
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 11 July 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.930701
This article is part of the Research Topic Experimental and Computational Processes in Surgery View all 10 articles
Objective: This study aims to study the correlation between serum β2-glycoprotein I (β2-GPI)/oxidized low-density lipoprotein (oxLDL) and the risk of cerebral infarction in patients with type 2 diabetes (T2DM).
Methods: From January 2019 to March 2021, 56 patients with T2DM combined with cerebral infarction were chosen as a diabetic cerebral infarction (DCI) group, and 60 patients with simple T2DM were chosen as a T2DM group. In addition, 60 healthy volunteers were recruited as a control group. The essential information of each group was collected, and the serum β2-GPI/oxLDL and inflammatory factor levels in each group were compared. The clinical factors that affect the risk of ischemic cerebral infarction in patients with T2DM were analyzed by a logistic model.
Results: Compared with the control group, the level of serum β2-GPI/oxLDL in the T2DM and DCI groups increased significantly, P < 0.001. Compared with the T2DM group, the serum β2-GPI/oxLDL level in the DCI group increased significantly, P < 0.05. The result of Pearson’s correlation analysis showed that serum β2-GPI/oxLDL was positively correlated with total cholesterol, triglycerides, fasting blood glucose, 2-h postprandial blood glucose, glycosylated hemoglobin, interleukin-6, and tumor necrosis factor (TNF)-α (all P’s < 0.05). Serum TNF-α and β2-GPI/oxLDL were independent risk variates for DCI (P < 0.05). Based on the receiver operating characteristic curve analysis, the values of the area under the curve for TNF-α, serum β2-GPI/oxLDL, and the combined diagnosis of DCI were 0.653 (0.552–0.753), 0.680 (0.583–0.777), 0.739 (0.647–0.831), respectively.
Conclusion: In DCI patients, the levels of serum oxLDL/β2-GPI are significantly increased. Serum oxLDL/β2-GPI is an independent risk factor that affects the occurrence of DCI. In addition, the serum β2-GPI/oxLDL level implicates the lipid metabolism and inflammatory status of the internal environment of DCI patients to a certain extent.
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by progressive insulin resistance and hyperglycemia and is proposed as an independent risk factor for cerebrovascular disease (1–2). T2DM is the most common type of diabetes and is also known as adult-onset diabetes because it occurs mostly in adults. The disease is caused by various causes that lead to insufficient insulin secretion in the body or the body cannot use insulin effectively, resulting in a continuous increase in blood sugar levels, eyes, and other organs. Mild forms of diabetes can be controlled with dietary intervention without medication (3). The largest autopsy study to date of cerebral infarction deceased confirmed a 1.57% increased risk of cerebral infarction associated with T2DM.
At present, cerebral infarction in T2DM is diagnosed mainly by imaging, but the imaging examination has certain limitations. It is of great significance to patients with T2DM if there are suitable serum indicators that accurately predict the risk of cerebral infarction. Low-density lipoprotein is a lipoprotein particle that carries cholesterol into peripheral tissue cells. When low-density lipoprotein, especially oxidized low-density lipoprotein (oxLDL), is in excess, the cholesterol it carries will accumulate on the arterial wall, which is easy to cause arteriosclerosis in the long run. Therefore, LDL is called “bad cholesterol.” oxLDL aggravates the inflammatory response and promotes the accumulation of cholesterol in lysosomes as well, eventually leading to cell death (4), which is a key factor in the occurrence of cardiovascular diseases. β2-Glycoprotein I (β2-GPI) activates platelets by interacting with cell surface phospholipids (phosphatidylserine, phosphatidylethanolamine) or platelet membrane receptors and exacerbates the progression of cardiovascular disease (5). oxLDL binds β2-GPI to form an oxLDL/β2-GPI complex, which induces atherosclerosis and the formation of foam cells. Studies have shown that elevated serum β2-GPI/oxLDL is associated with the occurrence of cerebral complications in T2DM patients (6). The purpose of this study was to deeply analyze the role of serum β2-GPI/oxLDL in T2DM complicated with cerebral infarction and to study the effect of serum β2-GPI/oxLDL in predicting cerebral infarction in T2DM patients.
