- 1Department of Urology, Second Affiliated Hospital of Naval Medical University, Shanghai, China
- 2Urology Centre, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
Objective: The limitations of tissue retraction and the amount of surgical working space have a great impact on extraperitoneal single-port robotic-assisted radical prostatectomy (sp-RARP) with the multiport robotic surgical system. We used an extraperitoneal tissue retraction technique to achieve tissue exposure and working space expansion. This study evaluated the safety, feasibility, and efficacy of the extraperitoneal tissue retraction technique in extraperitoneal pure sp-RARP with the da Vinci Si surgical system.
Methods: Data from 42 patients were analyzed retrospectively from December 2018 to February 2020. The extraperitoneal tissue retraction technique was not used in 20 patients (group I) and was used in 22 patients (group II). Preoperative, intraoperative, and postoperative data were collected. The oncological and functional data during late follow-up were recorded.
Results: All patients successfully underwent extraperitoneal pure sp-RARP. No patients required conversion to a multiport surgery or placement of additional assistant ports. The two groups were similar regarding baseline features. The median operation time in group I was significantly longer than that in group II (P < 0.001). The estimated blood loss volume in group I was significantly higher than that in group II (P < 0.001). There were no serious complications in either group. There were four cases of peritoneal tears in group I and none in group II (P = 0.043). The surgical margin and lymph nodes were negative in both groups. The oncological and functional outcomes were similar between the two groups 6 months after the procedure.
Conclusions: The extraperitoneal tissue retraction technique is safe and feasible. The technique promotes tissue exposure and expands the surgical working space, which is important for achieving extraperitoneal pure sp-RARP with the da Vinci Si surgical system, especially for beginners. The short-term oncological and functional outcomes were within acceptable ranges. The long-term effects of this technique need further evaluation.
Introduction
Robot-assisted radical prostatectomy (RARP) is the standard treatment for localized prostate cancer (PCa) (1, 2). Currently, we are in a technology-driven era aimed at balancing the maximization of oncological results with the minimization of surgery-related impacts on patient quality of life (3, 4). Single-port RARP (sp-RARP) is considered the direction for future development (5, 6). The use of single-port surgery can not only reduce trauma and speed up postoperative recovery but also increase patient satisfaction in terms of cosmetics and reduce their psychological trauma (4, 7, 8).
The new da Vinci SP surgical system has shown great potential for sp-RARP (9, 10). However, the SP platform is not approved for use in China. Most medical centers still use the da Vinci Si/Xi system for RP. These platforms are not specifically designed for single-port surgery, and many restrictions are associated with single-port surgery (11–13). In a previous study, we successfully completed a series of extraperitoneal pure sp-RARP procedures without adding an auxiliary port using the da Vinci Si system and showed its advantages in terms of cosmetics, pain, and postoperative recovery time, which was rapid (14, 15). Due to the lack of a fourth robotic arm, we found that tissue exposure and working space constraints posed great challenges to the use of extraperitoneal pure sp-RARP. The need for a means of tissue retraction that can overcome exposure and challenges with extraperitoneal space was evident. By learning from the experience of White et al. (11) and through refinements of the technique, we adopted an extraperitoneal tissue retraction technique to help operators achieve extraperitoneal pure sp-RARP.
The purpose of this study was to verify the safety and feasibility and describe the details of the extraperitoneal tissue retraction technique. We hope to better define the precise role of the extraperitoneal tissue retraction technique and encourage more doctors to explore the use of extraperitoneal pure sp-RARP.
Materials and methods
Patients and data collection
The data of 42 consecutive patients with localized PCa who underwent extraperitoneal pure sp-RARP from December 2018 to February 2020 were analyzed retrospectively. Twenty patients were not treated with the extraperitoneal tissue suspension technique (group I), and 22 patients were treated with the extraperitoneal tissue suspension technique (group II). The exclusion criteria included a previous infraumbilical midline incision, body mass index (BMI) of >40 kg/m2, preoperative prostate-specific antigen (PSA) levels of >20 ng/ml, biopsy Gleason score of >7, prior prostate treatment, or preoperative evidence of extraprostatic disease. Preoperative assessment, staging, and risk stratification were performed using physical examination, prostate biopsy results, PSA levels, and imaging examination. After a comprehensive discussion, informed consent was obtained.
