
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 08 July 2022
Sec. Pediatric Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.930581
This article is part of the Research Topic Case Reports in Pediatric Surgery 2022 View all 21 articles
 Xiaoming Liu1
Xiaoming Liu1 Qianqian Sun1
Qianqian Sun1 Wenjing Sun1
Wenjing Sun1 Qiong Niu2
Qiong Niu2 Zhu Wang3
Zhu Wang3 Chen Liu4
Chen Liu4 Tingliang Fu5
Tingliang Fu5 Lei Geng5*
Lei Geng5* Xiaomei Li1*
Xiaomei Li1*
Introduction: Unintentional injuries remain a leading cause of disability among children. Although most of the pediatric patients suffering blunt liver injury can be successfully treated with non-operative therapy, the diagnosis and management of delayed life-threatening hemobilia following severe blunt liver injury, especially in the pediatric population, remain a challenge for clinicians.
Case Presentation: A previously healthy 2-year-old girl suffered a severe blunt liver injury related to an electric bike, which was inadvertently activated by herself. She initially received non-operative therapy and was in a stable condition in the first 2 weeks. On the 16th and 22nd postinjury days, the patient presented with life-threatening massive hemobilia, which was confirmed via repeat emergent gastroscopy and hepatic arterial angiography. An emergency selective transarterial embolization of the involved branch of the left hepatic artery was successfully performed. The patient recovered uneventfully, and long-term follow-up was needed owing to a mild dilatation of the left intrahepatic bile duct.
Discussion: Incidental injury in children should be considered as a major public health issue and preventive measures should be taken to reduce its occurrence. Delayed massive hemobilia after severe blunt liver trauma is rare, and its accurate and timely diagnosis via emergency hepatic arterial angiography and selective angioembolization may allow prompt and optimal management to achieve good outcomes in the pediatric population.
The World Health Organization (WHO) has estimated that in the year 2004, more than tens of millions of children worldwide suffered injuries, and unintentional injuries remained a leading cause of disability among children (1). Implementation of preventive strategies will help reduce incidental injury and improve children's survival rates and health, which is vital to avoid far-reaching impacts on children's prospects and their parents’ livelihood (1–4). Today, electric bikes (e-bikes) are widely available (5, 6); however, its higher traveling speed may increase the risk of accidents and the severity of injury (6–11). Herein, we describe a rare case of a girl who suffered an e-bike-related severe blunt liver injury complicated by delayed massive hemobilia. We discuss the diagnosis and management of severe blunt liver injury associated with delayed life-threatening hemobilia in the pediatric population and review the literature in the English language from the Pubmed database.
A previously healthy girl aged 2 years weighing 15 kg was brought to our pediatric emergency department and admitted 5 h after her chest and upper abdomen were squeezed by an e-bike with high traveling speed in her family yard. The e-bike was inadvertently activated by herself, and it suddenly moved forward and pushed her chest and abdomen into a brick wall. On admission, she was conscious but looked pale. There were signs of hemorrhagic shock, blood pressure of 103/49 mmHg, heart rate of 167 beats/min, and respiratory rate of 42 breaths/min. Bruises were observed on her right lateral chest wall. Her abdomen revealed slight tenderness and was distended. The laboratory tests showed a red blood cell (RBC) count of 3.29 × 1012/L, hematocrit of 25%, and a hemoglobin (Hb) level of 90 g/L. Liver function test revealed an elevated liver enzyme, aspartate aminotransferase (AST) of 2,898 IU/L, and alanine aminotransferase (ALT) of 2,247 IU/L. Chest and abdominal plain radiograph was unremarkable. Chest and abdominal plain computed tomography (CT) revealed bilateral traumatic wet lungs and irregular lacerations in segments VI, IVA, and II, graded as III and IV, respectively, by the American Association for the Surgery of Trauma's Organ Injury Scaling (AAST-OIS) (12) (Figure 1). After admission, the Hb level declined to 71 g/L. Point-by-care ultrasonography demonstrated that the involved area of the left hepatic lobe was 5.5 cm × 4.8 cm × 1.5 cm and that of the right lobe was 4.8 cm × 4.4 cm × 4.9 cm with hemoperitoneum. Initial fluid resuscitation, blood transfusion of two units of concentrated RBCs, and hemostatics were given immediately. The patient received non-invasive ventilation with high volume oxygen inhalation and absolute bed rest. The Hb level was within the normal range. The patient received non-operative management and recovered uneventfully. On the 11th postinjury day, she was transferred to a conventional ward.
