients (26/29) had a long history of cholelithiasis. Notably, the major symptoms in CEF patients included abdominal pain (common in right upper quadrant), nausea and vomiting. Additionally, decreased passing gas and defecation was quite common among CEF patients with GI.
Despite major advancement in imaging techniques and gastrointestinal endoscopy, preoperative diagnosis of a CEF remains challenge. And the accurate preoperative diagnosis is of great importance for surgeons to avoid unanticipated complex, prolonged and headache procedure. Li et al. (4) and Gonzalez-Urquijo et al. (9) reported that successful preoperative diagnosis of CEF in 31% and 40%, respectively. In the present study, we found a preoperative diagnosis of 58.6% (17/22), which was slightly higher than that reported in previous literatures mentioned above. The experience of our institution regarding improvement of preoperative diagnosis is as follows: (1) Long time (especially > 5 years) of repeated episodes of cholecystitis is a risk factor, thus should capture surgeons' attention; (2) Although accurate diagnosis by US detection can be difficult, signs such as thick-walled gallbladder, gallbladder atrophy and pneumobilia/pneumo-gallbladder are valuable clues of CEF; (3) Abdominal CT (plain or contrast-enhanced scan), especially coronal reconstruction, is imperative to effective identification with signs of ill-defined borderline between gallbladder and digestive tract. Moreover, when combined with thick-walled gallbladder, Pneumobilia, pneumo-gallbladder or air-fluid level in gallbladder, the surgeon should highly suspect the presence of CEF; (4) MRCP has similar signs to CT scan. Notably, the MRCP is much more helpful when a CEF is combined with choledocholithiasis or Mirizzi syndrome; (5) Upper gastrointestinal imaging, gastroscopy/colonoscopy should be considered when a CEF is suspected, which can observe the fistula or communication between gastrointestinal and gallbladder; and (6) For patients who present with intestinal obstruction, typical signs which is also known as Rigler's triad (15–17), including bowel loop dilatation, impaction of rim-calcified or total-calcified gallstone associating with the above signs, make the diagnosis relatively easy.
Therapeutic principle for patients with CEF and CEF with GI is vastly different. Notably, cholecystectomy and closure of fistula is advocated, for CEF patients, either by an open or a laparoscopic procedure based on the surgeons' experience and patients' condition. (2–4, 9) Traditionally, most scholars advise CEF should be managed in an open manner because of the dense adhesion and the obscured Calot's triangle (4, 18, 19). Fortunately, numerous studies have shown that laparoscopic surgery is not a contraindication for CEF, owing to the recent advancement of technology coupled with widespread application of laparoscopy (2, 9, 10). Furthermore, Vanessa et al. (20) described a case report regarding CEF managed by robotic surgery. However, other studies have shown that the rate of conversion to open can be very high due to heavy inflammation, bleeding and difficulty in intestinal suturing (4, 21, 22). In this study, thirteen patients (44.8%) successfully accepted laparoscopic procedure, while eight (27.6%) underwent conversion to open procedure. The method of closing the sinus tract mainly involves sutures, applying Endo-GIA device and Graham patch (4, 9, 23). In the present study, majority sinus tracts (90%) were closed by simple interrupted sutures. One special case was a 57-year-old woman who both presented with CDF (fistula size: 2 cm) and CGF (fistula size: 1 cm). Even worse, the two fistulas were so close which made the simply interrupted sutures or Graham patch repair too difficult and insecure. Finally, a Billroth II (completely fistula resection) was performed, and the patient discharged without complication on post-operation day 18 (POD 18).
For CEF patients with GI, surgical treatment approach has generated a sustained debate on whether the biliary surgery should be carried out in the meantime to relieve bowel obstruction (11, 24). Current surgical procedures are summarized as follows: (1) enterolithotomy (2) enterolithotomy, cholecystectomy, and fistula repair (one-stage procedure) and (3) enterolithotomy with cholecystertomy + fistula repair later (two-stage procedure) (1). To date, the literature on CEF with GI is composed mainly of cases series or case report and whether the fistula should be repaired or not remains unanswered. Moreover, none of the studies are randomized control trial and the selections of the surgical strategy are likely to be influenced by the surgical bias (1, 14). Simple enterolithotomy is associated with lower operative morbidity and mortality, especially for the aged patients or patients with concomitant disease (1, 24, 25). Clavien et al. (24) suggests that one stage procedure is safe and worth promoting when the local and general conditions permit. Two stage procedure, in fact, is not common and the delayed cholecystectomy and fistula repair is suitable in case of symptoms persistence (1, 25, 26). The results from the present study showed that seven (58.3%) and 4 patients (33.3%) accepted simple enterolithotomy and one-stage procedure, respectively, indicating that enterolithotomy is the most commonly preferred procedure.