The baseline characteristics of the patients, operation time (from skin incision to skin closure), estimated blood loss (EBL), peritoneal tear, intraoperative complications, and duration of hospital stay were collected. Complications were assessed intraoperatively or postoperatively using the Clavien–Dindo classification system and were classified as major (grade ≥III) or minor (grade ≤II) (16). Pathology data, including the final pathological stage, positive surgical margins (PSM), and lymph node invasion, were recorded. All patients were followed up regularly to monitor the state of urinary continence and biochemical recurrence. Urinary continence was evaluated by the number of urine pads used per day (17). No use or the use of no more than one pad/day was considered to reflect good urinary continence. All the patients underwent sp-RARP by two surgeons who were experienced in multiport robotic surgery but did not go beyond their learning curve with sp-RARP. The patients signed a written agreement to participate. The patients undergoing single-port surgery were advised to receive additional assistance ports as needed during the operation.
Surgical procedure
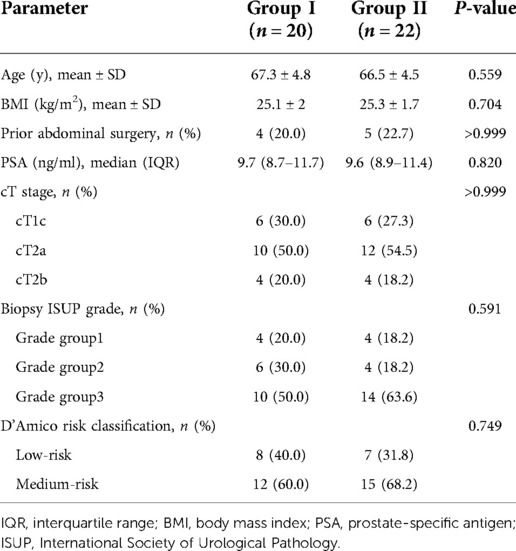
All patients underwent surgery with the da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, United States). A 4–5 cm transverse incision was made approximately 5 cm above the pubic symphysis (Figure 1A). Then, an 8-cm quadri-channel laparoscopic port (Lagis Inc., Taichung, China) was placed. The scope holder arm and two primary robotic arms were used (Figure 1B). The patients were placed in a low lithotomy position with no steep Trendelenburg position. Prophylactic single-dose intravenous antibiotics (e.g., cephalosporins) and subcutaneous prophylactic heparin (2,000 IU) were administered prior to surgery. Patients with PSA levels of >10 ng/ml underwent pelvic lymph node dissection. The main surgical steps of the operation were described in our previous report (14). A drainage tube was routinely placed (Figure 1C).
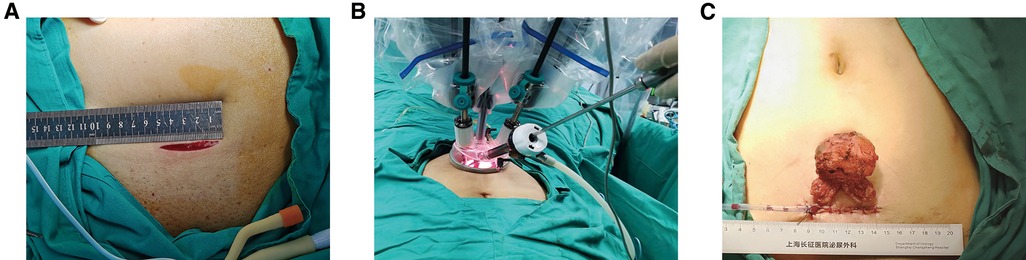
Figure 1. Illustration of extraperitoneal pure sp-RARP. (A) A 4–5 cm transverse abdominal incision approximately 5 cm above the pubic symphysis. (B) Intraoperative installation showing a scope holder arm and the two primary robotic arms. (C) Wound closure with drainage placed in the single-port incision. sp-RARP, single-port robotic-assisted radical prostatectomy.