From January 2019 to March 2021, a total of 56 patients with T2DM and cerebral infarction admitted to our hospital were included in the diabetic cerebral infarction (DCI) group, which includes 14 patients with cardio-embolism, 25 cases of large artery atherosclerotic, 11 cases of small artery occlusion type, and 6 cases of unknown etiology. There were 27 cases of anterior circulation infarction and 29 cases of posterior circulation infarction. A total of 60 patients only with T2DM were included in the T2DM group, and healthy volunteers without a history of diabetes and cerebral infarction who were age- and sex-matched with the above two groups of patients were included in the control group (n = 60). The average age of the DCI group was 65.86 ± 8.74 (43–81 years), with 33 males and 23 females. The average age of the T2DM group was 65.17 ± 10.55 (41–81 years), with 36 males and 24 females. The average age of the control group was 65.18 ± 9.50 (44–82 years), with 34 males and 26 females. Inclusion criteria are as follows: (1) the diagnostic criteria for T2DM conform to the “China Guidelines for the Prevention and Treatment of Type 2 Diabetes (2017 Edition)” (7); (2) the diagnostic criteria for cerebral infarction conform to the “Chinese Guidelines for Diagnosis and Treatment of Cerebral Infarction with Integrated Traditional Chinese and Western Medicine (2017)” (8), and cerebral infarction has occurred within 1 month; (3) 18–85 years old; (4) patients in the T2DM group had no history of cerebral infarction and clinical symptoms and signs of diabetic cerebral infarction; and (5) the physical, electrocardiogram, ultrasound, and biochemical examinations of the volunteers in the control group were all normal. Exclusion criteria are as follows: patients with cerebral hemorrhage on head imaging, gestational diabetes mellitus, diabetic macrovascular (including cardiovascular accident and lower extremity arterial disease) and microvascular (including diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy) complications, type 1 diabetes, infectious disease, chronic liver disease, chronic kidney disease, malignant tumor, or any hereditary disease that affects lipid metabolism. This study was approved by the Ethics Review Board of our institution, and all participants provided written informed consent prior to the study. All methods were performed according to approved guidelines and regulations.
General information about the patient was collected, including age, gender, body mass index (BMI), duration of T2DM, history of cardiovascular disease (history of hypertension, family history of coronary heart disease, history of arrhythmia, family history of hyperlipidemia or diabetes mellitus history), smoking history, drinking history, and biochemical indicators (fasting blood glucose [PBG], 2-h postprandial blood glucose [2-h PG], fasting insulin [Fins], total cholesterol, triglycerides, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and glycosylated hemoglobin [hemoglobin A1C, HbA1c]).
The concentration of serum β2-GPI/oxLDL was determined by a sandwich enzyme-linked immunosorbent assay. First, blood samples were collected after 12 h of fasting, centrifuged at 1,500 r/min (radius: 8 cm) for 15 min, and stored at –80 °C. The polyclonal antibody against human β2-GPI was immobilized on a 96-well plate, and the polyclonal antibody against apolipoprotein B was used as the detection antibody. About 500 μl of serum was incubated at room temperature for 2 h, polyethylene glycol was added, and the samples were incubated overnight (4°C). The sample was centrifuged (10,000 r/min, radius: 8 cm, 20 min), and the obtained precipitate was resuspended in 0.01 mol/L PBS. β2-GPI antibody (2.5 μg/ml) was added to the samples, followed by incubation at 37°C for 2 h and then at 4°C overnight. The diluted samples (1:40) were added to the wells and blocked with 1% gelatin. BSA was added and incubated for 2 h. Standard β2-GPI/ox-LDL complexes were added to the wells and incubated overnight at 4°C to obtain a standard curve. Horseradish peroxidase-labeled goat antirabbit LDL polyclonal antibody was added to the wells, and TMBUS was added after incubation for 3 h (room temperature). The absorbance was read at 450 nm by using a microplate reader, and the serum oxLDL/β2-GPI complex concentration was calculated based on the standard curve.