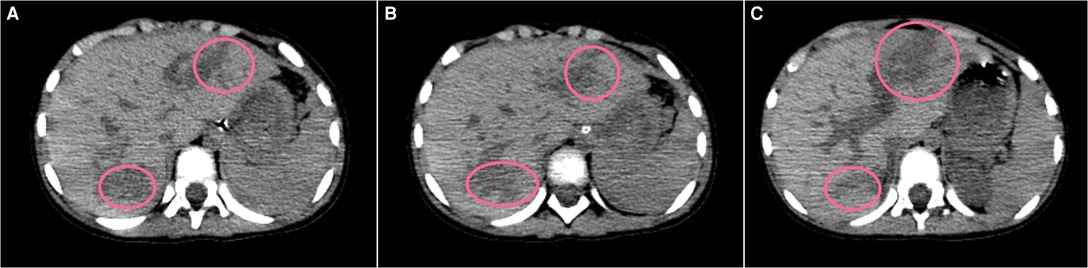
Figure 1. Plain CT scan (liver window A to C) showing irregular low-density lesions (pink circles) in SIVA and II, AAST Grade IV laceration and SVI, and AAST Grade III laceration.
On the 16th postinjury day, the patient suddenly presented abdominal discomfort and hematemesis. Then, massive bloody stool occurred with hemorrhagic shock. The Hb level declined to 52 g/L with hyperbilirubinemia (total bilirubin 99.8 mmol/L, direct bilirubin 82.3 mmol/L). Urgent fluid resuscitation, blood transfusion, systemic hemostatics, and oxygen inhalation were given. Emergent gastroscopy revealed no obvious bleeding from the duodenal papilla, and erosive gastroduodenitis was suspected. The source of gastrointestinal bleeding was unclear, but the bleeding ceased spontaneously. The Hb level was 103 g/L following multiple blood transfusions of 6 units of concentrated RBCs. On the 22nd postinjury day, massive bloody stool reoccurred. The Hb level dramatically declined to 51 g/L. Emergent re-gastroscopy showed active bleeding from the duodenal papilla (Figure 2A). The diagnosis of traumatic massive hemobilia was confirmed. After receiving fluid resuscitation and blood transfusions of 5 units of concentrated RBCs, the patient underwent an emergency hepatic arteriography. A pseudoaneurysm from a branch of the left hepatic artery was confirmed and contrast extravasation under the diaphragm was also observed. The involved arterial branch was selectively embolized using gelatin sponge particles, spring coil, and free coil. Post-embolization angiography showed a complete occlusion of the pseudoaneurysm and no contrast medium extravasation (Figures 2B,C). The patient recovered uneventfully and was discharged on the 5th post-embolization day.
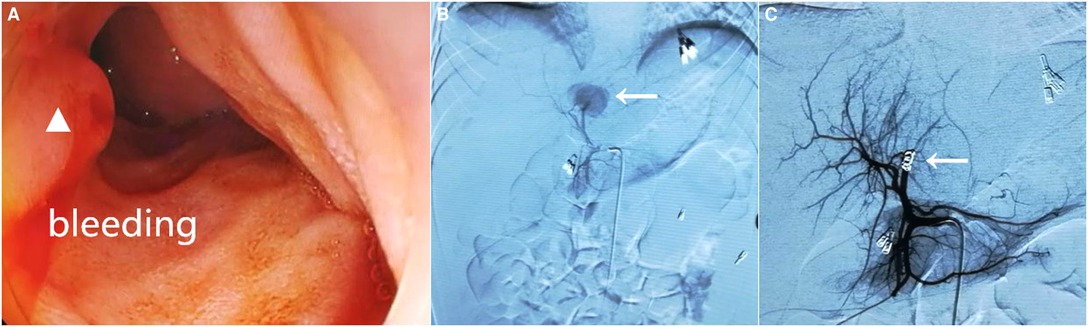
Figure 2. Gastroduodenoscopy revealing active bleeding with fresh blood from the duodenal papilla (white triangle) (A). Hepatic arteriography showed contrast extravasation from the branch of the left hepatic artery (white arrow) (B) and selective transcatheter arterial embolization was successfully implemented (C, white arrow).
After a one-year follow-up, regular ultrasonography and color Doppler ultrasound showed that a heterogeneous echogenicity in the parenchyma of the left hepatic lobe and the inner diameter of the portal vein was normal. Echogenicity of blood clots in the gallbladder lumen gradually disappeared at 2 months post-embolization. A mild dilatation of the left intrahepatic bile duct was found at the 6th week after trauma, and long-term follow-up was needed.
The Pubmed database revealed that there were about 35 pediatric patients who suffered blunt liver injury complicated by hepatic artery pseudoaneurysm and/or delayed life-threatening hemobilia (12–41). The clinical characteristics of these patients, including those of the present one, are summarized in Table 1.
The WHO reports that many children suffering nonfatal injuries are left with some form of disability, even with lifelong consequences (1–3). E-bike, as one of the new personal mobility devices, is being widely used for the last two decades (42). This convenient mode of transportation leads to a high ratio of e-bike-related casualties (9, 10, 43–45). If e-bike riders leave the key in situ after riding, toddlers may easily activate it inadvertently owing to their high curiosity and imitative ability. This can lead to their being exposed to a high risk of severe injuries. In the present case series, bicycle riding and fall-related high-grade liver injury occurred in nearly half of the patients (16/35), as shown in Table 1.
The patient in this study who suffered an e-bike-related severe blunt liver injury, accompanied by a wet lung, was initially treated non-operatively during the first 2 weeks after trauma, as described in previous reports (15, 16, 46). However, at the 3rd week after trauma, the patient suddenly presented with massive upper gastrointestinal bleeding with hemorrhagic shock, which required a high volume of blood transfusion. The diagnosis and positioning of the ruptured hepatic artery pseudoaneurysm were confirmed via repeat urgent gastroscopy and emergency hepatic angiography in the second episode of bleeding. Fortunately, a selective transcatheter angiographic embolization was successfully performed with good outcome.
Traumatic hepatic artery pseudoaneurysm, as a complication, may present as a clinically silent hemorrhage or as a sudden massive hemorrhage. Prompt recognition and a multidisciplinary approach with an experienced team are needed to manage this rare but life-threatening condition, especially in the pediatric population (17, 18, 32, 34, 36–39, 47, 48). Patients with AAST-OIS high-grade blunt hepatic injury seem to be at a higher risk of developing pseudoaneurysm based on a retrospective study. Pseudoaneurysm is usually identified within 2 weeks after liver trauma, as shown in Table 1. Therefore, routine surveillance may be indicated and should benefit patients because some of them present with sudden massive hemobilia after discharge (13, 48–52). Patients with traumatic hemobilia may present with a triad of the upper abdominal pain, hemobilia, and obstructive jaundice (19, 53, 54). However, there are a variety of presentations according to the source and degree of hemorrhage (36, 51, 55). The early diagnosis of hemobilia mainly depends on a high index of suspicion for those who suffered blunt liver injury followed by upper gastrointestinal bleeding and biliary symptoms (35, 49). Occasionally, pseudoaneurysm may rupture into the peritoneal cavity (15, 33). However, in the present case, hepatic angiographic imaging revealed a pseudoaneurysm from one branch of the left hepatic artery, and contrast media extravasation was seen under the diaphragm, suggesting that there were communications between the involved artery to both bile ducts and the abdominal cavity. This angiographic finding is extremely rare (42, 55) and should be managed accordingly.
A precise diagnosis is mainly made via contrast-enhanced CT and/or angiography (34, 36, 55). As far as the role of gastroscopy in the diagnosis of hemobilia is concerned, blood clots in the duodenum may often be revealed (25, 33). There are only two of the nine cases revealing bleeding from Vater's ampulla, as shown in Table 1. Repeat gastroscopy may be helpful to make the diagnosis of hemobilia. The selective use of point-of-care ultrasound imaging in children with high-grade liver injury may allow to identify hepatic artery pseudoaneurysms, which might minimize the risk of life-threatening hemorrhage (19). “Yin-yang” sign is the classic manifestation in color Doppler ultrasound for artery pseudoaneurysm (56).
Management of traumatic hemobilia is aimed at stopping bleeding and relieving biliary obstruction (36). Conservative management includes fluid resuscitation, blood transfusion, correction of coagulopathy, and adequate drainage (36). Endoscopic nasobiliary drainage without sphincterotomy is an optimal method to treat traumatic hepatobiliary injuries in patients with stable hemodynamics (57). Hemobilia may stop because of blood clot formation after the diversion of bile with nasobiliary drainage (57). Selective transcatheter arterial embolization as a useful therapeutic method for traumatic hepatic pseudoaneurysm and/or its rupture into the biliary duct remains the first-line choice for the majority of cases (17, 31, 35, 36, 39, 40, 50, 55, 58), with 26/35 (74.28%) of patients undergoing successful angiographic embolization, as shown in Table 1. Surgical procedures still play an important role in the management of patients with unstable hemodynamics or failure following selective angiographic embolization (22, 27, 36, 50, 59). A flowchart (Figure 3) was recommended to provide a suitable approach to deal with this rare but life-threatening condition in accordance with the literature (15, 17, 33, 36, 46, 50, 52, 54–64) and our preliminary experience.
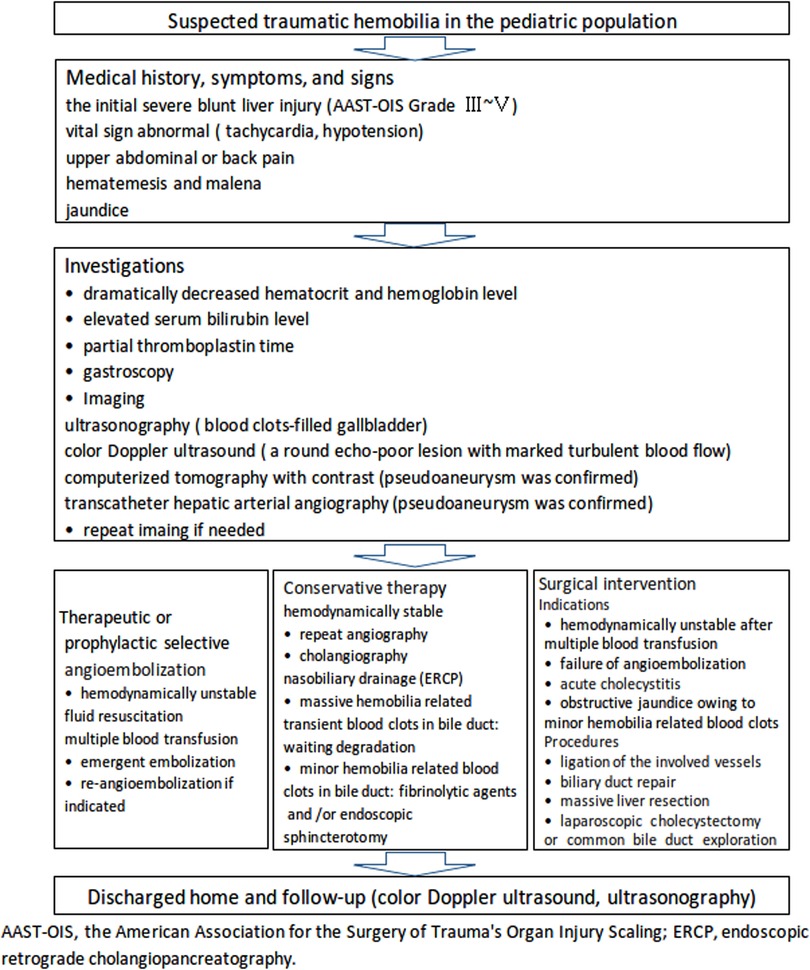
Figure 3. A recommended flowchart for the diagnosis and management of traumatic hemobilia in the pediatric population based on the literature and our preliminary experience.
Short-term complications, such as cholecystitis, gallbladder necrosis, liver ischemia, abscess, rebleeding, and febrility should be properly treated (19, 36, 52, 54, 59). Long-term follow-up is needed as complications may occur after liver trauma and angioembolization (36, 39).
In conclusion, massive upper gastrointestinal bleeding in patients with a history of severe blunt liver trauma should be highly suspicious of hemobilia. The diagnosis can be made via CT angiography and hepatic artery angiography (16, 17, 36, 41). Based on the age-specific characteristics of children, a multidisciplinary team, especially experienced interventional radiologists, is vital for the diagnosis and management of this rare but life-threatening condition to achieve a good outcome (18, 20, 37). Moreover, the management of e-bike-related injuries should not be underestimated in the pediatric population (5, 8, 9). Preventive measures, such as the promotion of safety devices and safety education, should be taken to reduce unintentional injuries among children.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not provided for this study on human participants because ethical review and approval were waived for this research as it was a case report. The parents of the patient signed an informed consent form before the medical management. Written informed consent was obtained from the individuals next of kin for the publication of any potentially identifiable images or data included in this article.
XML and XL did the conceptualization of the study. XML, QS, WS, QN, ZW, and LG conducted the investigations. XML, CL, and TLF were involved in writing and preparing the original draft. XML, XL, LG, and TLF wrote the review and did the editing. XL and LG performed the supervision. All authors have read and agreed to the published version of the manuscript.
The authors thank the patient and their family and appreciate the cooperation of all staff. We also thank Professor Xu Wang from the Department of Nuclear Medicine, Radiology, Binzhou Medical University Hospital, for his support in rechecking the CT imaging.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Peden M, Oyegbite K, Ozanne-Smith J, Hyder AA, Branche C, Rahman AKMF, et al. World report on child injury prevention. Geneva: World Health Organization (2008).
2. McClure R, Kegler S, Davey T, Clay F. Contextual determinants of childhood injury: a systematic review of studies with multilevel analytic methods. Am J Public Health. (2015) 105:e37–43. doi: 10.2105/AJPH.2015.302883
3. Crawley-Coha T. Childhood injury: a status report. J Pediatr Nurs. (2001) 16:371–4. doi: 10.1053 /jpdn.2001.27608
4. Zhou X. The choice of core control algorithm for 2-wheeled auto-balancing electric vehicle. Office Automation. (2013) 4:56–7 (in Chinese). doi: CNKI:SUN:BGDH.0.2013-08-020
5. Spörri E, Halvachizadeh S, Gamble JG, Berk T, Allemann F, Pape HC, et al. Comparison of injury patterns between electric bicycle, bicycle and motorcycle accidents. J Clin Med. (2021) 10:3359. doi: 10.3390/jcm10153359
6. Tan AL, Trauma Coordinators and Trauma Service Representatives, Nadkarni N, Wong TH. The price of personal mobility: burden of injury and mortality from personal mobility devices in Singapore – a nationwide cohort study. BMC Public Health. (2019) 19:880. doi: 10.1186/ s12889-019-7210-6
7. Papoutsi S, Martinolli L, Braun CT, Exadaktylos AK. E-bike injuries: experience from an urban emergency department – a retrospective study from Switzerland. Emerg Med Int. (2014) 2014:850236. doi: 10.1155/2014/850236
8. Zhou SA, Ho AFW, Ong MEH, Liu N, Pek PP, Wang YQ, et al. Electric bicycle-related injuries presenting to a provincial hospital in China: a retrospective study. Medicine (Baltimore). (2017) 96:e7395. doi: 10.1097/MD.0000000000007395
9. Feng Z, Raghuwanshi RP, Xu Z, Huang D, Zhang C, Jin T. Electric-bicycle-related injury: a rising traffic injury burden in China. Inj Prev. (2010) 16:417–9. doi: 10.1136/ip.2009.024646
10. Zhao J, Tu EJ, McMurray C, Sleigh A. Rising mortality from injury in urban China: demographic burden, underlying causes and policy implications. Bull World Health Organ. (2012) 90:461–7. doi: 10.2471/BLT.11.093849
11. Wu O, Liu QM. Electric bicycle related injury and risk factors in Hangzhou. J Environ Occup Med. (2012) 29:591–4, 599. doi: 10.13213/j.cnki.jeom.2012.09.006. (in Chinese).
12. Tinkoff G, Esposito TJ, Reed J, Kilgo P, Fildes J, Pasquale M, et al. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg. (2008) 207:646–55. doi: 10.1016/j.jamcollsurg. 2008.06.342
13. Notrica DM, Eubanks JW 3rd, Tuggle DW, Maxson RT, Letton RW, Garcia NM, et al. Nonoperative management of blunt liver and spleen injury in children: evaluation of the ATOMAC guideline using GRADE. J Trauma Acute Care Surg. (2015) 79:683–93. doi: 10.1097/TA.0000000000000808
14. Linnaus ME, Langlais CS, Garcia NM, Alder AC, Eubanks JW 3rd, Maxson RT, et al. Failure of nonoperative management of pediatric blunt liver and spleen injuries: a prospective Arizona-Texas-Oklahoma-Memphis-Arkansas Consortium study. J Trauma Acute Care Surg. (2017) 82:672–9. doi: 10.1097/TA.0000000000001375
15. Giss SR, Dobrilovic N, Brown RL, Garcia VF. Complications of nonoperative management of pediatric blunt hepatic injury: diagnosis, management, and outcomes. J Trauma. (2006) 61:334–9. doi: 10.1097/01.ta.0000197605.27190.2c
16. van As AB, Millar AJ. Management of paediatric liver trauma. Pediatr Surg Int. (2017) 33:445–53. doi: 10.1007/s00383-016-4046-3
17. Hardcastle TC, Reitz D, Hollander DD, Rodseth R, Muckart DJ. Posttraumatic intrahepatic pseudoaneurysm in a child managed by coil angioembolization: a case report and literature review. J Pediatr Surg. (2010)45:e1–3. doi: 10.1016/j.jpedsurg.2010.02.119
18. Schouten van der Velden AP, de Ruijter WM, Janssen CM, Schultze Kool LJ, Tan EC. Hemobilia as a late complication after blunt abdominal trauma: a case report and review of the literature. J Emerg Med. (2010) 39:592–5. doi: 10.1016/j.jemermed.2008.08.015
19. Safavi A, Beaudry P, Jamieson D, Murphy JJ. Traumatic pseudoaneurysms of the liver and spleen in children: is routine screening warranted? J Pediatr Surg. (2011) 46:938–41. doi: 10.1016/j.jpedsurg.2011.02.035
20. Ong CC, Toh L, Lo RH, Yap TL, Narasimhan K. Primary hepatic artery embolization in pediatric blunt hepatic trauma. J Pediatr Surg. (2012) 47:2316–20. doi: 10.1016/j.jpedsurg.2012.09.050
21. Sidhu MK, Shaw DW, Daly CP, Waldhausen JH, Coldwell D. Post-traumatic hepatic pseudoaneurysms in children. Pediatr Radiol. (1999) 29:46–52. doi: 10.1007/s002470050532
22. Bajpai M, Bhatnagar V, Mitra DK, Upadhyaya P. Surgical management of traumatic hemobilia in children by direct ligation of the bleeding vessel. J Pediatr Surg. (1989) 24:436–7. doi: 10.1016/s0022-3468(89)80396-3
23. Nielsen SL, Mygind T, Miskowiak J. Blood pool scintigraphy and arterial embolization in traumatic hemobilia. Eur J Nucl Med. (1982) 7:389–90. doi: 10.1007/BF00255660
24. Gupta LB, Puri AS. Management of traumatic hemobilia with embolization. Indian Pediatr. (2006) 43:825–7. PMID: 17033124
25. Prasad A, Prasad A, Kumar P, Kumar S. Post-traumatic pseudoaneurysm of hepatic artery: an unusual cause of upper gastrointestinal bleeding. Indian Pediatr. (2020) 57:370–2. doi: 10.1007/s13312-020-1796-8
26. Nielsen ML, Mygind T. Selective arterial embolization in traumatic hemobilia. World J Surg. (1980) 4:357–61. doi: 10.1007/BF02393406
27. Poulos E, Wilkinson LH, Simms AG 2nd, Floyd VT. Traumatic hemobilia treated by massive liver resection. Arch Surg. (1964) 88:596–601. doi: 10.1001/archsurg.1964.01310220086014
28. Souliotis PT, Pettgrew AH, Chamberlain JW. Traumatic hemobilia. N Engl J Med. (1963) 268:565–8. doi: 10.1056/NEJM196303142681101
29. Gow KW, Murphy JJ 3rd, Blair GK, Stringer DA, Culham JA, Fraser GC. Splanchnic artery pseudo-aneurysms secondary to blunt abdominal trauma in children. J Pediatr Surg. (1996) 31:812–5. doi: 10.1016/s0022-3468(96)90140-2
30. Hacker HW, Schwöbel MG, Allgayer B. Pseudoaneurysm rupture after liver injury in a 14-year-old boy. Eur J Pediatr Surg. (2008) 18:126–8. doi: 10.1055/s-2007-965743
31. Goldblatt M, Goldin AR, Shaff MI. Percutaneous embolization for the management of hepatic artery aneurysms. Gastroenterology. (1977) 73:1142–6. doi: 10.1016/S0016-5085(19)31873-6
32. Ahmed M, Elkahly M, Dada S, Mahmoud A, Chin M. Hepatic artery pseudoaneurysm following gunshot injury with early rupture. Cureus. (2021) 13:e15866. doi: 10.7759/cureus.15866
33. Thong-Ngam D, Shusang V, Wongkusoltham P, Brown L, Kullavanijaya P. Hemobilia: four case reports and review of the literature. J Med Assoc Thai. (2001) 84:438–44. PMID: 11460949
34. Bardes JM, Caranasos TG, Vaughan RA. Hepatic artery pseudoaneurysm: delayed presentation after bicycle accident. J Trauma. (2011) 71:783. doi: 10.1097/TA.0b013e31822b095a.
35. Hong Duc P, Xuan Dung P, Quang Huy H. Post-blunt traumatic hemobilia from pseudoaneurysm successfully treated with embolization. Cureus. (2020) 12:e7961. doi: 10.7759/cureus.7961
36. Green MH, Duell RM, Johnson CD, Jamieson NV. Haemobilia. Br J Surg. (2001) 88:773–86. doi: 10.1046/j.1365-2168.2001.01756.x
37. El Hajj II, Sherman S, Pyko M, Lehman GA. Life threatening hemobilia after endoscopic retrograde cholangiopancreatography (ERCP). Dig Liver Dis. (2017) 49:1336–7. doi: 10. 1016/j.dld.2017.09.001
38. Ogbemudia B, Raymond J, Hatcher LS, Vetor AN, Rouse T, Carroll AE, et al. Assessing outpatient follow-up care compliance, complications, and sequelae in children hospitalized for isolated traumatic abdominal injuries. J Pediatr Surg. (2019) 54:1617–20. doi: 10.1 016/j. jpedsurg.2018.09.001
39. Srivastava DN, Sharma S, Pal S, Thulkar S, Seith A, Bandhu S, et al. Transcatheter arterial embolization in the management of hemobilia. Abdom Imaging. (2006) 31:439–48. doi: 10.1 007/s00261-005-0392-7
40. Yi IK, Miao FL, Wong J, Narasimhan KL, Lo RH, Yee L, et al. Prophylactic embolization of hepatic artery pseudoaneurysm after blunt abdominal trauma in a child. J Pediatr Surg. (2010) 45:837–9. doi: 10.1016/j.jpedsurg.2010.01.021
41. Basile KE, Sivit CJ, Sachs PB, Stallion A. Hepatic arterial pseudoaneurysm: a rare complication of blunt abdominal trauma in children. Pediatr Radiol. (1999) 29:306–8. doi: 10.1007/s0024700 50594
42. Payne JE Jr, Kemmerer WT. Hepatic artery aneurysm with rupture into general peritoneal cavity. Case report of successful treatment. J Trauma. (1967) 7:793–7. doi: 10.1097/00005373-196709000-00016
43. Zmora O, Peleg K, Klein Y. Pediatric electric bicycle injuries and comparison to other pediatric traffic injuries. Traffic Inj Prev. (2019) 20:540–3. doi: 10.1080/15389588.2019.1608361
44. Gross I, Weiss DJ, Eliasi E, Bala M, Hashavya S. E-bike-related trauma in children and adults. J Emerg Med. (2018) 54:793–8. doi: 10.1016/j.jemermed.2017.12.012
45. Hermon K, Capua T, Glatstein M, Scolnik D, Tavor O, Rimon A. Pediatric electric bicycle injuries: the experience of a large urban tertiary care pediatric hospital. Pediatr Emerg Care. (2020) 36:e343–5. doi: 10.1097/PEC.0000000000001395
46. Du W, Yang J, Powis B, Zheng X, Ozanne-Smith J, Bilston L, et al. Epidemiological profile of hospitalised injuries among electric bicycle riders admitted to a rural hospital in Suzhou: a cross-sectional study. Inj Prev. (2014) 20:128–33. doi: 10.1136/injuryprev-2012-040618
47. Carrillo EH, Richardson JD. Liver injuries. In: Cameron JL, editor. Current surgical therapy. 8th ed. Baltimore, Maryland, USA: Elsevier Mosby (2004). p. 955–9.
48. Falkoff GE, Taylor KJ, Morse S. Hepatic artery pseudoaneurysm: diagnosis with real-time and pulsed doppler US. Radiology. (1986) 158:55–6. doi: 10.1148/radiology.158.1.3510028
49. Sul YH, Kim Y. Management for traumatic hepatic injury diagnosed by contrast-enhanced ultrasonography in a patient with an occluded coeliac axis: a case report. J Int Med Res. (2021) 49:3000605211019926. doi: 10.1177/03000605211019926
50. Murugesan SD, Sathyanesan J, Lakshmanan A, Ramaswami S, Perumal S, Perumal SU, et al. Massive hemobilia: a diagnostic and therapeutic challenge. World J Surg. (2014) 38:1755–62. doi: 10.1007/s00268-013-2435-5
51. Wagner ML, Streit S, Makley AT, Pritts TA, Goodman MD. Hepatic pseudoaneurysm incidence after liver trauma. J Surg Res. (2020) 256:623–8. doi: 10.1016/j.jss.2020.07.054
52. Villarreal DH, Norwood S, McAuley C, Berne JD. Hemobilia and subsequent hemocholecystitis complicating blunt hepatic injury. J Trauma. (2007) 62:E18–9. doi: 10.1097/01.ta.0000231980.68400.b6
53. Moodley J, Singh B, Lalloo S, Pershad S, Robbs JV. Non-operative management of haemobilia. Br J Surg. (2001) 88:1073–6. doi: 10.1046/j.0007-1323.2001.01825.x
54. Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep. (2010) 12:121–9. doi: 10.1007/s11894-010-0092-5
55. Tzeng WS, Wu RH, Chang JM, Lin CY, Koay LB, Uen YH, et al. Transcatheter arterial embolization for hemorrhage caused by injury of the hepatic artery. J Gastroenterol Hepatol. (2005) 20:1062–8. doi: 10.1111/j.1440-1746.2005.03768.x
56. Finley DS, Hinojosa MW, Paya M, Imagawa DK. Hepatic artery pseudoaneurysm: a report of seven cases and a review of the literature. Surg Today. (2005) 35(7):543–7. doi: 10.1007/s00595-005-2987-6
57. Singh V. Endoscopic management of traumatic hemobilia. J Trauma. (2007) 62:1045–7. doi: 10.1097/01.ta.0000246881.67799.4f
58. Mahmood I, Kasim M, El-Menyar A, Nabir S, Afifi I, Abdelrahman H, et al. Late development of giant hepatic artery pseudoaneurysm following abdominal trauma due to tire blast: case report and literature review. J Surg Case Rep. (2021) 2021:rjab564. doi: 10.1093/jscr/rjab564
59. Patel MS, Shetty V, Shelake A, Deshpande AA. Early presentation of ruptured post-traumatic hepatic artery pseudoaneurysm. J Postgrad Med. (2018) 64:250–2. doi: 10.4103/jpgm.JPGM_81_18
60. Shope TR, Bass TL, Haluck RS. Laparoscopic management of traumatic hemorrhagic cholecystitis. JSLS. (2004) 8:93–5.
61. Vultaggio F, Morère PH, Constantin C, Christodoulou M, Roulin D. Gastrointestinal bleeding and obstructive jaundice: think of hepatic artery aneurysm. World J Gastrointest Surg. (2016) 8:467–71. doi: 10.4240/wjgs.v8.i6.467
62. Kanani A, Sandve KO, Søreide K. Management of severe liver injuries: push, pack, pringle - and plug!. Scand J Trauma Resusc Emerg Med. (2021) 29:93. doi: 10.1186/s13049-021-00907-0
63. Forlee MV, Krige JE, Welman CJ, Beningfield SJ. Haemobilia after penetrating and blunt liver injury: treatment with selective hepatic artery embolisation. Injury. (2004) 35:23–8. doi: 10.1016/s0020-1383(03)00156-6
Keywords: blunt hepatic trauma, delayed massive hemobilia, injury prevention, toddler, case report
Citation: Liu X, Sun Q, Sun W, Niu Q, Wang Z, Liu C, Fu T, Geng L and Li X (2022) Severe Blunt Liver Injury Complicated by Delayed Massive Hemobilia in a Toddler: A Case Report and Literature Review. Front. Surg. 9:930581. doi: 10.3389/fsurg.2022.930581
Received: 28 April 2022; Accepted: 21 June 2022;
Published: 8 July 2022.
Edited by:
Luca Pio, St. Jude Children’s Research Hospital, United StatesReviewed by:
Simon Kargl,Copyright © 2022 Liu, Sun, Sun, Niu, Wang, Liu, Fu, Geng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Geng MzgxODExNDFAcXEuY29t Xiaomei Li bGlseWx4bUAxNjMuY29t
Specialty section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.