Key procedure of the extraperitoneal tissue retraction technique
The marionette technique was carried out by inserting retraction sutures into the needle of a 20 ml syringe (Figure 2A). When the tissue needed to be retracted, the needle was inserted vertically into the abdominal wall close to the midline of the pubic symphysis, and the retraction sutures were pulled out and fixed onto the tissue through a hem-o-lock (Figure 2B). The retraction sutures were fixed outside the abdominal wall with a vascular clamp. The position of the sutures was adjusted to retract the desired tissues according to the operation procedure.
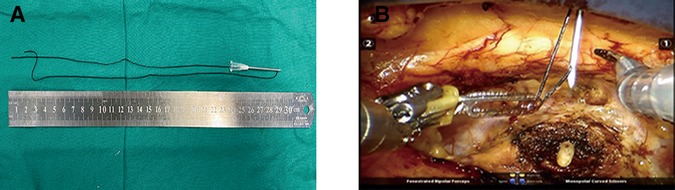
Figure 2. Homemade abdominal puncture device and its application points. (A) The abdominal puncture needle was made by inserting retraction sutures into the needle of a 20 ml syringe. (B) The needle was inserted vertically into the abdominal wall near the midline of the pubic symphysis, and the retraction sutures were pulled out by a robotic arm.
An extraperitoneal tissue retraction technique might be required at some critical stages of extraperitoneal pure sp-RARP. First, during bladder neck dissection, retractor pressure was applied to the bladder and catheter, which facilitated visualization of the vesicoprostatic junction. Without the assistance of the fourth arm in the extraperitoneal pure sp-RARP, one robotic arm needed to act as a retractor, which might have increased the risk of detrusor fiber damage and bleeding (Figure 3A). We fixed the retraction sutures and the catheter together to ensure a certain suspension tension (Figure 3B), which was used to replace the role of the fourth arm in conventional RARP. Second, the surgical field needed not to be disturbed when separating the vas deferens and seminal vesicles. A clear surgical field could be maintained with the use of conventional RARP with the help of the assistant port and the fourth arm, which were absent in sp-RARP. We needed the help of an extraperitoneal tissue retraction technique to expose the space. When one side of the seminal vesicle and vas deferens were dissected, the retraction sutures, dissected seminal vesicle, and vas deferens were suspended together. This approach was beneficial to the dissection of the vas deferens and seminal vesicles on the other side (Figure 3C). Third, in the process of the neurovascular bundle (NVB) sparing, because of the need for multiple operations and fine movements, we required greater exposure to the surgical visual field. The prostate, seminal vesicles, and vas deferens needed to be retracted using the extraperitoneal tissue retraction technique (Figure 3D). Finally, separating the ampullae from the seminal vesicles could challenge the visualization of the posterior Denonvilliers' fascia, particularly in patients with large seminal vesicles. Robotic arms were used to retract the ampullae and their attached seminal vesicles anteriorly to help identify the anterior rectal wall in epR-spRP (Figure 3E). Incising Denonvilliers' fascia with one remaining robotic arm increases the risk of rectal injury by entering the wrong layer, and especially increases the risk of tissue adhesion. The sutures were used to retract the seminal vesicle and vas deferens anteriorly, which promoted Denonvilliers' fascia exposure and reduced rectal injury (Figure 3F). The key procedures of the extraperitoneal tissue retraction technique were shown in the video as Supplementary material.
Figure 3. Key procedure of the extraperitoneal tissue retraction technique. (A) Because the assistance of the fourth arm in extraperitoneal pure sp-RARP was absent, one robotic arm needed to act as a retractor to stretch the catheter. (B) The retraction sutures and catheter were fixed together during dissection of the bladder neck. (C) The retraction sutures and prostate needed to be fixed together, which facilitates the dissection of the vas deferens and seminal vesicles. (D) During dissection of the NVB, the prostate, seminal vesicles, and vas deferens were suspended. (E) Use of robotic arms to retract the ampullae and their attached seminal vesicles anterior to help identify the anterior rectal wall in extraperitoneal pure sp-RARP. (F) The prostate, seminal vesicles, and vas deferens were retracted to expose the visual field and visualize Denonvilliers' fascia. sp-RARP: single-port robotic-assisted radical prostatectomy; NVB: neurovascular bundles.
Statistical analyses
Normally distributed data were analyzed with the one-sample Kolmogorov–Smirnov test. Univariate analysis was performed using parametric (Student's t-test) and nonparametric (Mann–Whitney U test) tests for continuous variables, and the chi-square test (or Fisher exact test) was used for categorical variables, as appropriate. Statistical significance was set at P < 0.05.
Results
Baseline characteristics
There was no difference in age, BMI, preoperative PSA levels, or clinical stage between the two groups. We routinely performed prostate magnetic resonance imaging and whole-body bone scans before RP to rule out the occurrence of local metastasis and bone metastasis. Preoperative prostate magnetic resonance imaging and whole-body bone scans showed no metastasis in any patient. According to the D’Amico risk classification for PCa (18), there were eight low-risk patients in group I and seven low-risk patients in group II, and all the others were medium-risk patients. In group I, four patients (20.0%) had a history of abdominal surgery. In group II, five patients (22.7%) had a history of abdominal surgery. The perioperative baseline characteristics of the patients are shown in Table 1.
Surgical outcomes
All patients successfully underwent extraperitoneal pure sp-RARP. No patient required additional port placement. No patient's treatment was changed to multiport RARP or open surgery. Lymph node dissection was performed in 12 patients in group I and 15 patients in group II. The median operation time in group I was significantly longer than that in group II (175.0 vs. 131.5 min; P < 0.001). The EBL in group I was also significantly higher than that in group II (163.4 vs. 117.6 ml, P < 0.001). No patient required transfusion. There was no difference in the duration of hospital stay between the two groups (P = 0.519). The incidence of complications in the two groups was similar, and there were no complications worse than Clavien–Dindo grade II. NVB sparing was not significantly different between the groups. There were four cases of peritoneal tears in group I and none in group II (P = 0.043). The surgical margin and lymph nodes were negative in both groups. The surgical results and postoperative pathological stages are shown in Table 2.
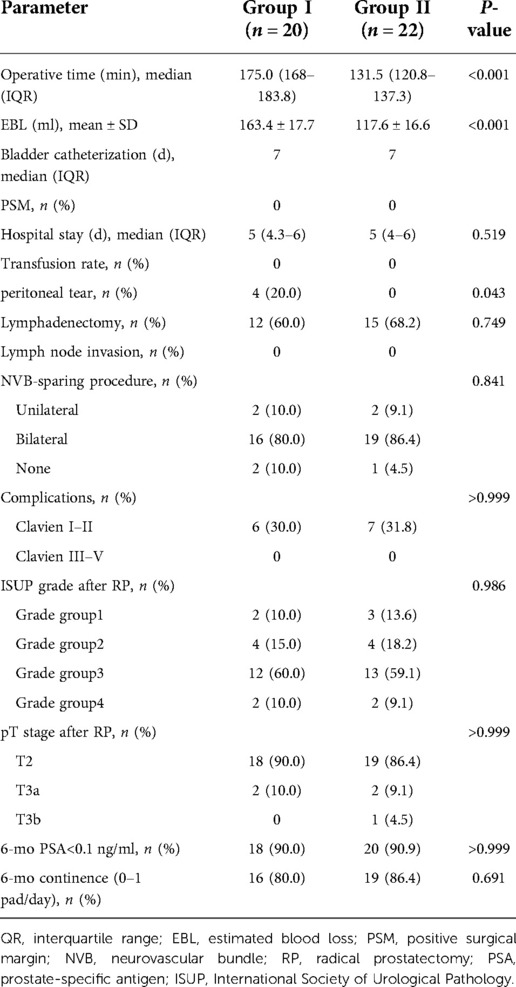
Table 2. Comparisons of intraoperative and postoperative data, complications between not using extraperitoneal tissue retraction technique (group I) and using extraperitoneal tissue retraction technique (group II).
