- Department of Cardiac Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
Objective: Fibroblast growth factor-23 (FGF-23) mediates vascular endothelial injury, inflammatory infiltration, and atherosclerosis, which could reflect major adverse cardiac and cerebrovascular event (MACCE) risk in several cardiovascular diseases. This study aims to further investigate the perioperative change of FGF-23, as well as its association with clinical characteristics and MACCE risk in unprotected left main coronary artery disease (ULMCAD) patients receiving coronary artery bypass grafting (CABG).
Methods: A total of 226 ULMCAD patients who underwent CABG were enrolled. Serum samples of the patients were collected on the day before CABG, the third day (D3) after CABG, and at discharge; then, the FGF-23 level was determined by enzyme-linked immunosorbent assay. The MACCE rate was recorded during a median follow-up of 25.5 (range: 2.0–46.0) months.
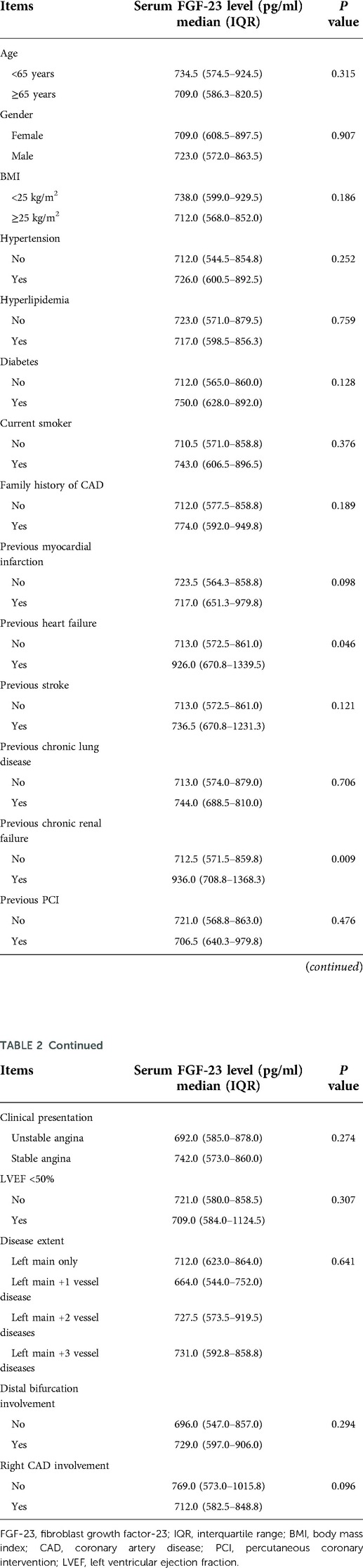
Results: The median, interquartile range (IQR), and range of FGF-23 level in ULMCAD patients receiving CABG were 717.0, 582.5–869.8, and 407.0–1765.0 pg/ml, respectively. FGF-23 level was increased in patients with both previous heart failure (P = 0.046) and chronic renal failure (P = 0.009) compared to those without. FGF-23 level increased from before surgery [median (IQR): 712.5 (574.5–879.8) pg/ml] to D3 [median (IQR): 844.0 (666.0–1072.5) pg/ml], then declined at discharge [median (IQR): 764.5 (569.3–986.8) pg/ml] (P < 0.001). Meanwhile, the preoperative FGF-23 level (P = 0.028), but not the FGF-23 level at discharge (P = 0.067) was positively correlated with the cumulative MACCE rate. Multivariable Cox's analyses found that preoperative FGF-23 level could independently predict cumulative MACCE rate [P = 0.015, hazards ratio (HR) = 2.940].
Conclusion: Preoperative FGF-23 level predicts higher MACCE risk in ULMCAD patients undergoing CABG surgery.
Introduction
Unprotected left main coronary artery disease (ULMCAD) is a highly lethal cardiovascular disease, which is defined as more than 50% stenosis of the left coronary artery, and without patent bypass graft to the left branches (1). In recent decades, remarkable progress has been made in coronary revascularization of ULMCAD and coronary artery bypass grafting (CABG) is one of the revascularization strategies that has been considered the main treatment modality for ULMCAD patients (2–4). However, even after CABG surgery, major adverse cardiac and cerebrovascular event (MACCE) occurs frequently in patients with ULMCAD, which brings a huge challenge to clinicians (5–7). Consequently, it is crucial to find potential biomarkers to early assess the risk of MACCE and thereby improve the management of ULMCAD patients undergoing CABG.