In the present study, failure of fistula repair was the most serious complication, which eventually resulted in intraperitoneal infection. In total, three patients presented with fistula recurrence, of which two and one had CCF on POD 6 and CDF on POD 5, respectively. Two patients were successfully treated through catheter drainage, while one (CDF) recovered after conservative treatment. One patient presented with jaundice, abdominal pain and bile leakage and was confirmed a residual stone in CBD on POD 3 and the ERCP and sphincterotomy was performed. We observed no death case in this study, which was markedly less than that reported 6.7%–25% in the literature (9, 27).
In conclusion, CEF is a rare complication of gallstone disease, that is occasionally found during operation. High grade suspicion is indispensable for the diagnosis of CEF. Moreover, a combination of US, CT, MRCP, gastrointestinal imaging and gastroscopy/colonoscopy could make the diagnosis more accurate. Surgical management of CEF, regardless of whether it is laparoscopy or open, is essential and should be performed by experienced surgeons. At present, there is no consensus on the best surgical approach of CEF, and the main difficulty is to successfully repair the fistula. For CEF patients with GI, the primary goal is to relieve the intestinal obstruction and fistula closure should be avoided unless the patients' conditions are good enough.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by TCM Hospital affiliated to Zhejiang Chinese Medical University (No.2022KY070). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HH and SH contributed to the design of the study. JC and JZ were in charge of data collection. SH and YH contributed to the pictures’ adjustment, statistical analysis and paper writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Medical Science and Technology Planning Project of Health Planning of Zhejiang, China under grant no. 2021KY927; the Chinese Medicine Research Program pf Zhejiang Province under grant no. 2020ZB030.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nuno-Guzman CM, Marin-Contreras ME, Figueroa-Sanchez M, Corona JL. Gallstone ileus, clinical presentation, diagnostic and treatment approach. World J Gastrointest Surg. (2016) 8(1):65–76. doi: 10.4240/wjgs.v8.i1.65
2. Angrisani L, Corcione F, Tartaglia A, Tricarico A, Rendano F, Vincenti R, et al. Cholecystoenteric fistula (CF) is not a contraindication for laparoscopic surgery. Surg Endosc. (2001) 15(9):1038–41. doi: 10.1007/s004640000317
3. Chowbey P, Bandyopadhyay S, Sharma A, Khullar R, Soni V, Baijal M, et al. Laparoscopic management of cholecystoenteric fistulas. J Laparoendosc Adv Surg Tech A. (2006) 16(5):467–72. doi: 10.1089/lap.2006.16.467
4. Li XY, Zhao X, Zheng P, Kao XM, Xiang XS, Ji W. Laparoscopic management of cholecystoenteric fistula: a single-center experience. J Int Med Res. (2017) 45(3):1090–7. doi: 10.1177/0300060517699038
5. Costi R, Randone B, Violi V, Scatton O, Sarli L, Soubrane O, et al. Cholecystocolonic fistula: facts and myths. A review of the 231 published cases. J Hepatobiliary Pancreat Surg. (2009) 16(1):8–18. doi: 10.1007/s00534-008-0014-1
6. Sarli L, Pietra N, Costi R, Gobbi S. Gallstone ileus: laparoscopic-assisted enterolithotomy. J Am Coll Surg. (1998) 186(3):370–1. doi: 10.1016/s1072-7515(97)00151-8
7. Martin F. Intestinal obstruction due to gall-stones: with report of three successful cases. Ann Surg. (1912) 55(5):725–43. doi: 10.1097/00000658-191205000-00005
8. O’Brien JW, Webb LA, Evans L, Speakman C, Shaikh I. Gallstone ileus caused by cholecystocolonic fistula and gallstone impaction in the sigmoid colon: review of the literature and novel surgical treatment with trephine loop colostomy. Case Rep Gastroenterol. (2017) 11(1):95–102. doi: 10.1159/000456656
9. Gonzalez-Urquijo M, Rodarte-Shade M, Lozano-Balderas G, Gil-Galindo G. Cholecystoenteric fistula with and without gallstone ileus: a case series. Hepatobiliary Pancreat Dis Int. (2020) 19(1):36–40. doi: 10.1016/j.hbpd.2019.12.004
10. Wang W-K, Yeh C-N, Jan Y-Y. Successful laparoscopic management for cholecystoenteric fistula. World J Gastroenterol. (2006) 12(5):772–5. doi: 10.3748/wjg.v12.i5.772
11. Abou-Saif A, Al-Kawas FH. Complications of gallstone disease: mirizzi syndrome, cholecystocholedochal fistula, and gallstone ileus. Am J Gastroenterol. (2002) 97(2):249–54. doi: 10.1111/j.1572-0241.2002.05451.x
12. Alemi F, Seiser N, Ayloo S. Gallstone disease: cholecystitis, mirizzi syndrome, bouveret syndrome, gallstone ileus. Surg Clin North Am. (2019) 99(2):231–44. doi: 10.1016/j.suc.2018.12.006
13. Mulita F, Tchabashvili L, Bousis D, Kehagias D, Kaplanis C, Liolis E, et al. Gallstone ileus: a rare cause of small intestine obstruction. Clin Case Rep. (2021) 9(11):e04924. doi: 10.1002/ccr3.4924
14. Ravikumar R, Williams JG. The operative management of gallstone ileus. Ann R Coll Surg Engl. (2010) 92(4):279–81. doi: 10.1308/003588410X12664192076377
15. Lassandro F, Gagliardi N, Scuderi M, Pinto A, Gatta G, Mazzeo R. Gallstone ileus analysis of radiological findings in 27 patients. Eur J Radiol. (2004) 50(1):23–9. doi: 10.1016/j.ejrad.2003.11.011
16. Wang L, Dong P, Zhang Y, Tian B. Gallstone ileus displaying the typical Rigler triad and an occult second ectopic stone: a case report. Medicine. (2017) 96(45):e8541. doi: 10.1097/md.0000000000008541
17. Beuran M, Ivanov I, Venter MD. Gallstone ileus–clinical and therapeutic aspects. J Med Life. (2010) 3(4):365–71.
19. Beksac K, Erkan A, Kaynaroglu V. Double incomplete internal biliary fistula: coexisting cholecystogastric and cholecystoduodenal fistula. Case Rep Surg. (2016) 2016:5108471. doi: 10.1155/2016/5108471
20. Baratta VM, Kurbatov V, Le Blanc JM, Bowker B, Yavorek G. Robotic cholecystectomy and cholecystoenteric fistula closure in a female with remote cholangitis. J Surg Case Rep. (2019) 2019(8):rjz231. doi: 10.1093/jscr/rjz231
21. Moreno Ruiz F, del Rey Moreno A, Suescun García R, Martínez Ferriz J, Hidalgo Garrido J, Espadas Padial B, et al. Treatment of cholecystoduodenal fistula in the era of laparoscopy. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. (2001) 93(11):715–20.
22. Goh BKP, Low TY, Teo JY, Lee SY, Chan CY, Chung AYF, et al. Initial single institution experience with robotic biliary surgery and bilio-enteric anastomosis in southeast Asia. ANZ J Surg. (2019) 89(4):E142–6. doi: 10.1111/ans.15135
23. Hill BC, Burke LH, Ertl CW. Cholecystoduodenal fistula in a patient post-Roux-en-Y gastric bypass. BMJ Case Rep. (2013) 2013. doi: 10.1136/bcr-2013-200562
24. Clavien PA, Richon J, Burgan S, Rohner A. Gallstone ileus. Br J Surg. (1990) 77(7):737–42. doi: 10.1002/bjs.1800770707
25. Reisner RM, Cohen JR. Gallstone ileus: a review of 1001 reported cases. Am Surg. (1994) 60(6):441–6.
26. Rodríguez-Sanjuán JC, Casado F, Fernández MJ, Morales DJ, Naranjo A. Cholecystectomy and fistula closure versus enterolithotomy alone in gallstone ileus. Br J Surg. (1997) 84(5):634–7.
Keywords: cholecystoenteric fistula, cholelithiasis, complication, gallstone ileus, surgical management
Citation: Huang S, Han Y, Chen J, Zhang J and Huang H (2022) Surgical Management of Cholecystoenteric Fistula in Patients With and Without Gallstone Ileus: An Experience of 29 Cases. Front. Surg. 9:950292. doi: 10.3389/fsurg.2022.950292
Received: 22 May 2022; Accepted: 24 June 2022;
Published: 8 July 2022.
Edited by:
Ioannis Kehagias, University of Patras, GreeceReviewed by:
Georgios-Ioannis Verras, University of Edinburgh, United KingdomFrancesk Mulita, General University Hospital of Patras, Greece
Copyright © 2022 Huang, Han, Chen, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Huang 17888214580@aliyun.com
†These authors have contributed equally to this work and share first authorship.
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Shi-fei Huang†
Shi-fei Huang†