Six-month oncological and functional outcomes
All patients had enough follow-up for 6-month postoperative PSA levels and continence data. The rate of PSA levels of <0.1 ng/ml was over 90% in both groups (P > 0.999). The rate of patients who used 0–1 pads/day was not different between the two groups (P = 0.691). The oncological and functional results are shown in Table 2.
Discussion
The advantages of robotic surgical systems include reduced instrument crossover, excellent ergonomic value, restored instrument triangulation, and improved effectiveness of single-port surgery (19, 20). The extraperitoneal approach RARP avoids entering the peritoneal cavity has minimal influence on intestinal function, and does not require a steep Trendelenburg position, which is more beneficial for postoperative recovery (21, 22). However, there are significant limitations to using a multiport surgical platform for single-port surgery, especially during instrument collisions and intraoperative sutures (20). Furthermore, the extraperitoneal working space is narrower and more limited (23). Expanding the working space and exposing the tissue are very important for the use of extraperitoneal pure sp-RARP with the da Vinci Si surgical system. In the absence of the new SP platform, we believe that the extraperitoneal tissue retraction technique is an effective method for solving the problem of limited surgical working space and tissue retraction in extraperitoneal pure sp-RARP, especially for beginners.
At present, a straight needle is used in some tissue retraction methods (10, 11). Nevertheless, the straight needle is relatively long and needs to pass through the abdominal wall twice, which may lead to an increase in the risk of injury in the narrow extraperitoneal space. Steinberg et al. (24) used a magnetic retractor to assist in tissue retraction in robotic prostatectomy with the new SP system. We used a 20 ml syringe needle as a handy tool to assist in tissue retraction. The length of the needle is very suitable for the depth of the extraperitoneal space. The needle does not need to be removed from the abdominal wall twice, which reduces the risk of inferior epigastric vessel injury. The surgeon can adjust the position of the suture according to the operation process to make the surgery more autonomous. However, our tissue retraction equipment was easy to manufacture and conferred no additional cost.
The operation time and bleeding volume are important indicators of the safety and feasibility of a technique. Wilson et al. reported that the operation time and EBL associated with extraperitoneal radical prostatectomy via the SP platform were 198.0 min and 179 ml, respectively (25). A high-volume surgical center showed that the average operation time and average EBL of conventional extraperitoneal robotic RP were 146 min and 100 ml, respectively (26). The operation time (175.0 min) and EBL (163.4 ml) exceeded our expectations in patients who did not receive the extraperitoneal tissue retraction technique in our study. Due to the lack of the fourth arm and additional assistant ports, tissue retraction and space exposure of the narrow pelvis are strictly limited. Additionally, the collision of instruments occurs in the upright environment of the robotic arm. The bedside assistant cannot be used to focus on suction/irrigation and clip application. The unstable visual field of the operation may be an important factor leading to an increase in operation time and bleeding volume. The extraperitoneal tissue retraction technique benefits tissue exposure, increases the surgical space, and maintains a stable surgical view. The results showed that the operation time and EBL decreased significantly after the extraperitoneal tissue retraction technique.
Injury to inferior epigastric vessels caused by abdominal puncture is also a concern (27). There was no inferior epigastric vessel injury or bleeding in our patients that were subjected to the extraperitoneal tissue retraction technique. Our experience was that the puncture point was as close to the midline of the pubic symphysis as possible, and the needle was inserted vertically. In our study, no major complications were observed in either group. Although no significant reduction in NVB sparing was observed in patients that did not undergo the extraperitoneal tissue retraction technique, more time was required to eliminate interference and perform a detailed dissection. This finding might be related to the reduction in visual field interference that occurs with the extraperitoneal tissue retraction technique.