An enzyme-linked immunosorbent assay kit (Diaclone, France) was used to detect tumor necrosis factor (TNF)-α and interleukin (IL)-6 in serum, the samples were detected by a Lablifeer/ew 2007, Varioskan LUX multimode microplate reader (CA, USA), and the concentrations of IL-6 and TNF-α in the sample were calculated based on the fitted concentration–absorbance curve. C-reactive protein (CRP) was detected by immuno-turbidimetry using a Hitachi 7020 automatic analyzer (Hitachi Kokusai Electric Inc., Tokyo, Japan).
SPSS 20.0 software was used for data analysis; the data were tested for normality first, and the continuous variables conforming to the normal distribution were expressed as ). Comparisons between groups were performed using an independent sample t-test or one-way ANOVA, and data with skewed normal distribution were expressed as M50 (P25, P75). Comparisons between groups were performed using the Mann–Whitney U or the Kruskal–Wallis test. The enumeration data were expressed in the form of cases (percentages), the comparison between groups was performed by the chi-square test, and Pearson’s analysis was used to measure the correlation of each index. Logistic model analysis of clinical factors affecting the risk of ischemic cerebral infarction in patients with T2DM was carried out, and the receiver operating characteristic (ROC) curve was used to analyze the diagnostic efficacy of serum β2-GPI/oxLDL for DCI. When P < 0.05, the data were statistically different.
There were no significant differences in age, gender, smoking history, drinking history, T2DM course, cardiovascular disease history, total cholesterol, triglyceride, and HDL-C among the three groups (P > 0.05). Compared with the control group, the BMI, LDL-C, PBG, 2-h PG, HbAlc, Fins, CRP, TNF-α, and IL-6 of the T2DM group and DCI group were significantly increased, with P values less than 0.05. Compared with those in the T2DM group, the TNF-α and CRP levels in the DCI group were significantly increased, and the P values were all less than 0.05, as shown in Table 1.
The level of serum β2-GPI/oxLDL in the control group, in the T2DM group, and in the DCI group was 0.79 (0.57, 1.03), 1.09 (0.88, 1.28), and 1.34 (1.03, 1.68) mmol/L, respectively. The difference was statistically significant among the three groups (Z = 53.504, P < 0.001). Compared with that in the control group, the concentration of serum β2-GPI/oxLDL in T2DM and DCI groups increased significantly (P < 0.001). Compared with that in the T2DM group, the level of serum β2-GPI/oxLDL in the DCI group was significantly increased (P < 0.05), as shown in Table 2.
The result of Pearson’s correlation analysis showed that serum β2-GPI/oxLDL was positively correlated with total cholesterol, triglyceride, PBG, 2hPG, HbAlc, IL-6, and TNF-α (P < 0.05), as shown in Table 3.

Table 3. Correlation of serum β2-GPI/oxLDL concentration with blood lipids, blood sugar, and inflammatory markers.
Taking cerebral infarction as a dependent variable (occurrence = 1, no occurrence = 0), the basic clinical data were included in the univariate logistic model analysis, and the results showed that HbAlc, serum TNF-α, and β2-GPI/oxLDL were closely related to the occurrence of DCI (P < 0.05). The above independent variables were included in the multivariate model analysis, and the results showed that elevated serum TNF-α and β2-GPI/oxLDL levels were independent risk factors for DCI (P < 0.05), as shown in Table 4.
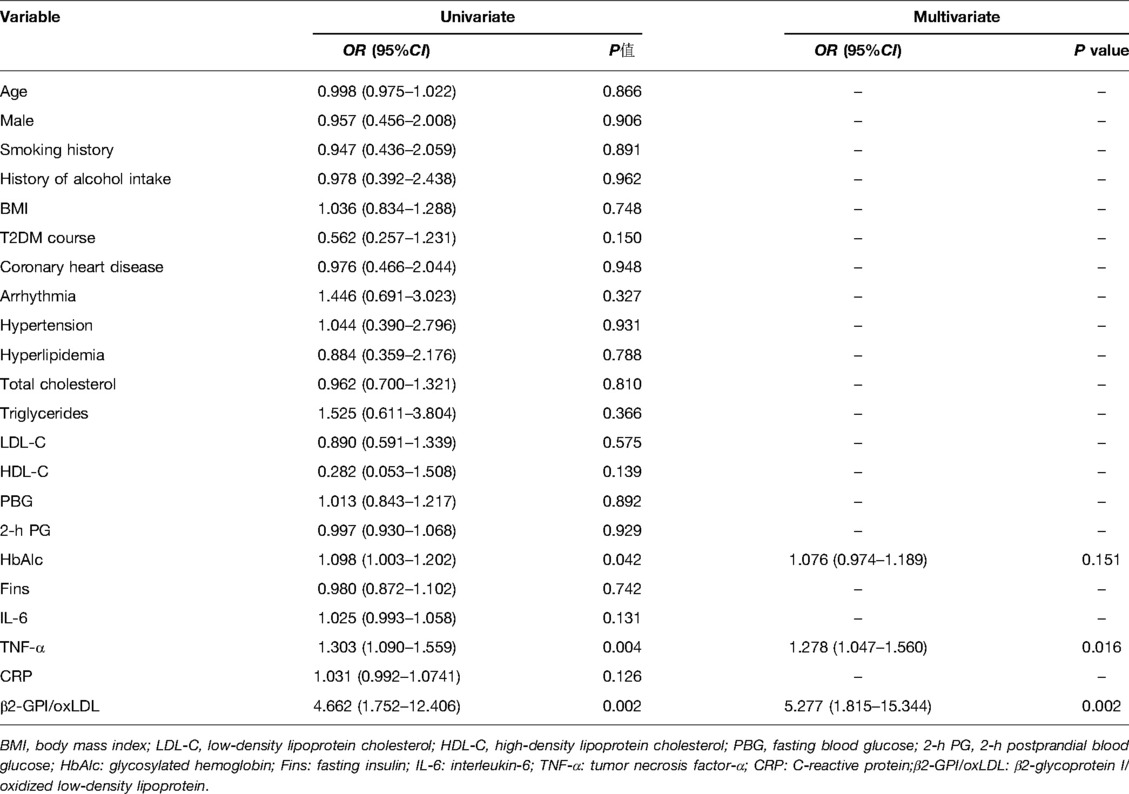
Table 4. Univariate and multivariate logistic model analyses of clinical factors affecting cerebral infarction.
According to the ROC curve analysis, the area under the curve of serum β2-GPI/oxLDL and their combination in the diagnosis of DCI was 0.680 (0.583–0.777), the sensitivity was 0.589, the specificity was 0.750, the cutoff value was 1.256, and the Youden index was 0.339 (P < 0.05), as shown in Figure 1.
Studies have reported that diabetes is an independent risk factor for cerebral infarction (9). The results of this study showed that serum β2-GPI/oxLDL was closely related to the occurrence of cerebral infarction in patients with T2DM. An elevated serum β2-GPI/oxLDL level is an independent risk factor for DCI, and serum β2-GPI/oxLDL is also related to lipids and inflammatory factors in DCI patients. The persistent inflammatory response may be the pathological basis of DCI. In addition, the detection of serum β2-GPI/oxLDL levels is helpful for the diagnosis of clinical DCI.