Fibroblast growth factor-23 (FGF-23) is a bone-derived hormone, which is a fundamental regulator of phosphate and vitamin D homeostasis (8, 9). Recently, studies have shown that FGF-23 has a regulatory role in the pathological process of various cardiovascular diseases, and it has the potential to become a clinical biomarker for predicting the risk of MACCE (10–12). For example, FGF-23 regulates cardiac hypertrophy by acting directly on FGF receptor 4 (FGFR4), while FGFR4 activation requires klotho as a cofactor (13). Meanwhile, serum FGF-23 is associated with major adverse cardiovascular events (MACE) risk in patients undergoing coronary angiography (14). Moreover, plasma FGF-23 is a risk factor for MACCE in patients with end-stage renal disease (ESRD) receiving continuous ambulatory peritoneal dialysis (CAPD) (11). Based on the above considerations, it could be hypothesized that FGF-23 might also be associated with the risk of MACCE in ULMCAD patients. However, no research found this to be the case.
Therefore, this study aims to investigate the perioperative variation of serum FGF-23 and its linkage with clinical features as well as MACCE risk in ULMCAD patients receiving CABG.
Methods
Patients
This study consecutively included 226 ULMCAD patients who received their first-ever CABG from May 2017 to December 2020. Patients who met the following criteria were eligible for inclusion: (a) angiographical diagnosis of ULMCAD, which was defined as the narrow of left main coronary artery >50% and without patent bypass grafts to its branches (15); (b) aged over 18 years; (c) planned to receive CABG; (d) willing to provide peripheral blood (PB) samples; (e) willing to perform follow-up visit required by the protocol. The exclusion criteria were as follows: (a) had ST-elevation myocardial infarction within 24 h; (b) had a high risk of CABG or had contraindications of CABG; (c) had a prior history of CABG; (d) during pregnant or lactating. The study was permitted by Ethics Committee. The written informed consents were collected from patients or guardians.
Data collection
Clinical characteristics of the eligible patients were recorded after recruitment, which included demographics, hypertension, hyperlipidemia, diabetes, smoke status, family history of coronary artery disease (CAD), previous myocardial infarction, previous heart failure, previous stroke, previous chronic lung disease, previous chronic renal failure, previous PCI, clinical presentation, left ventricular ejection fraction (LVEF), disease extent; distal bifurcation involvement, and right CAD involvement.
Samples collection and detection
PB samples were collected from the eligible patients on the day before CABG (n = 226), 3 days (D3) after CABG (n = 219), and on the day of discharge (n = 217). Then, the collected PB samples were centrifuged at 3,500 revolutions per minute for 10 min to isolate serum samples, and the serum samples were stored at −80 °C for further detection. Sequentially, serum samples were used to measure FGF-23 level by enzyme-linked immunosorbent assay (ELISA) using Human FGF-23 DuoSet ELISA (DY2604-05, sensitivity: 8.0 pg/ml, Bio-Techne China Co. Ltd., Minneapolis, MN, USA). The experimentation was performed in strict accordance with the manufacturer's instructions. In the analysis, the median value of FGF-23 level was used to classify the patients as high group and low group.
Coronary artery bypass grafting operation
According to the 2011 CABG Guideline issued by the American College of Cardiology Foundation (16), CABG procedures were carried out with standard techniques. Briefly, a midline thoracotomy was performed, and the left internal mammary artery was separated from the left heart outside the pleura, then the great saphenous vein or radial artery was grafted. Sequentially, distal vascular anastomosis was constructed with the choice of on- or off-pump.
Follow-up and assessment
Patients were followed up continuously by clinic visits every 3–6 months until 31 December 2021, and the median follow-up period was 25.5 months. Based on follow-up, major adverse cardiac and cerebrovascular event (MACCE) was recorded, which was defined as the composite of death for any reason, myocardial infarction, stroke, and repeat revascularization (17). In addition, the cumulative MACCE rate was calculated for evaluation. During the study period, approximately 31 patients were lost to follow-up, and we used censored data to calculate the accumulating MACCE rate.
Statistics
In order to avoid the deviation caused by short follow-up, patients lost to follow-up within 1 year were excluded from the analysis. Statistical analysis was completed by SPSS V.22.0 (IBM Corp., Armonk, NY, USA), and figure plotting was fulfilled by GraphPad Prism V.7.02 (GraphPad Software Inc., USA). The correlation of FGF-23 level with clinical characteristics was estimated using the Kruskal–Wallis H rank sum test or Wilcoxon rank sum test. Association of FGF-23 level with cumulative MACCE rate was measured using the Kaplan-Meier method and log-rank test. Change in FGF-23 level over time was analyzed using the Friedman test, followed by post hoc comparisons with the Bonferroni test. Factors affecting the cumulative MACCE rate were determined using forward-stepwise multivariable Cox's proportional hazards regression analyses (the factors shown in Table 1 were included), and the independent prognostic factors were further determined by the receiver operating characteristic (ROC) curve. Statistical significance was declared as a two-side P value <0.05.
Results
Patient's characteristics
The mean age of ULMCAD patients was 64.8 ± 7.6 years, with 49 (21.7%) females and 177 (78.3%) males. Regarding the clinical characteristics of ULMCAD patients, there were 119 (52.7%) patients with unstable angina and 107 (47.3%) patients with stable angina. Meanwhile, there were 185 (81.9%) patients with LVEF ≥50%, while 41 (18.1%) patients with LVEF <50%. Relating to disease extent, there were 7 (3.1%) patients with left main only, 15 (6.6%) patients with left main +1 vessel diseases, 48 (21.2%) patients with left main +2 vessel diseases, and 156 (69.0%) patients with left main +3 vessel diseases. Concerning right CAD involvement, there were 52 (23.0%) patients without right CAD involvement and 174 (77.0%) patients with right CAD involvement. The detailed ULMCAD patients' characteristics are shown in Table 1.
Serum FGF-23 level distribution
The ULMCAD patients' serum FGF-23 level ranged from 407.0 to 1765.0 pg/ml, with a mean value of 761.3 ± 234.3 pg/ml and a median [interquartile range (IQR)] value of 717.0 (582.5–869.8) pg/ml. The specific distribution of serum FGF-23 in ULMCAD patients is shown in Figure 1.
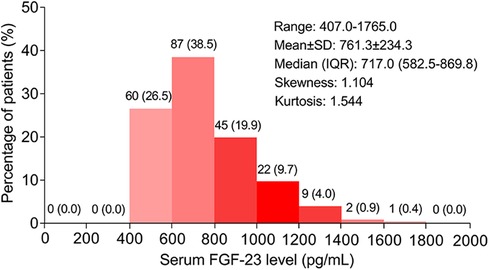
Figure 1. Distribution of serum FGF-23 level in unprotected left main coronary artery disease patients receiving coronary artery bypass grafting .
Comparison of FGF-23 levels in patients with different clinical characteristics
FGF-23 level was increased in ULMCAD patients with previous heart failure (P = 0.046) by contrast to those without. Meanwhile, FGF-23 was also elevated in ULMACD patients with previous chronic renal failure (P = 0.009) compared to those without (Table 2).
Perioperative variation of serum FGF-23 level
Serum FGF-23 level in ULMCAD patients showed an upward trend from before surgery [median (IQR) level: 712.5 (574.5–879.8) pg/ml] to D3 after surgery [median (IQR) level: 844.0 (666.0–1072.5) pg/ml], then there was a certain decline at discharge [median (IQR) level: 764.5 (569.3–986.8) pg/ml] (P < 0.001). Besides, further multiple comparisons exhibited that the FGF-23 level was increased at D3 after surgery compared to before surgery (P < 0.001). Meanwhile, no difference was found between the FGF-23 level before surgery and at discharge (P = 0.057). Moreover, the FGF-23 level was reduced at discharge compared with at D3 after surgery (P < 0.001) (Figure 2).
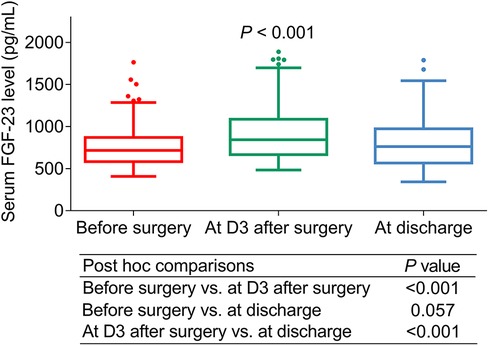
Figure 2. Serum FGF-23 level changed before and after coronary artery bypass grafting (CABG) surgery in unprotected left main coronary artery disease patients receiving CABG.
Correlation between FGF-23 (before surgery) level and cumulative MACCE rate
According to the median value of ULMCAD patients' FGF-23 level before surgery, they were divided into FGF-23 (before surgery) high and low groups. It was found that the cumulative MACCE rate was increased in the FGF-23 (before surgery) high group compared to the FGF-23 (before surgery) low group (P = 0.028) (Figure 3). Specifically, the 1-year, 2-year, and 3-year cumulative MACCE rate in FGF-23 (before surgery) high group was 8.8%, 15.3%, and 25.5%, respectively; while 1.8%, 4.8%, and 13.7% in FGF-23 (before surgery) low group, respectively.
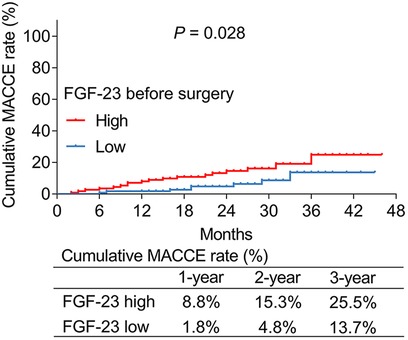
Figure 3. FGF-23 (before surgery) level was positively correlated with cumulative major adverse cardiac and cerebrovascular event rate in unprotected left main coronary artery disease patients receiving CABG.
Correlation between FGF-23 (at discharge) level and cumulative MACCE rate
According to the median value of ULMCAD patients' FGF-23 level at discharge, they were divided into FGF-23 (at discharge) high and low groups. It was found that the cumulative MACCE rate was increased slightly in FGF-23 (at discharge) high group compared to FGF-23 (at discharge) low group but did not reach statistical significance (P = 0.067) (Figure 4). Particularly, the 1-year, 2-year, and 3-year cumulative MACCE rate in FGF-23 (at discharge) high group was 7.3%, 13.6%, and 26.4%, respectively; but was 3.7%, 6.2%, and 14.5% in FGF-23 (at discharge) low group, respectively.
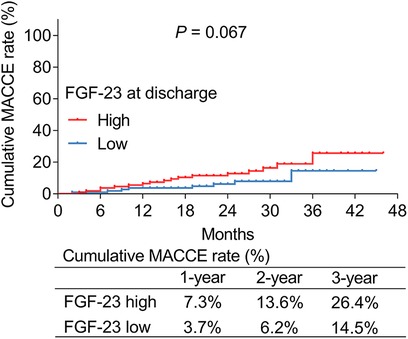
Figure 4. FGF-23 (at discharge) level exhibited a trend to be positively correlated with the cumulative major adverse cardiac and cerebrovascular event rate in unprotected left main coronary artery disease patients receiving coronary artery bypass grafting.
Adjustment by multivariate Cox's hazards regression analysis
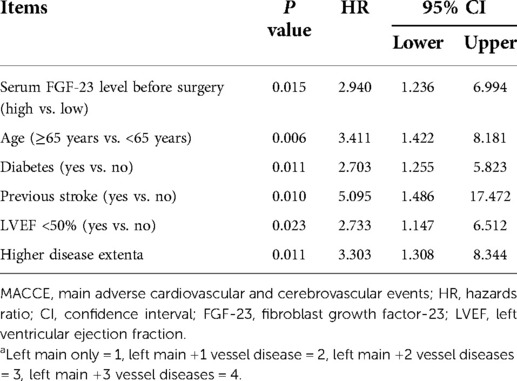
Multivariate Cox's proportional hazards regression analysis exhibited that serum FGF-23 before surgery level (high vs. low) [P = 0.015, hazards ratio (HR) = 2.940], age (≥65 years vs. <65 years) (P = 0.006, HR = 3.411), diabetes (yes vs. no) (P = 0.011, HR = 2.703), previous stroke (yes vs. no) (P = 0.010, HR = 5.095), LVEF <50% (yes vs. no) (P = 0.023, HR = 2.733), and higher disease extent (P = 0.011, HR = 3.303) were all independently associated with higher cumulating MACCE rate in ULMCAD patients receiving CABG (Table 3).
The ability of the combination of independent prognostic factors for estimating MACCE rate
The combination of FGF-23 with other independent prognostic factors had a certain ability to estimate the 3-year MACCE rate [area under the curve (AUC): 0.637, 95% confidence interval (CI): 0.525–0.750] (Figure 5A). Meanwhile, the combination of other independent prognostic factors apart from FGF-23 had no capacity to evaluate the 3-year MACCE rate in ULMCAD patients (AUC: 0.593, 95% CI: 0.480–0.706) (Figure 5B).
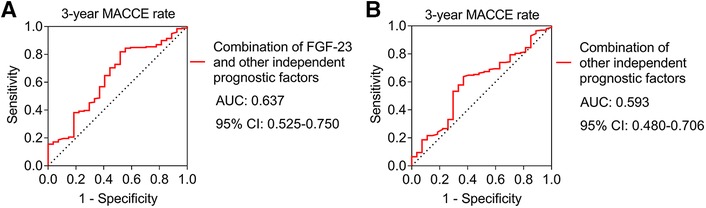
Figure 5. The combination of FGF-23 with other independent prognostic factors showed a certain ability to estimate the major adverse cardiac and cerebrovascular event (MACCE) rate in unprotected left main coronary artery disease patients receiving coronary artery bypass grafting. Receiver operating characteristic (ROC) curve of combination of FGF-23 and other independent factors for estimating 3-year MACCE rate (A); ROC curve of combination of other independent factors apart from FGF-23 for estimating 3-year MACCE rate (B).
Discussion
FGF-23 is a bone-derived hormone whose primary role is to regulate vitamin D metabolism and renal phosphate homeostasis; meanwhile, it has been reported that FGF-23 regulates the development of cardiovascular disease by motivating nitric oxide (NO) production, endothelial nitric oxide synthase (eNOS) expression, and cell proliferation in human aortic endothelial cells (9, 18). Moreover, a study claims that FGF-23 exhibits a higher expression in CAD patients than in non-CAD patients (19). Nevertheless, no relevant studies explore the variation of FGF-23 levels in ULMCAD patients before and after CABG. The present study observed that the FGF-23 level showed an upward trend after CABG surgery, then it decreased at discharge in ULMCAD patients. The possible reason might be that: FGF-23 was able to increase the inflammation through FGFR-mediated mechanisms, thereby reflecting the degree of inflammation to some extent (20); due to vascular injury after CABG, inflammation was higher in ULMCAD patients, which led to an increase in FGF-23 level; with the recovery from CABG surgery, the inflammation status was decreased and the abnormality of phosphate homeostasis was recovered; therefore, FGF-23 level was attenuated at discharge (21).
FGF-23 is reported to serve as a risk factor to reflect adverse cardiovascular events (10, 12, 22–24). However, no study reports the correlation between perioperative serum FGF-23 level and MACCE risk in ULMCAD patients undergoing CABG. The present study found that the preoperative FGF-23 level was independently correlated with a higher cumulating MACCE rate; meanwhile, the FGF-23 level at discharge showed a trend to be positively associated with the cumulative MACCE rate but did not reach statistical significance. The possible explanations could be that FGF-23 might facilitate the progression of atherosclerosis through several methods including regulating oxidative stress and proliferation of vascular endothelial cells, as well as promoting the infiltration of macrophages (18, 20, 25, 26). Therefore, preoperative FGF-23 could directly induce the incidence of MACCE. As a result, preoperative serum FGF-23 levels could independently predict MACCE risk in ULMCAD patients receiving CABG. Besides, the reason why the FGF-23 level at discharge was not linked to cumulative MACCE rate might be that: after CABG, FGF-23 in ULMCAD patients was disturbed by the surgery itself as well as by the recovery of trauma, therefore reducing its ability to predict MACCE risk at discharge. Moreover, this study also discovered that the 1-year, 2-year, and 3-year cumulative MACCE rates in ULMCAD patients were 5.3%, 10.1%, and 20.1%. Notably, a previous study reveals the 1-year, 2-year, and 3-year MACCE rates in ULMCAD patients are 7.1%, 11.2%, and 14.3% (27). The finding of this study was partly similar to previous data.
Heart failure is a clinical syndrome caused by abnormalities in the function or structure of the heart, clinically manifested by a lack of the heart's ability to provide adequate oxygen and blood to surrounding tissues (28). Chronic renal disease is a common disease that usually presents with symptoms of renal insufficiency at the end stage and is associated with a higher cardiovascular risk (29). The present study found that FGF-23 was positively correlated with both previous heart failure and previous chronic renal failure. The possible explanation might be that previous heart failure and chronic renal failure might have caused certain damage to the cardiovascular system; meanwhile, as mentioned above, FGF-23 was able to regulate vascular endothelial injury and inflammatory cell infiltration (18, 20, 30, 31), thereby reflecting the cardiovascular injury to some extent. Thus, FGF-23 was positively associated with previous heart failure and previous chronic renal failure.
Apart from FGF-23, some traditional prognostic factors could also predict MACCE risk in ULMCAD patients receiving CABG, including age ≥65 years, diabetes, previous stroke, LVEF <50%, and higher disease extent, which was in line with a previous study (3). The finding of this study further confirmed the prognostic value of these factors, and the ROC curve based on a combination of FGF-23 with these independent prognostic factors showed a certain ability to estimate the 3-year MACCE risk in ULMACD patients receiving CABG.
There were several limitations in this study. First, the follow-up period of this study was short; therefore, the predictive value of FGF-23 for long-term MACCE risk in ULMCAD patients receiving CABG was elusive. Second, this study only included ULMCAD patients who received CABG; whether FGF-23 also could serve as a predictive biomarker for MACCE in ULMCAD patients who received other treatments remained to be investigated. Third, since most of the ULMCAD patients receiving CABG were not local residents, it was difficult to consistently obtain their blood samples, which hampered the follow-up and the long-term recording of their serum FGF-23 levels. Fourth, the sample size of this study was relatively small; further studies could consider enrolling more ULMCAD patients receiving CABG to strengthen the findings. Fifth, to reduce the cost of the research, the ELISA detection was performed in batches; therefore, there might exist detection deviation among different batches. Sixth, although all CABGs were successful, there would be some differences in operation skills among surgeons, and these may indirectly affect the subsequent MACCE risk in ULMCAD patients receiving CABG.
In conclusion, preoperative serum FGF-23 level could independently predict MACCE risk in ULMCAD patients receiving CABG, suggesting that serum FGF-23 has potential value as a prognostic biomarker in ULMCAD management. However, further validation is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Zhongshan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CW designed the study. FW and CW collected and analyzed the data. RM prepared the figures and tables. FW and RM wrote the manuscript. CW revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collet C, Capodanno D, Onuma Y, Banning A, Stone GW, Taggart DP, et al. Left main coronary artery disease: pathophysiology, diagnosis, and treatment. Nat Rev Cardiol. (2018) 15:321–31. doi: 10.1038/s41569-018-0001-4
2. Lin TC, Lu TM, Huang FC, Hsu PF, Shih CC, Lin SJ, et al. Coronary artery bypass surgery versus percutaneous coronary intervention for left main coronary artery disease with chronic kidney disease. Int Heart J. (2018) 59:279–85. doi: 10.1536/ihj.17-260
3. Wang X, Zhao J, Wang H, Zhao Y, Fu X. Three-year clinical outcome of unprotected left main coronary artery disease patients complicated with chronic kidney disease treated by coronary artery bypass graft versus percutaneous coronary intervention. Ir J Med Sci. (2021) 190:89–6. doi: 10.1007/s11845-020-02257-9
4. Dimeling G, Bakaeen L, Khatri J, Bakaeen FG. CABG: when, why, and how? Cleve Clin J Med. (2021) 88:295–3. doi: 10.3949/ccjm.88a.20115
5. Hyun J, Kim JH, Jeong Y, Choe K, Lee J, Yang Y, et al. Long-term outcomes after PCI or CABG for left main coronary artery disease according to lesion location. JACC Cardiovasc Interv. (2020) 13:2825–36. doi: 10.1016/j.jcin.2020.08.021
6. Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice MC, Puskas J, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. (2019) 381:1820–30. doi: 10.1056/NEJMoa1909406
7. Holm NR, Makikallio T, Lindsay MM, Spence MS, Erglis A, Menown IBA, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet. (2020) 395:191–9. doi: 10.1016/S0140-6736(19)32972-1
8. Batra J, Buttar RS, Kaur P, Kreimerman J, Melamed ML. FGF-23 and cardiovascular disease: review of literature. Curr Opin Endocrinol Diabetes Obes. (2016) 23:423–9. doi: 10.1097/MED.0000000000000294
9. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. (2004) 19:429–35. doi: 10.1359/JBMR.0301264
10. Roy C, Lejeune S, Slimani A, de Meester C, Ahn As SA, Rousseau MF, et al. Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. (2020) 7:2494–507. doi: 10.1002/ehf2.12816
11. Liu D, Zhou S, Mao H. MicroRNA-497/fibroblast growth factor-23 axis, a predictive indictor for decreased major adverse cardiac and cerebral event risk in end-stage renal disease patients who underwent continuous ambulatory peritoneal dialysis. J Clin Lab Anal. (2020) 34:e23220. doi: 10.1002/jcla.23220
12. Vazquez-Sanchez S, Poveda J, Navarro-Garcia JA, Gonzalez-Lafuente L, Rodriguez-Sanchez E, Ruilope LM, et al. An overview of FGF-23 as a novel candidate biomarker of cardiovascular risk. Front Physiol. (2021) 12:632260. doi: 10.3389/fphys.2021.632260
13. Han X, Cai C, Xiao Z, Quarles LD. FGF23 Induced left ventricular hypertrophy mediated by FGFR4 signaling in the myocardium is attenuated by soluble Klotho in mice. J Mol Cell Cardiol. (2020) 138:66–74. doi: 10.1016/j.yjmcc.2019.11.149
14. Huang SS, Huang PH, Leu HB, Wu TC, Chen JW, Lin SJ. Significance of serum FGF-23 for risk assessment of contrast-associated acute kidney injury and clinical outcomes in patients undergoing coronary angiography. PLoS One. (2021) 16:e0254835. doi: 10.1371/journal.pone.0254835
15. Deb S, Wijeysundera HC, Ko DT, Tsubota H, Hill S, Fremes SE. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. (2013) 310:2086–95. doi: 10.1001/jama.2013.281718
16. Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. (2011) 124:e652–735. doi: 10.1161/CIR.0b013e31823c074e
17. Kang SH, Ahn JM, Lee CH, Lee PH, Kang SJ, Lee SW, et al. Differential event rates and independent predictors of long-term major cardiovascular events and death in 5795 patients with unprotected left main coronary artery disease treated with stents, bypass surgery, or medication: insights from a large international multicenter registry. Circ Cardiovasc Interv. (2017) 10:e004988. doi: 10.1161/CIRCINTERVENTIONS.116.004988
18. Chung CP, Chang YC, Ding Y, Lim K, Liu Q, Zhu L, et al. Alpha-Klotho expression determines nitric oxide synthesis in response to FGF-23 in human aortic endothelial cells. PLoS One. (2017) 12:e0176817. doi: 10.1371/journal.pone.0176817
19. Akin F, Celik O, Ayca B, Altun I, Diker VO, Biyik I, et al. Associations of fibroblast growth factor 23 and fetuin-A with coronary plaque burden and plaque composition in young adults. J Investig Med. (2015) 63:613–9. doi: 10.1097/JIM.0000000000000153
20. Han X, Li L, Yang J, King G, Xiao Z, Quarles LD. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett. (2016) 590:53–67. doi: 10.1002/1873-3468.12040
21. Lang F, Leibrock C, Pandyra AA, Stournaras C, Wagner CA, Foller M. Phosphate homeostasis, inflammation and the regulation of FGF-23. Kidney Blood Press Res. (2018) 43:1742–8. doi: 10.1159/000495393
22. Ter Maaten JM, Voors AA, Damman K, van der Meer P, Anker SD, Cleland JG, et al. Fibroblast growth factor 23 is related to profiles indicating volume overload, poor therapy optimization and prognosis in patients with new-onset and worsening heart failure. Int J Cardiol. (2018) 253:84–90. doi: 10.1016/j.ijcard.2017.10.010
23. Poelzl G, Trenkler C, Kliebhan J, Wuertinger P, Seger C, Kaser S, et al. FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur J Clin Invest. (2014) 44:1150–8. doi: 10.1111/eci.12349
24. Niizuma S, Iwanaga Y, Yahata T, Miyazaki S. Renocardiovascular biomarkers: from the perspective of managing chronic kidney disease and cardiovascular disease. Front Cardiovasc Med. (2017) 4:10. doi: 10.3389/fcvm.2017.00010
25. Bernheim J, Benchetrit S. The potential roles of FGF23 and Klotho in the prognosis of renal and cardiovascular diseases. Nephrol Dial Transplant. (2011) 26:2433–8. doi: 10.1093/ndt/gfr208
26. Del Porto F, Proietta M, di Gioia C, Cifani N, Dito R, Fantozzi C, et al. FGF-23 levels in patients with critical carotid artery stenosis. Intern Emerg Med. (2015) 10:437–44. doi: 10.1007/s11739-014-1183-3
27. Shen J, Chang C, Ma J, Feng Q. Potential of circulating proangiogenic microRNAs for predicting major adverse cardiac and cerebrovascular events in unprotected left main coronary artery disease patients who underwent coronary artery bypass grafting. Cardiology. (2021) 146:400–8. doi: 10.1159/000509275
28. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1002/ejhf.592
29. Ammirati AL. Chronic kidney disease. Rev Assoc Med Bras (1992). (2020) 66(Suppl 1):s03–9. doi: 10.1590/1806-9282.66.S1.3
30. Papagianni A. Fibroblast growth factor-23: a novel biomarker for cardiovascular disease in chronic kidney disease patients. Prilozi. (2017) 38:19–27. doi: 10.1515/prilozi-2017-0018
31. Imazu M, Takahama H, Asanuma H, Funada A, Sugano Y, Ohara T, et al. Pathophysiological impact of serum fibroblast growth factor 23 in patients with nonischemic cardiac disease and early chronic kidney disease. Am J Physiol Heart Circ Physiol. (2014) 307:H1504–11. doi: 10.1152/ajpheart.00331.2014
Keywords: unprotected left main coronary artery disease, coronary artery bypass grafting, fibroblast growth factor 23, major adverse cardiac and cerebrovascular event, risk factor
Citation: Wang F, Ma R and Wang C (2022) Perioperative variation in serum FGF-23 level and its correlation with MACCE risk in unprotected left main coronary artery disease patients receiving coronary artery bypassing grafting. Front. Surg. 9:937342. doi: 10.3389/fsurg.2022.937342
Received: 6 May 2022; Accepted: 27 July 2022;
Published: 5 September 2022.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Jie Ma, Second Hospital of Shanxi Medical University Affiliated to Shanxi Medical University, ChinaZheng Qin, Affiliated Hospital of Guizhou Medical University, China
Xiaoning Cui, Liaocheng People's Hospital, China
© 2022 Wang, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunsheng Wang d2FuZ2NodW5zaGVuZ0BmdWRhbi5lZHUuY24=
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
 Fanshun Wang
Fanshun Wang Chunsheng Wang
Chunsheng Wang