The PSM rate is our key concern and is directly related to the prognosis of PCa patients (28, 29). PSM was present in up to 17% of patients when experienced surgeons performed RP (30). In this study, there were no patients with PSM in either group. The rate of patients with PSA levels of <0.1 ng/ml in both groups was over 90% during the short follow-up period. More than 80% of the patients recovered continence within 6 months. The low PSM rate, the short-term oncological outcomes, and the recovery of urinary continence may be related to the proportion of low/medium-risk patients and the insufficient sample size in our study.
The limitations of this study should be mentioned. Overall, this was a retrospective study, and the sample size was small. The patients who underwent extraperitoneal pure sp-RARP with the da Vinci Si surgical system were highly screened individuals and adopted a relatively conservative treatment approach. Therefore, these results were preliminary, and the risk of selection bias was inevitable. During the study, we determined the trends in the clinical benefits provided by the extraperitoneal tissue retraction technique. Thus, only a small number of patients were not treated with the extraperitoneal tissue retraction technique, which may have caused bias in the results and weakened the conclusions regarding oncological and functional outcomes. However, this was considered the best course of action for the patients. This approach is still being investigated, and we will consider these limitations in further studies in a larger cohort of patients. We believe that this technique could be used in other single-port operations, such as during retroperitoneal single-port kidney and gynecological surgery. We encourage more doctors to explore the limitations of the technology and assess its potential benefits.
Conclusions
The extraperitoneal tissue retraction technique facilitates tissue exposure and expands the operation space in extraperitoneal pure sp-RARP with the da Vinci Si surgical system. It is safe, feasible, and effective, especially for beginners, to complete extraperitoneal pure sp-RARP. The short-term oncological and functional were promising but will require longer-term follow-up. Although we lack a new SP platform, we have demonstrated our commitment to maximizing the clinical benefit for patients. Randomized trials with adequate sample sizes and postoperative follow-up periods are necessary.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Second Affiliated Hospital of Naval Medical University, Shanghai, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GJ, DX, and SR contributed to the provision of study materials or patients, conception, and design. GJ, ZW, and JS contributed to the collection and assembly of data. GJ, WX, ZZ, and LY contributed to the data analysis and interpretation. GJ, ZW, JS, and SR contributed to the manuscript writing. GJ, DX, and SR contributed to the critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
This study was supported by the Medical Guidance Project of the Shanghai Science and Technology Committee (No. 19411967600) and the Clinical Research Special Youth Project of the Shanghai Municipal Health Commission (No. 20194Y0208).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.941104/full#supplementary-material.
References
1. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. (2021) 79(2):243–62. doi: 10.1016/j.eururo.2020.09.042
2. Novara G, Ficarra V, Rosen RC, Artibani W, Costello A, Eastham JA, et al. Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol. (2012) 62(3):431–52. doi: 10.1016/j.eururo.2012.05.044
3. Checcucci E, Amparore D, De Luca S, Autorino R, Fiori C, Porpiglia F. Precision prostate cancer surgery: an overview of new technologies and techniques. Minerva Urol Nefrol. (2019) 71(5):487–501. doi: 10.23736/S0393-2249.19.03365-4
4. Checcucci E, De Cillis S, Pecoraro A, Peretti D, Volpi G, Amparore D, et al. Single-port robot-assisted radical prostatectomy: a systematic review and pooled analysis of the preliminary experiences. BJU Int. (2020) 126(1):55–64. doi: 10.1111/bju.15069
5. Dobbs RW, Halgrimson WR, Talamini S, Vigneswaran HT, Wilson JO, Crivellaro S. Single-port robotic surgery: the next generation of minimally invasive urology. World J Urol. (2020) 38(4):897–905. doi: 10.1007/s00345-019-02898-1
6. Nelson RJ, Chavali JSS, Yerram N, Babbar P, Kaouk JH. Current status of robotic single-port surgery. Urol Ann. (2017) 9(3):217–22. doi: 10.4103/UA.UA_51_17
7. Bertolo R, Garisto J, Gettman M, Kaouk J. Novel system for robotic single-port surgery: feasibility and state of the art in urology. Eur Urol Focus. (2018) 4(5):669–73. doi: 10.1016/j.euf.2018.06.004
8. Huang MM, Schwen ZR, Biles MJ, Alam R, Gabrielson AT, Patel HD, et al. A comparative analysis of surgical scar cosmesis based on operative approach for radical prostatectomy. J Endourol. (2021) 35(2):138–43. doi: 10.1089/end.2020.0649
9. Saidian A, Fang AM, Hakim O, Magi-Galluzzi C, Nix JW, Rais-Bahrami S. Perioperative outcomes of single vs multi-port robotic assisted radical prostatectomy: a single institutional experience. J Urol. (2020) 204(3):490–5. doi: 10.1097/JU.0000000000000811
10. Agarwal DK, Sharma V, Toussi A, Viers BR, Tollefson MK, Gettman MT, et al. Initial experience with da Vinci single-port robot-assisted radical prostatectomies. Eur Urol. (2020) 77(3):373–9. doi: 10.1016/j.eururo.2019.04.001
11. White MA, Autorino R, Spana G, Hillyer S, Stein RJ, Kaouk JH. Robotic laparoendoscopic single site urological surgery: analysis of 50 consecutive cases. J Urol. (2012) 187(5):1696–701. doi: 10.1016/j.juro.2011.12.073
12. Kaouk JH, Haber GP, Autorino R, Crouzet S, Ouzzane A, Flamand V, et al. A novel robotic system for single-port urologic surgery: first clinical investigation. Eur Urol. (2014) 66(6):1033–43. doi: 10.1016/j.eururo.2014.06.039
13. Merseburger AS, Herrmann TR, Shariat SF, Kyriazis I, Nagele U, Traxer O, et al. EAU guidelines on robotic and single-site surgery in urology. Eur Urol. (2013) 64(2):277–91. doi: 10.1016/j.eururo.2013.05.034
14. Xu D-L, Ju G-Q, Wang Z-J, Shi J-Z, Zhang Z-Q, Wu Z-J, et al. A comparison of perioperative outcomes between extraperitoneal robotic single-port and multiport radical prostatectomy with the da Vinci Si surgical system. Asian J Androl. (2021) 23(0):1–8. doi: 10.4103/aja.aja_50_21
15. Chang YF, Gu D, Mei N, Xu WD, Lu XJ, Xiao YT, et al. Initial experience on extraperitoneal single-port robotic-assisted radical prostatectomy. Chin Med J (Engl). (2020) 134(2):231–3. doi: 10.1097/CM9.0000000000001145
16. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
17. Paparel P, Akin O, Sandhu JS, Otero JR, Serio AM, Scardino PT, et al. Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol. (2009) 55(3):629–37. doi: 10.1016/j.eururo.2008.08.057
18. Leyh-Bannurah SR, Budaus L, Zaffuto E, Pompe RS, Bandini M, Briganti A, et al. Adherence to pelvic lymph node dissection recommendations according to the National Comprehensive Cancer Network pelvic lymph node dissection guideline and the D’Amico lymph node invasion risk stratification. Urol Oncol. (2018) 36(2):81e17–e24. doi: 10.1016/j.urolonc.2017.10.022
19. Kaouk JH, Goel RK, Haber GP, Crouzet S, Stein RJ. Robotic single-port transumbilical surgery in humans: initial report. BJU Int. (2009) 103(3):366–9. doi: 10.1111/j.1464-410X.2008.07949.x
20. White MA, Haber GP, Autorino R, Khanna R, Forest S, Yang B, et al. Robotic laparoendoscopic single-site radical prostatectomy: technique and early outcomes. Eur Urol. (2010) 58(4):544–50. doi: 10.1016/j.eururo.2010.06.040
21. Semerjian A, Pavlovich CP. Extraperitoneal robot-assisted radical prostatectomy: indications, technique and outcomes. Curr Urol Rep. (2017) 18(6):42. doi: 10.1007/s11934-017-0689-4
22. Xylinas E, Ploussard G, Durand X, de la Taille A. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: a review of the current literature. Urol Oncol. (2013) 31(3):288–93. doi: 10.1016/j.urolonc.2010.07.004
23. Kurokawa S, Umemoto Y, Mizuno K, Okada A, Nakane A, Nishio H, et al. New steps of robot-assisted radical prostatectomy using the extraperitoneal approach: a propensity-score matched comparison between extraperitoneal and transperitoneal approach in Japanese patients. BMC Urol. (2017) 17(1):106. doi: 10.1186/s12894-017-0298-z
24. Steinberg RL, Johnson BA, Meskawi M, Gettman MT, Cadeddu JA. Magnet-assisted robotic prostatectomy using the da Vinci SP robot: an initial case series. J Endourol. (2019) 33(10):829–34. doi: 10.1089/end.2019.0263
25. Wilson CA, Aminsharifi A, Sawczyn G, Garisto JD, Yau R, Eltemamy M, et al. Outpatient extraperitoneal single-port robotic radical prostatectomy. Urology. (2020) 144:142–6. doi: 10.1016/j.urology.2020.06.029
26. Scarcia M, Zazzara M, Divenuto L, Cardo G, Portoghese F, Romano M, et al. Extraperitoneal robot-assisted radical prostatectomy: a high-volume surgical center experience. Minerva Urol Nefrol. (2018) 70(5):479–85. doi: 10.23736/S0393-2249.18.03114-4
27. Wong C, Merkur H. Inferior epigastric artery: surface anatomy, prevention and management of injury. Aust N Z J Obstet Gynaecol. (2016) 56(2):137–41. doi: 10.1111/ajo.12426
28. Wright JL, Dalkin BL, True LD, Ellis WJ, Stanford JL, Lange PH, et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. (2010) 183(6):2213–8. doi: 10.1016/j.juro.2010.02.017
29. Olde Heuvel J, de Wit-van der Veen BJ, Huizing DMV, van der Poel HG, van Leeuwen PJ, Bhairosing PA, et al. State-of-the-art intraoperative imaging technologies for prostate margin assessment: a systematic review. Eur Urol Focus. (2021) 7(4):733–41. doi: 10.1016/j.euf.2020.02.004
Keywords: extraperitoneal tissue retraction technique, single-port surgery, robotic-assisted radical prostatectomy, extraperitoneal pathway, minimally invasive surgery
Citation: Ju G, Wang Z, Shi J, Xu W, Zhang Z, Yin L, Xu D and Ren S (2022) Extraperitoneal tissue retraction technique: An effective assistant of extraperitoneal pure single-port robotic-assisted radical prostatectomy with the da Vinci Si surgical system. Front. Surg. 9:941104. doi: 10.3389/fsurg.2022.941104
Received: 11 May 2022; Accepted: 26 September 2022;
Published: 25 October 2022.
Edited by:
Jeffrey J. Leow, Tan Tock Seng Hospital, SingaporeReviewed by:
Sabine Brookman-May, Ludwig Maximilian University of Munich, GermanyYuzhe Tang, Harvard Medical School, United States
© 2022 Ju, Wang, Shi, Xu, Zhang, Yin, Xu and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shancheng Ren cmVuc2hhbmNoZW5nQGdtYWlsLmNvbQ== Dongliang Xu RHJfeHVkb25nbGlhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
 Guanqun Ju
Guanqun Ju Zhijun Wang1,†
Zhijun Wang1,† Dongliang Xu
Dongliang Xu Shancheng Ren
Shancheng Ren