Cerebral infarction is a common complication of T2DM, with acute onset and high mortality. At present, the diagnosis of DCI mainly relies on examination by imaging, but patients are generally in the attack stage when imaging examinations are performed. Therefore, minimally invasive and accurate biological indicators for the diagnosis of DCI are of great value in patients with T2DM. Hyperglycemia can lead to changes in blood rheology (e.g., reduced red blood cell deformability, increased platelet viscosity, etc.) in patients with T2DM, leading to microcirculation disturbances and a higher risk of stroke (10). In addition, high glucose, high fat, and other risk factors can promote the occurrence of atherosclerosis (AS), which is a complex inflammatory disease and the pathological basis of cardiovascular and cerebrovascular diseases. Hyperglycemia can lead to the production of reactive oxygen species (ROS). Glucose reacts with blood proteins to form glycation end products. It can also trigger the production of ROS. ROS can trigger a chain reaction leading to increased inflammation, chemical modification of lipoproteins, and reduced nitric oxide utilization, thereby increasing the risk of atherosclerosis in the blood vessels and in the brain (11).
AS is a disease characterized by foam cell formation, lipid accumulation, and inflammation (12). Several studies have demonstrated that oxLDL, endothelial dysfunction, and oxidative stress are the most prominent risk factors for AS (13). oxLDL plays a central role in the initiation and progression of atherosclerosis, as it mediates the promotion of several vascular cells (e.g., neutrophils, monocytes/macrophages, smooth muscle cells, endothelial cells, and platelets). Inflammatory and pro-regulatory effects (14). The primary mechanism of macrophage formation stems from disturbances in oxLDL uptake and lipid efflux. Under normal circumstances, plasma low-density lipoprotein exists in the blood. Under pathological conditions, LDL-C in the plasma passes through the damaged endothelium and enters the subintima of the blood vessel, where it is oxidized by reactive oxygen species to form oxLDL. oxLDL is toxic to cells and induces inflammatory gene expression that promotes foam cell formation. Pretreatment with ox-LDL can induce downregulation of human cord blood endothelial cell viability or activate cells to secrete chemical factors, cytokines, and inflammatory factors that promote early atherosclerotic plaque formation (15, 16). The rupture of unstable plaques is the direct cause of cerebral infarction, and elevated serum ox-LDL is a risk factor for the formation of carotid atherosclerotic plaques in patients with AS cerebral infarction (17).
β2-GPI is a highly glycosylated plasma protein. β2-GPI can bind to lipoproteins and participate in lipid metabolism. In patients with autoimmune diseases (such as systemic lupus erythematosus), β2-GPI can activate systemic lupus erythematosus—Th17 and Th1 responses in atherosclerotic lesions in patients with antiphospholipid syndrome—and affect the release of inflammatory factors such as IL-17, IL-12, etc., thereby affecting disease progression (18). The increase of anti-β2-GPI antibodies can accelerate the formation of AS plaques in ApoE-/- mice, and β2-GPI can bind to negatively charged oxLDL to form a complex. In patients with antiphospholipid syndrome, the oxLDL/β2-GPI complex was found to be a predictor of heart disease. The occurrence of vascular complications has a favorable effect and is a more substantial indicator (19). The oxLDL/β2-GPI/anti-β2-GPI antibody complex increases the conversion of macrophages to foam cells, so the oxLDL/β2-GPI/anti-β2-GPI antibody complex increases gradually with the progression of AS disease. Xie et al. (20) also demonstrated that elevated β2-GPI/oxLDL and oxLDL levels were independently associated with diabetic microvascular complications. The results of our study showed that serum TNF-α and IL-6 were significantly increased in DCI, and the serum TNF-α level was an independent risk factor for DCI, which indicated that persistent inflammatory response was closely related to the occurrence of DCI. In addition, serum β2-GPI/oxLDL was significantly increased in DCI patients, and serum β2-GPI/oxLDL was positively correlated with total cholesterol, triglyceride, PBG, 2hPG, HbAlc, IL-6, and TNF-α, indicating that serum β2-GPI/oxLDL can reflect the lipid status and inflammatory status of DCI patients to a certain extent.
In conclusion, serum oxLDL/β2-GPI levels were significantly increased in DCI patients, and serum oxLDL/β2-GPI was an independent risk factor for the occurrence of DCI. In addition, serum β2-GPI/oxLDL levels can also reflect the lipid metabolism status and inflammatory status of DCI patients to a certain extent. Persistent inflammatory response and lipid disturbance may be the pathological basis of DCI. Serum oxLDL/β2-GPI has the potential to become a biomarker for the diagnosis of DCI in T2DM patients and is worthy of clinical promotion.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the ethics committee of our hospital. The patients/participants provided written informed consent to participate in this study.
WK, YL, GL, and YZ are mainly responsible for the writing and research design of the article. GC and BL are mainly responsible for data analysis. The corresponding author is SK, and she is responsible for ensuring that the descriptions are accurate and agreed upon by all authors. All authors contributed to the article and approved the submitted version.
This study was supported by the Key Projects Supported by Hunan Provincial Health Commission (20201914).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lou Q, Yuan X, Hao S, Miller JD, Yan J, Zuo P, et al. Effects of glucose fluctuation targeted intervention on the prognosis of patients with type 2 diabetes following the first episode of cerebral infarction. J Diabetes Res. (2020) 2020:2532171. doi: 10.1155/2020/2532171
2. Liu X, Feng Y, Zhu X, Shi Y, Lin M, Song X, et al. Serum anion gap at admission predicts all-cause mortality in critically ill patients with cerebral infarction: evidence from the MIMIC-III database. Biomarkers. (2020) 25(4):1–8. doi: 10.1080/1354750X.2020.1842497
3. Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, et al. Diabetes is associated with cerebrovascular but not Alzheimer neuropathology. Alzheimers Dement. (2016) 12(8):882–9. doi: 10.1016/j.jalz.2015.12.006
4. Shih CM, Chen CC, Chu CK, Wang KH, Huang CY, Lee AW. The roles of lipoprotein in psoriasis. Int J Mol Sci. (2020) 21(3):859. doi: 10.3390/ijms21030859
5. Capozzi A, Manganelli V, Riitano G, Recalchi S, Truglia S, Alessandri C, et al. Tissue factor over-expression in platelets of patients with anti-phospholipid syndrome: induction role of anti-beta2-GPI antibodies. Clin Exp Immunol. (2019) 196(1):59–66. doi: 10.1111/cei.13248
6. Rosales-Hernandez A, Cheung A, Podgorny P, Chan C, Toth C. Absence of clinical relationship between oxidized low density lipoproteins and diabetic peripheral neuropathy: a case control study. Lipids Health Dis. (2014) 13:32. doi: 10.1186/1476-511X-13-32
7. Diabetes Society of Chinese Medical Association. Prevention and treatment guidelines of type 2 diabetes in China (2017 edition). Chin J Diab. (2018) 10(1):4–67. doi: 10.3760/cma.j.issn.1674-5809.2018.01.003
8. Professional Committee of Neurology of China Association of Integrative Medicine. Guidelines for the diagnosis and treatment of cerebral infarction in China (2017). Chin J Integr Med. (2018) 38(2):136–44. doi: 10.7661/j.cjim.20171221.483
9. Zhang Z, Qian M, Ge Z, Zhou P, Liu J, Chen J. Effects of blood glucose and glycosylated hemoglobin levels on intravenous thrombolysis in patients with acute cerebral infarction and type 2 diabetes mellitus. Pak J Med Sci. (2019) 35(3):862–867. doi: 10.12669/pjms.35.3.8
10. Zhang L, Wu Y, Qiu L, Liu Y, Li Q. Elevated levels of serum β2-glycoprotein I/oxidized low-density lipoprotein complexes are associated with cerebral infarction in patients with type 2 diabetes mellitus. Med Sci Monit. (2018) 24:1232–40. doi: 10.12659/MSM.907078
11. Lee JH, Yoon SR, Na GY, Jun M, Ahn MR, Cha JK, et al. Fasting glucose is a useful indicator for cerebrovascular risk in non-diabetic Koreans: association with oxidative stress and inflammation. Clin Nutr Res. (2016) 5(1):33–42. doi: 10.7762/cnr.2016.5.1.33
12. Zhong Y, Liu C, Feng J, Li JF, Fan ZC. Curcumin affects ox-LDL-induced IL-6, TNF-alpha, MCP-1 secretion and cholesterol efflux in THP-1 cells by suppressing the TLR4/NF-kappaB/miR33a signaling pathway. Exp Ther Med. (2020) 20(3):1856–70. doi: 10.3892/etm.2020.8915
13. Khatana C, Saini NK, Chakrabarti S, Saini V, Sharma A, Saini RV, et al. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid Med Cell Longev. (2020) 2020:5245308. doi: 10.1155/2020/5245308
14. Miyazaki A, Uehara T, Usami Y, Ishimine N, Sugano M, Tozuka M. Highly oxidized low-density lipoprotein does not facilitate platelet aggregation. J Int Med Res. (2020) 48(10):300060520958960. doi: 10.1177/0300060520958960
15. Jia Z, An L, Lu Y, Xu C, Wang S, Wang J, et al. Oxidized low density lipoprotein-induced atherogenic response of human umbilical vascular endothelial cells (HUVECs) was protected by atorvastatin by regulating miR-26a-5p/phosphatase and tensin homolog (PTEN). Med Sci Monit. (2019) 25:9836–43. doi: 10.12659/MSM.918405
16. Guo S, Lin Y, Ma X, Zhao Y, Jin A, Liu X, et al. Long-term safety and efficacy of antiplatelet therapy in patients with cerebral infarction with thrombocytopenia. Clin Appl Thromb Hemost. (2021) 27(12):107602962098006. doi: 10.1177/1076029620980067
17. Yan Z, Fu B, He D, Zhang Y, Liu J, Zhang X. The relationship between oxidized low-density lipoprotein and related ratio and acute cerebral infarction. Medicine (Baltimore). (2018) 97(39):e12642. doi: 10.1097/MD.0000000000012642
18. Benagiano M, Borghi MO, Romagnoli J, Mahler M, Bella CD, Grassi A, et al. Interleukin-17/Interleukin-21 and Interferon-gamma producing T cells specific for beta2 Glycoprotein I in atherosclerosis inflammation of systemic lupus erythematosus patients with antiphospholipid syndrome. Haematologica. (2019) 104(12):2519–27. doi: 10.3324/haematol.2018.209536
19. Zhang G, Cai Q, Zhou H, He C, Chen Y, Zhang P, et al. OxLDL/β2-GPI/anti-β2-GPI Ab complex induces inflammatory activation via the TLR4/NF-κB pathway in HUVECs. Mol Med Rep. (2021) 23(2):148. doi: 10.3892/mmr.2020.11787
Keywords: oxLDL, β2-GPI, cerebral infarction, type 2 diabetes, T2DM
Citation: Kuang W, Li Y, Liu G, Zhang Y, Chen G, Luo B and Kuang S (2022) Correlation Between Serum β2-GPI/oxLDL and the Risk of Cerebral Infarction in Patients with T2DM. Front. Surg. 9:930701. doi: 10.3389/fsurg.2022.930701
Received: 28 April 2022; Accepted: 2 June 2022;
Published: 11 July 2022.
Edited by:
Songwen Tan, Central South University, ChinaReviewed by:
Yang Liu, The First Medical Center of Chinese PLA General Hospital, ChinaCopyright © 2022 Kuang, Li, Liu, Zhang, Chen, Luo and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangyu Kuang c2h1YW5neXVrdWFuZ0AxNjMuY29t
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.