
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 22 July 2022
Sec. Genitourinary Surgery and Interventions
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.930160
 Zhan-yi Zhang1,†
Zhan-yi Zhang1,† Peng Hong1†
Peng Hong1† Shao-hui Deng1
Shao-hui Deng1 Shi-ying Tang1
Shi-ying Tang1 Zhuo Liu1
Zhuo Liu1 Hui-ying He2
Hui-ying He2 Lu-lin Ma1*
Lu-lin Ma1* Shu-dong Zhang1*
Shu-dong Zhang1* Xiao-jun Tian1*
Xiao-jun Tian1*
Background: Anastomosing hemangioma (AH) is a rare vascular tumor and occurs in various organs. It is difficult to distinguish AH from malignant tumors even through multimodal imaging examination. AH located in the inguinal region is even rare. We present the diagnosis and treatment of a patient with spermatic cord AH in detail and conduct a literature review.
Case Report: An 84-year-old Chinese man had swelling pain in his right scrotum. A hard and fixed mass was palpable in the right inguinal region. Preoperative radiological examination considered it a neurogenic or vascular tumor. Malignant soft tissue sarcoma could not be excluded. He underwent radical inguinal right orchiectomy under intraspinal anesthesia. The diagnosis of spermatic cord AH was confirmed by pathological examination. The patient recovered uneventfully and remained disease-free during an 18-month follow-up.
Conclusion: Spermatic cord AH is quite rare and could be misdiagnosed as a malignant tumor. Pathological evidence might be necessary. The optimal choice of treatment should be determined through a comprehensive assessment of both tumor and patient factors.
Anastomosing hemangioma (AH) is a rare subtype of vascular tumor. Montgomery and Epstein reported the first AH case in 2009 (1). Thereafter, a number of AH cases have been reported. It seems that AH is usually localized to the genitourinary system, especially in the kidney. Previous reports showed that AH contained a benign course (1–3), with few instances of recurrence or metastasis. However, there are many overlapping clinicopathological features between AH and malignant sarcomas, placing many hurdles to the diagnosis and treatment of AH. AH located in the inguinal region is even rare. In this report, we present a unique case of spermatic cord AH, conduct a brief English literature review about AH, and discuss the differential diagnosis of an inguinal mass according to radiological and pathological features. Besides, reasonable treatment choices are discussed. This study may prove favorable for the diagnosis and treatment of AH at an unusual site.
An 84-year-old Chinese man came to our clinic complaining of swelling and pain in his right scrotum for 1 week. He could touch a mass in his right inguinal region. Upon physical examination (PE), a 3.0 × 1.5 cm hard and fixed mass could be touched in his right inguinal region. Droppler ultrasound (US) examination was performed initially. A 2.9 × 1.2 cm solid-cystic (predominantly solid) mass could be observed in the right inguinal region. There were abundant blood flow signals within the mass. The spermatic cord was swollen with a high echo. No obvious hernia contents or enlarged lymph nodes were observed when abdominal pressure increased (Figure 1). The radiologists believed that neurogenic tumors could not be excluded and suggested an ultrasound-guided percutaneous biopsy.
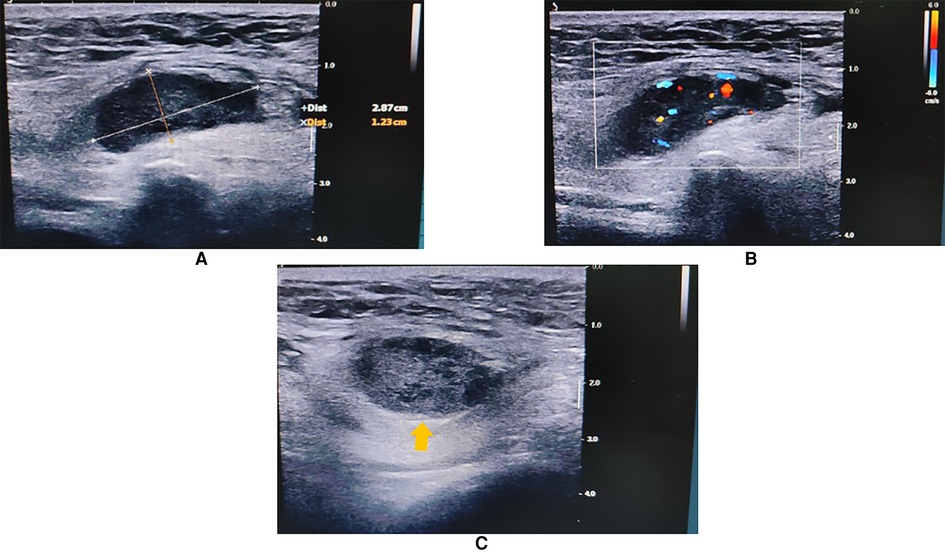
Figure 1. Inguinal US showing a 2.9 × 1.2 cm solid-cystic mass in the right inguinal region (arrow head). The spermatic cord was swollen with a high echo. (A) Long-axis view. (B) Color Droppler mode showing blood flow signals within the mass. (C) Short-axis view.
To learn about the properties, blood supply, and anatomical position of the mass, an abdominal and pelvic contrast-enhanced computed tomography (CE-CT) scan was performed thereafter. A 2.3 × 1.3 cm heterogeneously enhanced mass with soft tissue density was seen inside the right inguinal canal (Figure 2). Hypodense, non-enhanced cystic components could be observed within the mass. To further evaluate the involvement of adjacent soft tissues and lymph nodes, pelvic magnetic resonance imaging (MRI) was then completed. MRI showed a 2.3 × 1.3 cm round-like mass with low signal intensity in T1-weighted imaging (WI) and high signal intensity in T2-weighted imaging (WI). Upon diffusion-weighted imaging (DWI), the mass also presented high signal intensity. Meanwhile, perilesional effusions and edema could be observed. There were no enlarged lymph nodes on both CT and MRI scans (Figure 3). Based on imaging findings, a neurogenic tumor was initially considered by radiologists and might be clinically relevant to his scrotal discomfort. Considering its abundant blood flow, a vascular tumor was also taken into consideration. Moreover, its heterogeneous components and enhancing patterns raised concerns for malignant soft tissue sarcoma. Besides, the patient had a recent history of coronary heart disease (CHD). Therefore, a multidisciplinary team (MDT) discussion with radiologists, pathologists, general surgeons, and cardiologists in our center was conducted. Then, we felt that radical inguinal right orchiectomy might be the appropriate treatment for this patient. We informed the patient about the treatment options, benefits, and potential perioperative risks. He fully understood the benefits and risks of each treatment and chose to undergo surgery.
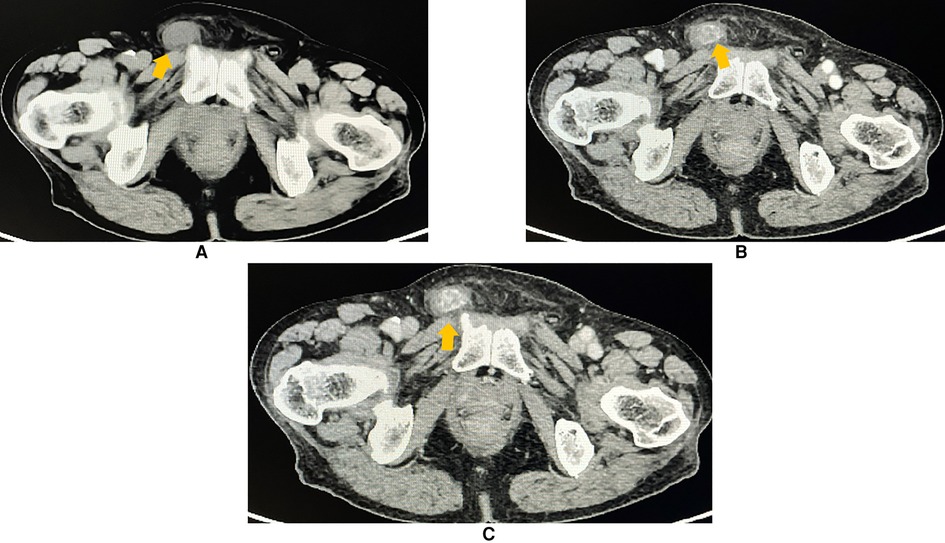
Figure 2. Contrast-enhanced CT (axial plane) revealing a 2.3 × 1.3 cm heterogeneous and an avidly enhanced mass with a soft-tissue density (arrowhead) in the right inguinal region. Perilesional effusions were observed. (A) Non-contrast phase. (B) Arterial phase of the contrast. (C) Venous phase of the contrast.
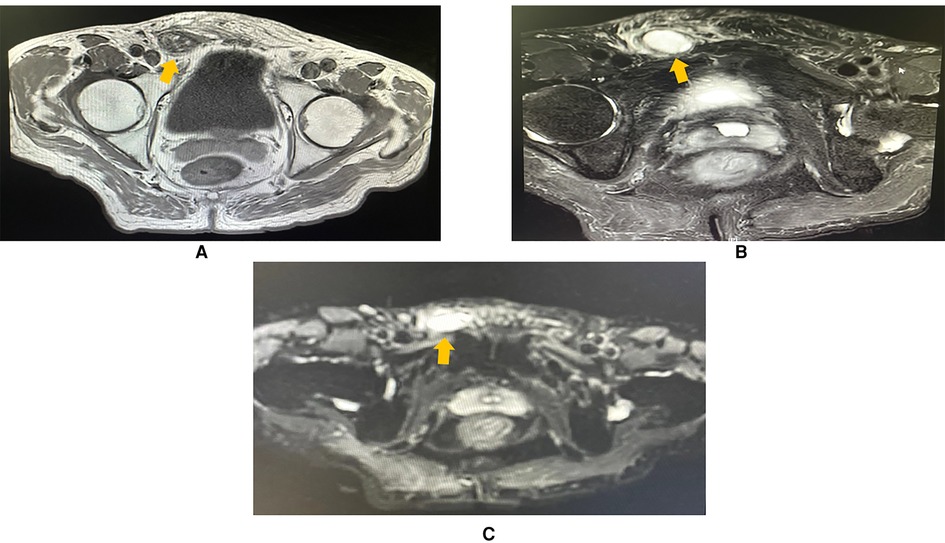
Figure 3. MRI imaging (axial plane) showing a 2.3 × 1.3 cm oval mass in the right inguinal region, with perilesional effusions and edema. (A) T1-weighted imaging showing that the lesion was hypointense (arrowhead). (B) T2-weighted imaging showing that the mass (arrowhead) was hyperintense. (C) The mass was hyperintense (arrowhead) in diffusion-weighted imaging (DWI).
After intraspinal anesthesia, an oblique incision was made along a Langer line, 2 cm superior and parallel to the right inguinal ligament. The inguinal canal was exposed after incising the external oblique aponeurosis. We transected the cremasteric muscle and exposed the spermatic cord. The mass was palpable within the spermatic cord. The spermatic cord was then dissected bluntly from the mass toward the cephalad direction. Gonadal vessels and vas deferens were separately ligated with absorbable sutures at the deep inguinal ring level. Blunt dissection of the spermatic cord in the caudad direction was performed thereafter. The right testis along with its tunica vaginalis was retracted into the surgical area and completely resected. After careful hemostasis, the fascial layers and skin were sutured. The ilioinguinal nerve was well identified and preserved during the whole surgical procedure.
Grossly, a 1.5 × 1 × 0.8 cm gray-white solid mass could be seen on the sagittal section of the resected specimen. The mass was 1 cm away from the cutting edge of the spermatic cord, 5.5 cm from the testis, and 5 cm from the epididymis. There was no clear boundary between the mass and its adjacent tissues. Microscopically, the mass showed typical features of a vascular tumor. It was composed of irregular, anastomosing capillary-sized vessels. The lumina of those vessels were lined by a monolayer of oval endothelial cells. Some of them showed a hobnail appearance. Those endothelial cells had a mild cellular morphology lacking nuclear atypia and mitoses. Focal short spindle stromal cell hyperplasia could be observed within the spermatic cord. Atrophy in adjacent seminiferous tubules was observed in the right testis. There was no obvious lesion in both resected testis and epididymis. The margin status was negative. Immunohistochemical (IHC) staining showed positive staining of endothelial markers—CD31, CD34, and ERG. Desmin, inhibin-α, S-100, and NSE were negative. The Ki-67 rate was low (approximately 2%) (Figure. 4). SMA was positive in the surrounding stromal cells. Based on the pathological findings, the diagnosis of AH was confirmed.
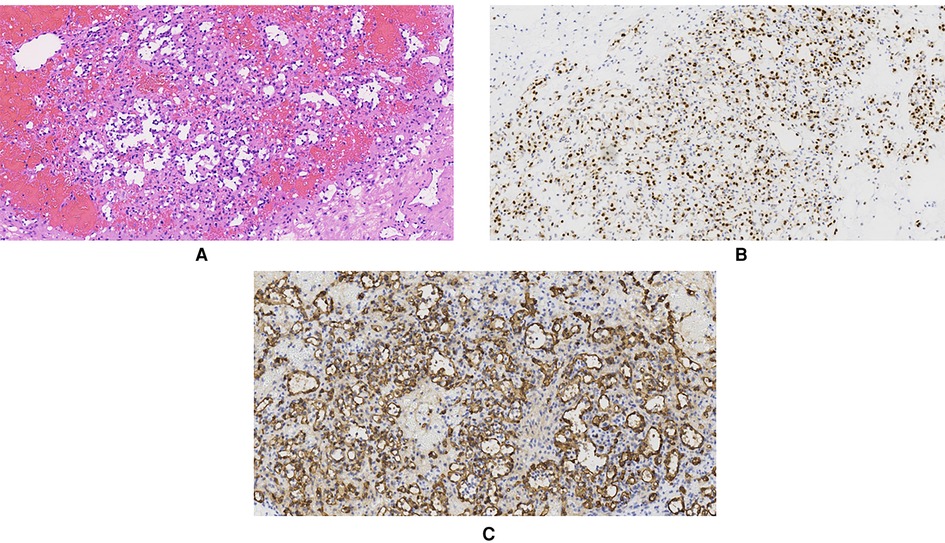
Figure 4. Pathological examination showing that the mass was composed of distinctive “anastomosing” capillary-sized vessels lined by “hobnail” endothelial cells. (A) Hematoxylin and eosin (HE) staining (×200). (B) Immunohistochemistry staining of ERG in endothelial cells (×200). (C) Immunohistochemistry staining of CD34 in endothelial cells (×20).
The patient recovered uneventfully after surgery and was discharged 4 days later. During the 18-month follow-up, the patient reported no local discomfort. PE and abdominal US examination showed no disease recurrence. He expressed satisfaction over the treatment. The timeline of the presented case is summarized in Supplementary Figure S1.
To learn more about this rare subtype of vascular tumor, we searched PubMed, Web of Science, and EMBASE for all AH cases reported in English before September 1, 2021. Fifty-three publications (1–53), including 137 genitourinary cases and 71 non-genitourinary cases, were identified (Supplementary Table S1).
AH patients’ age varies from 1 to 85 years. There are more male patients than females. Most genitourinary AHs locate in the kidney, while the para-vertebral region is the most frequently involved region in non-genitourinary patients (Supplementary Figure S2A). The tumor size ranges from 0.1 to 14.0 cm and tends to be larger in non-genitourinary patients (Supplementary Figure S2B). More than half of AH cases are incidentally found in routine examination. The common manifestations are local pain, palpable mass, hematuria, neurological deficit, hematoma, and so on (Supplementary Figure S2C). Moreover, 21.7% of genitourinary cases and 11.3% of non-genitourinary cases are coexistent with other tumors (Supplementary Figure S2D). In this article, we presented an 84-year-old man with a rare, symptomatic spermatic cord AH. The patient complained of discomfort in his scrotum, and a palpable mass was palpable in his right inguinal region. It could be deducted that the symptom in the scrotum might be caused by the inguinal mass. It is reported that spermatic cord masses are generally benign, and inguinal lipoma is the most common tumor. However, the incidence of malignancy is not low, which could reach up to 30% (54). It is also noteworthy that about 46% of soft tissue sarcoma is located in the thigh, buttock, and inguinal regions (55). Thus, although the incidence of sarcoma is only about 2/100,000 in China (56), we should still make a careful differential diagnosis of this inguinal mass.
Currently, there is a lack of specific imaging features for a definite diagnosis of AH, but some common presentation is already confirmed. AHs usually show a hypoechoic or anechoic cystic lesion on US (6, 20, 26, 57) and may have an enrichment of blood flow on edge (21). Nearly all AH patients underwent a regional CT examination. On CT scans, the majority of AHs are well-circumscribed, hyperdense nodules with or without centrally hypodense cystic components. The existence of post-contrast avid peripheral enhancement and the filing pattern of contrast agent from the peripheral region to the center could reveal the properties of hemangiomas (2, 36, 37). Some patients underwent MRI scans with or without gadolinium enhancement (3, 5, 26, 34, 36–38). AHs tend to be hypointense on T1WI and hyperintense on T2WI and DWI. Peripheral enhancement in the arterial phase and enhancement of central components in the venous phase could be observed, similar to the manifestations of CE-CT (5).
Our patient showed a hypoechoic mass with a prominent blood circulation signal on US. The mass was peripherally isodense with centrally hypodense cystic components on non-contrast images and could be heterogeneously and avidly enhanced. Along with its characteristics on MRI (hypointense T1 signal, hyperintense T2, and DWI signal), it showed similar imaging features to the previously reported AHs, while it lacked the typical filing pattern of hemangiomas on CE-CT. