- 1Department of Orthopaedics, Peking University Third Hospital, Beijing, China
- 2Beijing Key Laboratory of Spinal Disease Research, Beijing, China
- 3Engineering Research Center of Bone and Joint Precision Medicine, Ministry of Education, Beijing, China
Background: Ossification of the posterior longitudinal ligament (OPLL) and that of ligamentum flavum (OLF) are the main types of the ossification of spinal ligaments (OSL) that cause the thoracic myelopathy. Although several studies have investigated the relationship of body mass index (BMI) with the onset or severity of OSL, it remains unverified due to the contradictory results of existing evidence. A systematic review and meta-analysis were performed in this work to determine the relationship of BMI with the onset and severity of OSL.
Methods: PubMed, EMBASE, Web of Science, and Cochrane Library were comprehensively searched online for relevant studies focusing on the relationship of BMI with the onset or severity of the OSL. The difference in BMI of OSL (or severe OSL group) and non-OSL (or nonsevere OSL group) groups was evaluated using the mean difference (MD) with a corresponding 95% confidence interval (CI).
Results: Fifteen studies were included in this systematic review and meta-analysis. The BMI of the OSL group was significantly higher than that of the non-OSL group (MD = 1.70 kg/m2, 95% CI = 1.02–2.39 kg/m2, and P < 0.01). Similar results were observed in the subgroup analysis of female (P < 0.01), OPLL (P < 0.01), and OLF (P < 0.01) populations. Three studies reported a significant association of BMI with the ossification index of OSL and the standardized regression coefficient ranging from 0.11 to 0.43 (P < 0.05). Moreover, a significantly higher BMI was observed in the severe OSL group compared with that in the nonsevere OSL group (MD = 3.09, 95% CI, 0.22–5.97 kg/m2, and P = 0.04).
Conclusion: The significant association of high BMI with the onset and severity of OSL may provide new evidence and insights into the mechanism research and management of OSL.
Introduction
Ossification of spinal ligaments (OSL) is a characterized heterotopic disease that can lead to severe myelopathy by compressing the spinal cord (1). Ossification of the posterior longitudinal ligament (OPLL) and ossification of the ligamentum flavum (OLF) are common and clinically significant types of OSL that generally progress and easily result in spinal injury; meanwhile, patients diagnosed with OPLL or OLF often complain of the local neck or thoracic pain and stiffness, difficulty in walking, limb weakness, and even incontinence (2, 3).
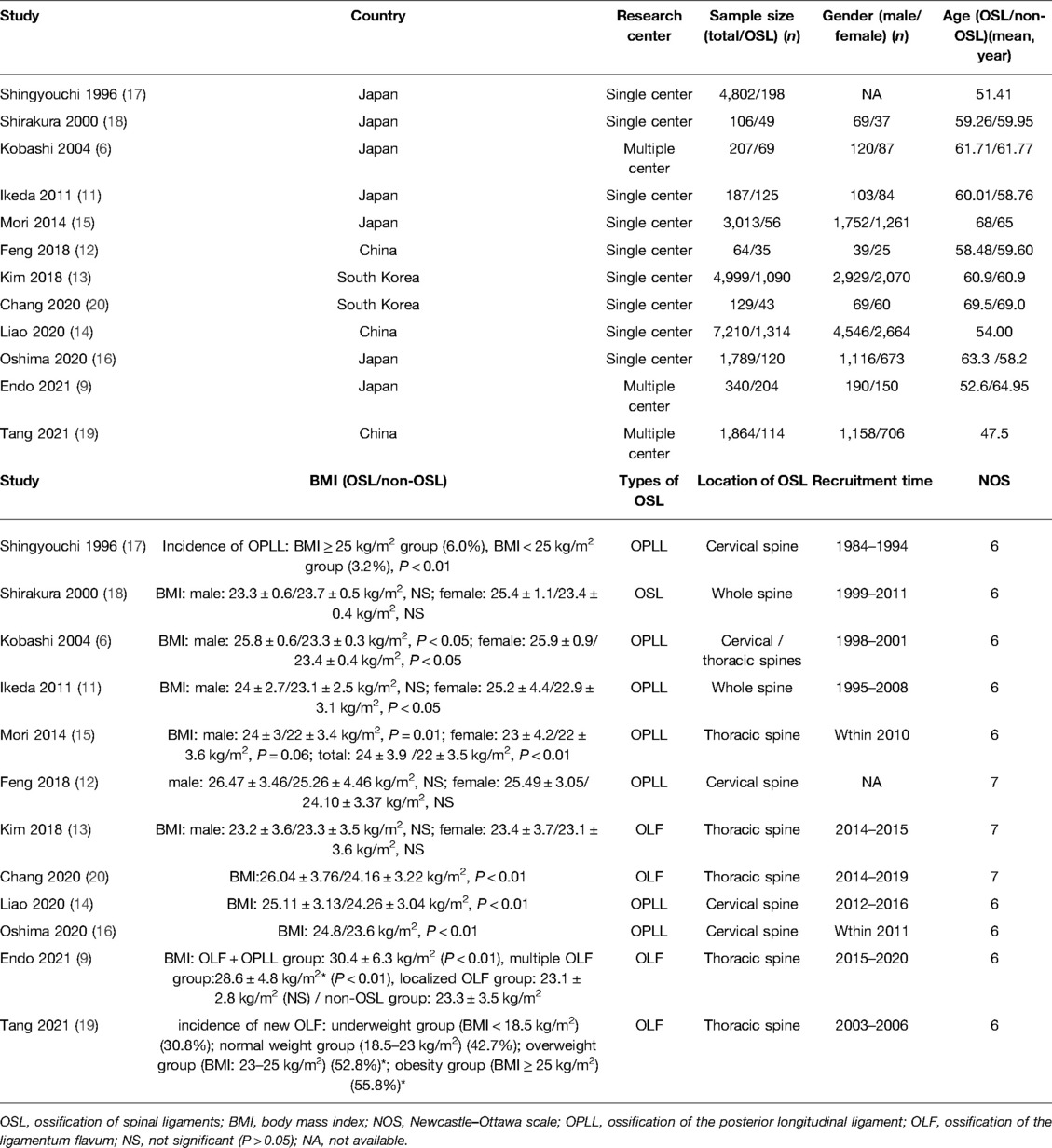
The precise pathogenesis of OSL remains unclear, and OSL is widely thought to be due to a mix of genetic and environmental factors (2–4). OSL typically occurs in Asians, especially the East Asian population, thereby indicating that genetic factors may play a vital role in its onset; however, many environmental factors, such as mechanical stress, metabolic disorders, gender, and age, may also contribute to the occurrence of OSL (5, 6). A few researchers have recently begun to focus on the relationship of obesity or body mass index (BMI) with the onset or severity of OSL because the clinical practice has presented the susceptibility of OSL in the obese population, although the results of existing studies are contradictory (6–20). Kobashi et al. (6) showed that the BMI of the OPLL group is significantly higher than that of the non-OPLL group in both male (25.8 kg/m2 vs. 23.3 kg/m2, P < 0.05) and female (25.9 kg/m2 vs. 23.4 kg/m2, P < 0.05) individuals. Similarly, Mori et al. (15) observed a significantly higher BMI in the OPLL group compared with that in the non-OPLL group (24 kg/m2 vs. 22 kg/m2, P < 0.05). However, Shirakura et al. (18) demonstrated that the BMI difference between OSL and non-OSL groups is nonsignificant (P > 0.05). Meanwhile, Feng et al. (12) also failed to observe the distinct difference in the BMI between OPLL and non-OPLL groups in both male (P > 0.05) and female (P > 0.05) individuals. Hirai et al. (10) observed a positive relationship between the BMI and ossification index in OPLL patients (P < 0.05) with consideration for the severity of OSL, thereby indicating that high BMI may increase the severity of OPLL. Similarly, Endo et al. (9) demonstrated there was a significantly higher BMI in patients with multilevel OLF compared with that in patients with localized OLF (P < 0.05). By contrast, Ikeda et al. (11) showed there was no significant difference in BMI between OPLL limited at the cervical spine and cervical OPLL extended to the thoracic or lumbar spine (P > 0.05). Therefore, the role of BMI in the onset and severity of OSL remains unverified. BMI is a typical measurement for the assessment of obesity, which is a very common metabolic disease that can be successfully managed with diet control and exercise (21). Obesity is associated with several human diseases, such as tumors, diabetes, and osteoarthritis (22–24). However, studies on the definite character of obesity in OSL are lacking. Hence, determining the role of BMI in the pathogenesis of OSL is essential in the prevention and treatment of this disease.
We performed a systematic review and meta-analysis in this study to determine the relationship of BMI with the onset and severity of OSL by integrating existing evidence. We speculated that high BMI could increase the risk of onset and severity of OSL.
Materials and Methods
This systematic review and meta-analysis was strictly conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (25) and registered in PROSPERO (https://www.crd.york.ac.uk/prospero/) (CRD42021289323). This study has been approved by the Ethics Committee of Peking University Third Hospital. The written informed consent was not necessary because all data were extracted from published studies.
Eligibility Criteria for Study Selection
Original investigations were eligible for inclusion if they reported data on the association of BMI with the onset or severity of OSL regardless of their study design. The following studies were directly excluded from this research: cadaver experiments, case reports, reviews, animal or cell experiments, non-English reports, and investigations containing duplicate patients for statistical analysis.
Information Source, Literature Search, and Study Selection
PubMed, EMBASE, Web of Science, and Cochrane Library were searched online on June 1, 2022 using the following keywords: “ossification of yellow ligament,” “ossification of ligamentum flavum,” “ossification of posterior longitudinal ligament,” “OPLL,” “ossification of the spinal ligament,” “BMI,” “body mass index,” and “obesity.” Details on the literature search in the PubMed database are listed in Supplementary Table S1. Eligible studies were then independently selected by two authors according to the eligibility criteria, and any disagreement was resolved by group discussion.
Data Collection
The following items were extracted from all included studies that met the inclusion criteria: authors' name, published year, country, research center, sample size, gender and age of patients, types of OSL, location of OSL, recruitment time of patients, and relevant data regarding the relationship of BMI with the onset or severity of OSL.
Risk of Bias in Individual Studies
The Newcastle–Ottawa scale (NOS), an assessment tool for cohort and case–control studies, was utilized in this work to evaluate the risk of bias in included studies (26). The NOS contains three main categories with a maximum of nine points, including the selection, comparability, and ascertainment of outcomes. NOS scores with 0–3, 4–6, and 7–9 stars were defined as high risk, moderate risk, and low risk, respectively (26).
Statistical Analysis
All analyses were performed using Review Manager 5.3 (Cochrane Collaboration, London, UK) and Stata 12.0 (StataCorp, College Station, TX). The difference in BMI between OSL (severe OSL group) and non-OSL (nonsevere OSL group) groups was evaluated using the mean difference (MD) with a confidence interval (CI) of 95%. Heterogeneity across the included studies was assessed using the chi-squared test, and a random-effects model was applied for the evident heterogeneity (P < 0.10, I2 ≥ 50%). Otherwise, a fixed-effect model should be used. Subgroup analyses stratified by gender and types of OSL were also performed to determine the association between BMI and onset of OSL further. Sensitivity analysis was conducted to check the stability of the pooled results. Publication bias among the included studies was assessed using Begg's and Egger's tests. A P value less than 0.05 indicated that the difference is statistically significant.
Results
Characteristics of the Included Studies
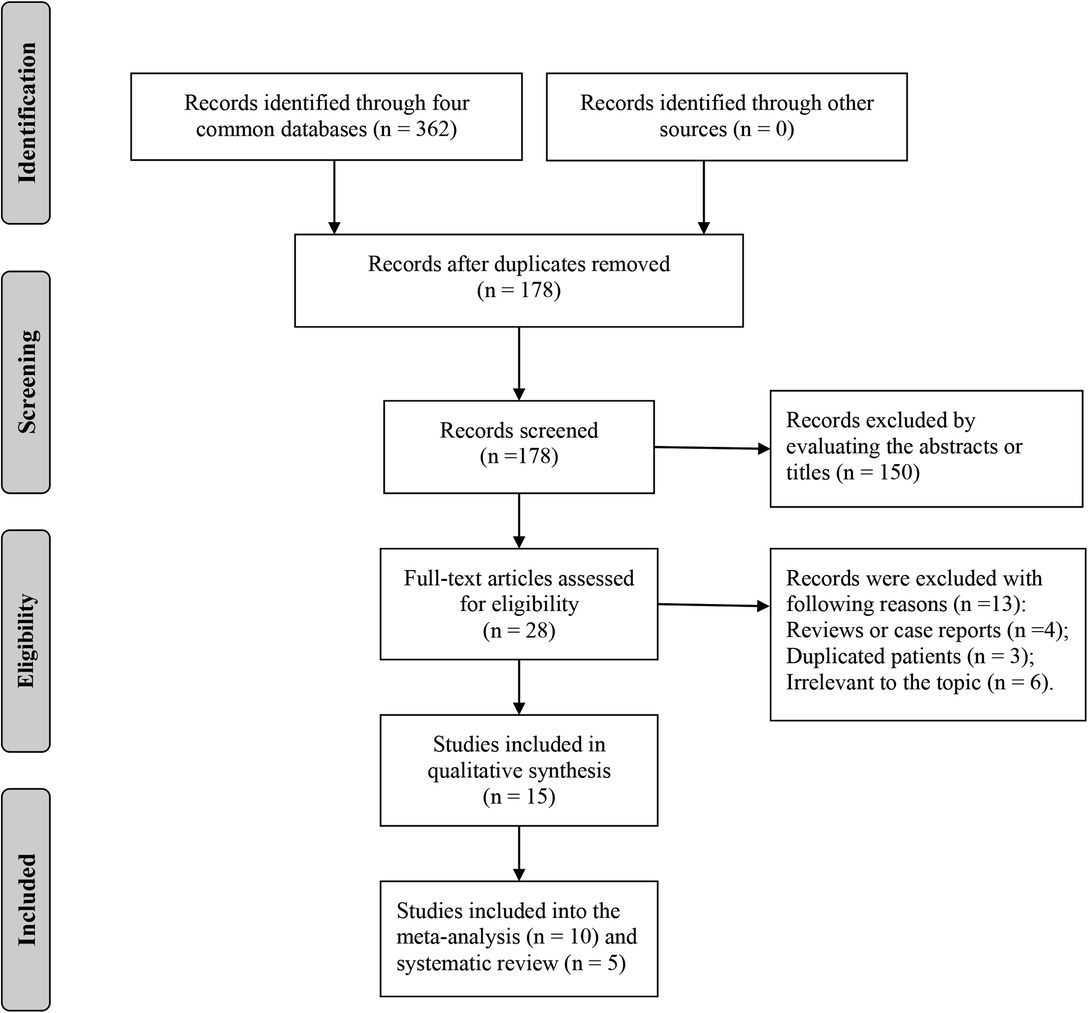
A total of 362 records were retrieved from PubMed, EMBASE, Web of Science, and Cochrane Library, and 15 studies were finally included in the systematic review and meta-analysis of this study (Figure 1) (6–20). Characteristics of the included studies are listed in Table 1. Ten studies focused on the OPLL (6, 7, 9–12, 14–17) and four studies focused on the OLF (9, 13, 19, 20) with respect to the types of OSL. Twelve studies containing 24,710 individuals explored the association between the BMI and onset of OSL (6, 9, 11–20), and five studies on 797 OSL patients explored the association between the BMI and severity of OSL (7–11). Notably, Ikeda et al. (11) and Endo et al. (9) both simultaneously analyzed the relationship of BMI with the onset and severity of OSL. Five studies with a NOS score of 7 were considered low risk (8, 10, 12, 13, 20) and ten studies with a NOS score of 6 were considered moderate risk (6, 7, 9, 11, 14–19) in terms of the risk of bias. The NOS details for each study are listed in Supplementary Table S2.
Relationship of BMI with the Onset of OSL
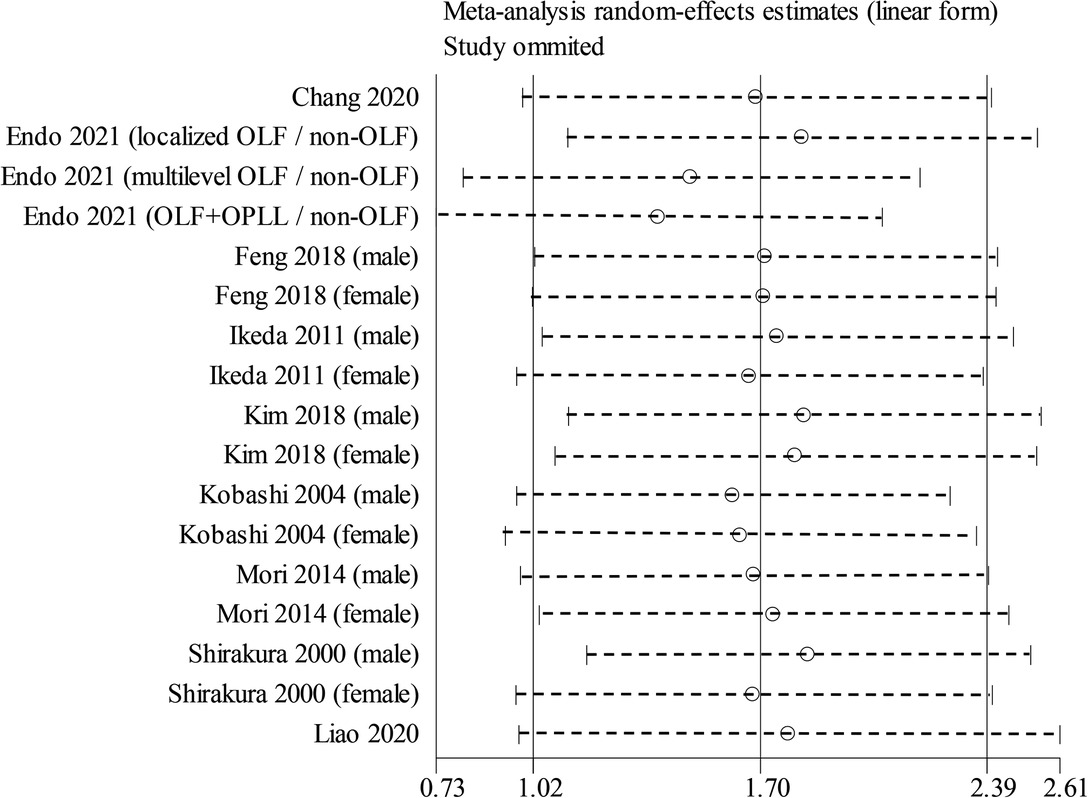
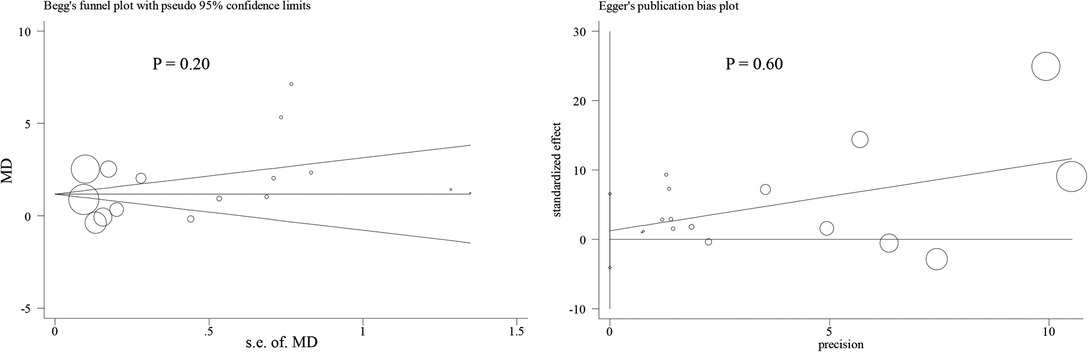
Nine studies containing 17 case–control pairs that compared the BMI between OSL and non-OSL groups were included in the meta-analysis (6, 9, 11–15, 18, 20). The results showed that patients in the OSL group demonstrated a significantly higher BMI compared with those in the non-OSL group (P < 0.01), with an MD of 1.70 kg/m2 (95% CI and 1.02–2.39 kg/m2) (Figure 2). Subgroup analyses according to the gender and types of OSL were subsequently performed. The BMI of the OSL group was significantly higher than that of the non-OSL group in female individuals (MD = 1.58, 95% CI, 0.54–2.63 kg/m2, and P < 0.01) but nonsignificant in male individuals (MD = 0.98, 95% CI, −0.46–2.42 kg/m2, and P = 0.18) (Figure 3). The subgroup analysis based on types of OSL showed there was a significantly higher BMI is observed in both OPLL (MD = 1.68, 95% CI, 0.91–2.46 kg/m2, and P < 0.01) and OLF (MD = 2.20, 95% CI, 0.77–3.62 kg/m2, and P < 0.01) groups compared with that in the non-OSL group (Figure 4). Moreover, the results of the meta-analysis were considered stable according to the sensitivity analysis (Figure 5) and without evident publication bias among included studies based on Begg's (P = 0.20) and Egger's (P = 0.60) tests (Figure 6).
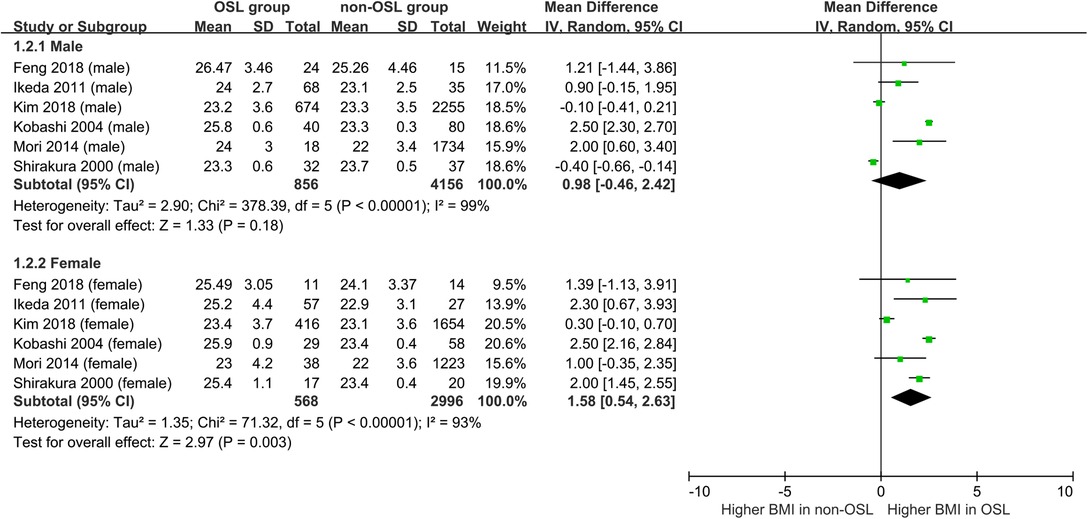
Figure 3. Subgroup analysis stratified by the gender for the relationship of BMI with the onset of OSL (top, males; bottom, females).
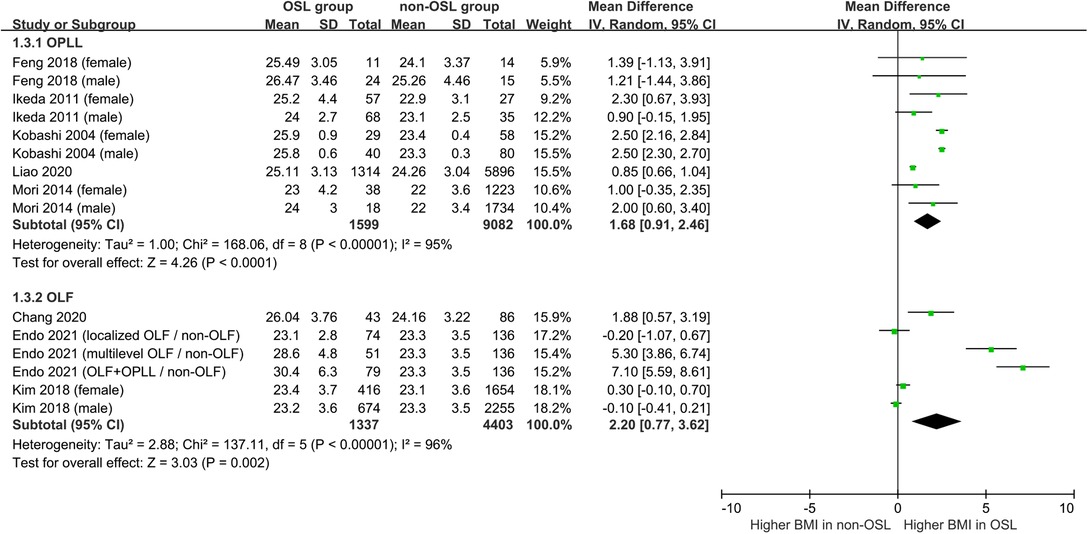
Figure 4. Subgroup analysis stratified by the type of OSL for the relationship of BMI with the onset of OSL (top, OPLL; bottom, OLF).
Three studies were excluded from this meta-analysis because of the different forms of data (16, 17, 19). Shingyouchi et al. (17) observed a significantly higher incidence of OPLL in the obesity group (BMI ≥ 25 kg/m2) compared with that in the nonobesity group (BMI < 25 kg/m2) (6.0% vs. 3.2%, P < 0.01). Tang et al. (19) also showed that new OLF typically occurs in obese (BMI ≥ 25 kg/m2) (55.8%) and overweight (23 ≤ BMI < 25 kg/m2) (52.8%) individuals compared with that in normal BMI (18.5 ≤ BMI < 23 kg/m2) (42.7%) and underweight (BMI < 18.5 kg/m2) (30.8%) (P = 0.02) individuals. Oshima et al. (16) reported that the BMI of OPLL individuals is significantly higher than that of non-OPLL individuals (24.8 kg/m2 vs. 23.6 kg/m2, P < 0.01).
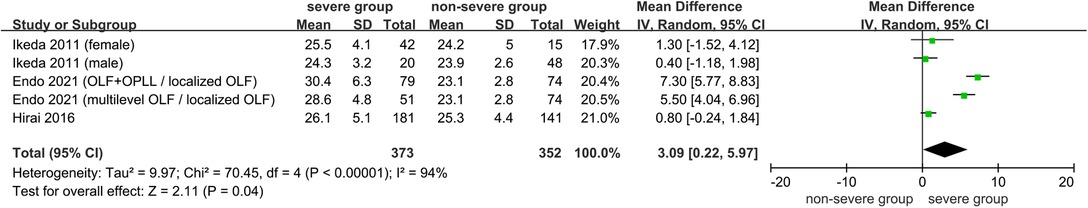
Relationship of BMI with the Severity of OSL
Five studies investigated the association between BMI and severity of OSL (7–11) (Table 2). Three studies assessed the relationship of BMI with the ossification index of OSL (7, 9, 10), which was calculated using the number of vertebral bodies and intervertebral disc level affected by the ossification site (27); meanwhile, the positive association of BMI with the ossification index of OPLL was observed using a standardized regression coefficient ranging from 0.11 to 0.43 in multiple regression analysis (P < 0.05) (7, 9, 10).
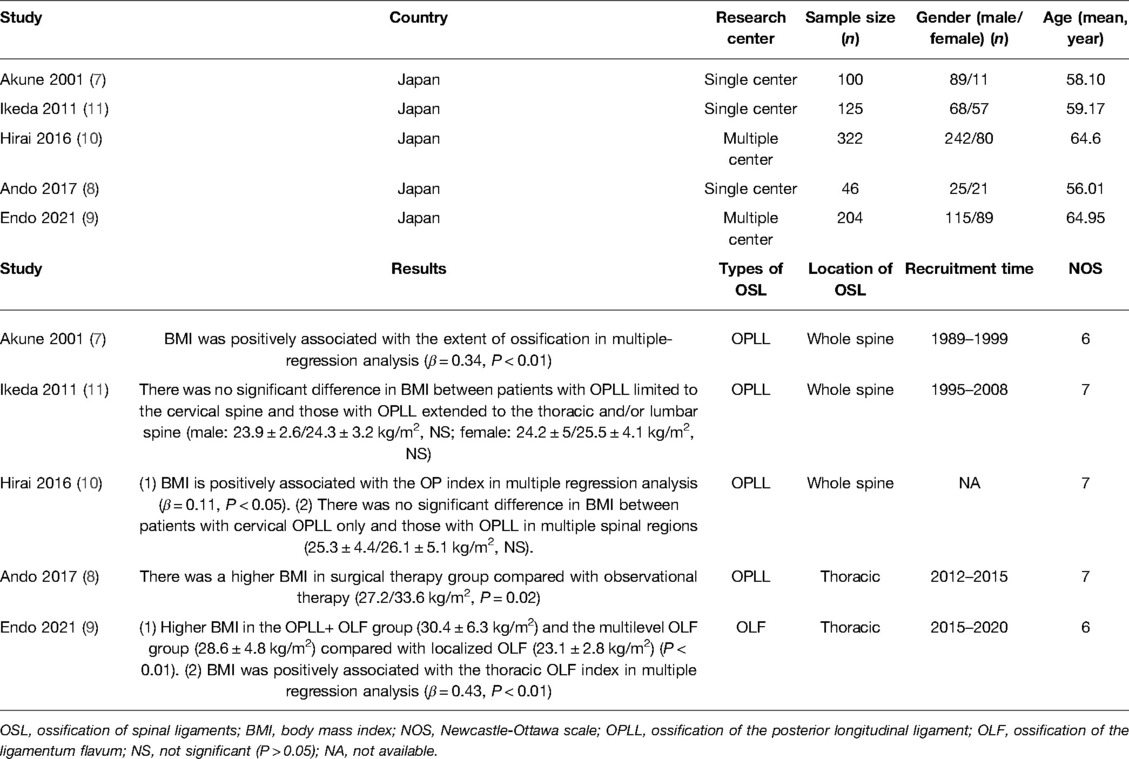
Table 2. Characteristics of included studies about the relationship of BMI with the severity of OSL.
Three studies comparing the BMI between severe OSL and nonsevere OSL groups were included in the meta-analysis (9–11). The results showed that the BMI in the severe OSL group is significantly higher than that in the nonsevere OSL group (MD = 3.09, 95% CI, 0.22–5.97 kg/m2, and P = 0.04) (Figure 7). Ando et al. (8) also revealed that the BMI of patients treated with surgical therapy for severe myelopathy is significantly higher than that of patients treated with observation therapy for the absence of or mild myelopathy (33.6 kg/m2 vs. 27.2 kg/m2, P = 0.02); however, this study was excluded from the meta-analysis due to the lack of standard deviation of BMI.
Discussion
OSL is a characterized heterotopic ossification disease with multiple potential risk factors likely associated with its pathogenesis (2, 3). Although several studies have explored the relationship between BMI and OSL, the results are conflicting and no definite conclusions have been obtained (6–20). Our study integrated the existing evidence and revealed that the BMI of the OSL group was higher than that of the non-OSL group (P < 0.01), thereby indicating that a high BMI may increase the risk of OSL. Moreover, a significant relationship existed between BMI and the ossification index of OPLL in three studies (P < 0.05), and the severe OSL group presents a higher BMI than the nonsevere OSL group (P = 0.04), thereby suggesting that a high BMI may aggravate the severity of OSL. Hence, our results showed that a high BMI would likely increase the onset and severity of OSL. To the best of our knowledge, this was the first study to perform a systematic review and meta-analysis to determine the relationship between BMI and the onset and severity of OSL.
We discovered that the significantly higher BMI of OSL individuals compared with that of non-OSL individuals might play an important role in the pathogenesis of this disease. Although previous studies indicated that a high BMI or obesity is involved in the pathology of several human diseases, such as tumors, diabetes, and cardiovascular diseases (28, 29), the underlying mechanism of a high BMI or obesity contributing to the pathogenesis of OSL remains unclear to date (12, 30). Leptin is secreted by adipose tissue and elevated in patients with OPLL and can promote the osteogenesis of OPLL cells via the ERK1/2, p38 MAPK, and/or JNK signaling pathways (12). Hyperleptinemia is a common characteristic of obese patients, and serum leptin is significantly increased in OPLL females compared to non-OPLL female controls (11). Similarly, hyperleptinemia was also observed in OLF patients, and the leptin-induced osteogenic effect in thoracic OLF cells may be associated with the activation of STAT3, JNK, and ERK1/2 (30). Furthermore, obesity is associated with a low-grade inflammation status, and adipose tissue can secrete several proinflammatory factors, such as C-reactive protein, tumor necrosis factor-α, and interleukin-6, which may exert crucial functions in the pathogenesis of OPLL or OLF (31, 32). It should be noted that although the OSL group typically exhibited a higher BMI than the non-OSL group in male individuals, this statistically nonsignificant difference requires further investigation. The following hypotheses are formed from these results: First, evident heterogeneity was observed across the included studies, and a random effect model may reduce the accuracy of the results. Second, the mean BMI of male individuals in the OSL group was low at 23.50 kg/m2, which may be insufficient to increase the risk of OSL. Additional studies should be conducted to determine the role of high BMI or obesity in the pathogenesis of OSL further.
We also explored the association of BMI with the severity of OSL, and three studies showed a positive association between BMI and the ossification index (P < 0.05), thereby indicating that high BMI likely promotes the severity of OSL (7, 9, 10). The results of our meta-analysis also presented that a high BMI is significantly associated with the severity of OSL. The association of high BMI with the severity of OSL may be interpreted by some published research studies (33, 34). Endo et al. (33) revealed the occurrence of a higher BMI in the early-onset OPLL group compared with that in the common OPLL group (34.2 kg/m2 vs. 25.6 kg/m2, P < 0.05). Katsumi et al. (34) reported that a high BMI is a potential risk factor for the annual rate of lesion increase in OPLL patients (P = 0.03); therefore, the higher severity of OSL in patients with higher BMI may be due to the earlier onset and faster progression of OSL compared with patients with lower BMI. Meanwhile, Tang et al. (19) presented that 22.8% (68/298 segments) of OLF spontaneously disappeared and the BMI of these patients reduced from 23.7 kg/m2 to 23.4 kg/m2 within the 5-year follow-up findings (P < 0.01); hence, OSL will likely instinctively improve and even disappear in nonobese patients. Notably, only one study assessed the severity of OSL based on the clinical symptoms instead of radiological data (8); however, previous studies have shown that evident symptoms are absent in a specific proportion of OSL cases (35). Therefore, future studies can focus on determining whether a high BMI can affect the severity of clinical symptoms in OSL.
The strengths of this systematic review and meta-analysis include the novelty of this topic, large population, strict methodology, and comprehensive subgroups. However, the current study also presents the following limitations. First, the majority of included studies were case–control types without a prospectively designed algorithm; hence, selection bias of individuals was unavoidable. Second, the characteristics of non-OSL individuals differed significantly across all studies. For instance, non-OSL individuals were patients with lumbar spondylosis instead of healthy patients in Chang et al. (20). As a result, the lenient selection of participants may affect the results of the current study. Third, high heterogeneity among the included studies and the use of a random-effects model for the meta-analysis may have affected the accuracy of the results. Fourth, the applicability of our conclusion to other races may be limited because all studies were performed on Asian populations. However, it should be noted that real-world data indicated that OSL frequently occurs in Asian populations, especially in the East Asian context (3). Fifth, the majority of the included studies explored the association of the BMI value instead of obesity or nonobesity with the onset or severity of OSL; as a result, an optimal cutoff value of the BMI remains unknown for the prediction of onset and severity of OSL. Finally, a selection bias may be induced because all the included studies were published in English.
Conclusion
High BMI values might be significantly associated with the onset and severity of OSL. The results of this study can provide new evidence and insights into the prevention and treatment of OSL. Future studies can focus on further exploring the underlying mechanism of a high BMI value in the pathogenesis and severity of OSL, the effect of high BMI on the onset of OSL in other races and male individuals, the relationship of BMI with the severity of clinical symptoms of OSL, and the optional cutoff value of BMI to predict the onset and severity of OSL.
Data Availability Statement
The original contributions presented in the study are included in the article/Suplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This study has been approved by the Ethics Committee of Peking University Third Hospital. The written informed consent was not necessary because all data were extracted from published studies.
Author Contributions
WL designed and supervised the study. YZ, QX, and JL extracted, assembled, and analyzed the data. YZ and SJ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82172480).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.941672/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kawaguchi Y. Biomarkers of ossification of the spinal ligament. Global Spine J. (2019) 9(6):650–7. doi: 10.1177/2192568218791799
2. Hirabayashi S. Ossification of the ligamentum flavum. Spine Surg Relat Res. (2017) 1(4):158–63. doi: 10.22603/ssrr.1.2016-0031
3. Saetia K, Cho D, Lee S, Kim DH, Kim SD. Ossification of the posterior longitudinal ligament: a review. Neurosurg Focus. (2011) 30(3):E1. doi: 10.3171/2010.11.FOCUS10276
4. Abiola R, Rubery P, Mesfin A. Ossification of the posterior longitudinal ligament: etiology, diagnosis, and outcomes of nonoperative and operative management. Global Spine J. (2016) 6(2):195–204. doi: 10.1055/s-0035-1556580
5. Tsukamoto N, Maeda T, Miura H, Jingushi S, Hosokawa A, Harimaya K, et al. Repetitive tensile stress to rat caudal vertebrae inducing cartilage formation in the spinal ligaments: a possible role of mechanical stress in the development of ossification of the spinal ligaments. J Neurosurg Spine. (2006) 5(3):234–42. doi: 10.3171/spi.2006.5.3.234
6. Kobashi G, Washio M, Okamoto K, Sasaki S, Yokoyama T, Miyake Y, et al. High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: a case-control study in multiple hospitals. Spine (Phila Pa 1976). (2004) 29(9):1006–10. doi: 10.1097/00007632-200405010-00011
7. Akune T, Ogata N, Seichi A, Ohnishi I, Nakamura K, Kawaguchi H. Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg A. (2001) 83(10):1537–44. doi: 10.2106/00004623-200110000-00013
8. Ando K, Imagama S, Kobayashi K, Hida T, Ito K, Tsushima M, et al. Comparative study of surgical treatment and nonsurgical follow up for thoracic ossification of the posterior longitudinal ligament: radiological and clinical evaluation. Spine. (2017) 42(6):407–10. doi: 10.1097/BRS.0000000000001769
9. Endo T, Koike Y, Hisada Y, Fujita R, Suzuki R, Tanaka M, et al. Aggravation of ossified ligamentum flavum lesion is associated with the degree of obesity. Global Spine J. (2021):21925682211031514. doi: 10.1177/21925682211031514. [Epub ahead of print].
10. Hirai T, Yoshii T, Iwanami A, Takeuchi K, Mori K, Yamada T, et al. Prevalence and distribution of ossified lesions in the whole spine of patients with cervical ossification of the posterior longitudinal ligament a multicenter study (JOSL CT study). PLoS ONE. (2016) 11(8). doi: 10.1371/journal.pone.0160117
11. Ikeda Y, Nakajima A, Aiba A, Koda M, Okawa A, Takahashi K, et al. Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur Spine J. (2011) 20(9):1450–8. doi: 10.1007/s00586-011-1688-7
12. Feng B, Cao SL, Zhai JL, Ren Y, Hu JH, Tian Y, et al. Roles and mechanisms of leptin in osteogenic stimulation in cervical ossification of the posterior longitudinal ligament. J Orthop Surg Res. (2018) 13(1):165. doi: 10.1186/s13018-018-0864-4
13. Kim SI, Ha KY, Lee JW, Kim YH. Prevalence and related clinical factors of thoracic ossification of the ligamentum flavum – a computed tomography-based cross-sectional study. Spine J. (2018) 18(4):551–7. doi: 10.1016/j.spinee.2017.08.240
14. Liao X, Jin Z, Shi L, Zhao Y, Zhou S, Chen D, et al. Prevalence of ossification of posterior longitudinal ligament in patients with degenerative cervical myelopathy: cervical spine 3D CT observations in 7210 cases. Spine. (2020) 45(19):1320–8. doi: 10.1097/BRS.0000000000003526
15. Mori K, Imai S, Kasahara T, Nishizawa K, Mimura T, Matsusue Y. Prevalence, distribution, and morphology of thoracic ossification of the posterior longitudinal ligament in Japanese: results of ct-based cross-sectional study. Spine. (2014) 39(5):394–9. doi: 10.1097/BRS.0000000000000153
16. Oshima Y, Doi T, Kato S, Taniguchi Y, Matsubayashi Y, Nakajima K, et al. Association between ossification of the longitudinal ligament of the cervical spine and arteriosclerosis in the carotid artery. Sci Rep. (2020) 10(1):3369. doi: 10.1038/s41598-020-60248-3
17. Shingyouchi Y, Nagahama A, Niida M. Ligamentous ossification of the cervical spine in the late middle-aged Japanese men: its relation to body mass index and glucose metabolism. Spine. (1996) 21(21):2474–8. doi: 10.1097/00007632-199611010-00013
18. Shirakura Y, Sugiyama T, Tanaka H, Taguchi T, Kawai S. Hyperleptinemia in female patients with ossification of spinal ligaments. Biochem Biophys Res Commun. (2000) 267(3):752–5. doi: 10.1006/bbrc.1999.2027
19. Tang CYK, Cheung KMC, Samartzis D, Cheung JPY. The natural history of ossification of yellow ligament of the thoracic spine on MRI: a population-based cohort study. Global Spine J. (2021) 11(3):321–30. doi: 10.1177/2192568220903766
20. Chang SY, Kim Y, Kim J, Kim H, Kim HJ, Yeom JS, et al. Sagittal alignment in patients with thoracic myelopathy caused by the ossification of the ligamentum flavum: a retrospective matched case-control study. Spine (Phila Pa 1976). (2021) 46(5):300–6. doi: 10.1097/BRS.0000000000003791
21. Gjermeni E, Kirstein AS, Kolbig F, Kirchhof M, Bundalian L, Katzmann JL, et al. Obesity-an update on the basic pathophysiology and review of recent therapeutic advances. Biomolecules. (2021) 11(10). doi: 10.3390/biom11101426
22. Wei Y, Zhan Y, Löfvenborg JE, Tuomi T, Carlsson S. Birthweight, BMI in adulthood and latent autoimmune diabetes in adults: a Mendelian randomisation study. Diabetologia. (2022):10.1007/s00125-022-05725-2. doi: 10.1007/s00125-022-05725-2. [Epub ahead of print]
23. Lee S, Jang J, Abe SK, Rahman S, Saito E, Islam R, et al. Association between body mass index and oesophageal cancer mortality: a pooled analysis of prospective cohort studies with >800 000 individuals in the Asia Cohort Consortium. Int J Epidemiol. (2022):dyac023. doi: 10.1093/ije/dyac023. [Epub ahead of print].
24. Khor A, Ma CA, Hong C, Hui LL, Leung YY. Diabetes mellitus is not a risk factor for osteoarthritis. RMD Open. (2020) 6(1). doi: 10.1136/rmdopen-2019-001030
25. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319(4):388–96. doi: 10.1001/jama.2017.19163
26. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
27. Kawaguchi Y, Nakano M, Yasuda T, Seki S, Hori T, Kimura T. Ossification of the posterior longitudinal ligament in not only the cervical spine, but also other spinal regions: analysis using multidetector computed tomography of the whole spine. Spine (Phila Pa 1976). (2013) 38(23):E1477–82. doi: 10.1097/BRS.0b013e3182a54f00
28. Bendor CD, Bardugo A, Pinhas-Hamiel O, Afek A, Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. (2020) 19(1):79. doi: 10.1186/s12933-020-01052-1
29. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. (2017) 127(1):1–4. doi: 10.1172/JCI92035
30. Fan D, Chen Z, Chen Y, Shang Y. Mechanistic roles of leptin in osteogenic stimulation in thoracic ligament flavum cells. J Biol Chem. (2007) 282(41):29958–66. doi: 10.1074/jbc.M611779200
31. Kawaguchi Y, Nakano M, Yasuda T, Seki S, Suzuki K, Yahara Y, et al. Serum biomarkers in patients with ossification of the posterior longitudinal ligament (OPLL): inflammation in OPLL. PLoS One. (2017) 12(5):e0174881. doi: 10.1371/journal.pone.0174881
32. Ren L, Hu H, Sun X, Li F, Zhou JJ, Wang YM. The roles of inflammatory cytokines in the pathogenesis of ossification of ligamentum flavum. Am J Transl Res. (2013) 5(6):582–5.
33. Endo T, Imagama S, Kato S, Kaito T, Sakai H, Ikegawa S, et al. Association between vitamin A intake and disease severity in early-onset heterotopic ossification of the posterior longitudinal ligament of the spine. Global Spine J. (2021):2192568221989300. doi: 10.1177/2192568221989300. [Epub ahead of print]
34. Katsumi K, Watanabe K, Izumi T, Hirano T, Ohashi M, Mizouchi T, et al. Natural history of the ossification of cervical posterior longitudinal ligament: a three dimensional analysis. Int Orthop. (2018) 42(4):835–42. doi: 10.1007/s00264-017-3667-z
Keywords: ossification of the ligamentum flavum, ossification of the posterior longitudinal ligament, body mass index, onset, severity, systematic review, meta-analysis
Citation: Zhao Y, Xiang Q, Lin J, Jiang S and Li W (2022) High Body Mass Index Is Associated with an Increased Risk of the Onset and Severity of Ossification of Spinal Ligaments. Front. Surg. 9:941672. doi: 10.3389/fsurg.2022.941672
Received: 11 May 2022; Accepted: 21 June 2022;
Published: 22 July 2022.
Edited by:
Qiling Yuan, Xi'an Jiaotong University, ChinaReviewed by:
Xiao-ming Zhao, Xian Jiaotong University, ChinaZiquan Li, Peking Union Medical College Hospital (CAMS), China
Jian Weng, Peking University, China
Chensheng Qiu, Medical College of Wisconsin, United States
Copyright © 2022 Zhao, Xiang, Lin, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weishi Li cHVoM2xpd2Vpc2hpQDE2My5jb20=
†These authors have contributed equally to this work.
Specialty section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Abbreviations: OSL, ossification of the spinal ligament; OPLL, ossification of the posterior longitudinal ligament; OLF, ossification of the ligamentum flavum; BMI, body mass index; NOS, Newcastle–Ottawa scale; MD, mean difference; CI, confidence interval.
 Yongzhao Zhao1,2,3,†
Yongzhao Zhao1,2,3,† Jialiang Lin
Jialiang Lin Weishi Li
Weishi Li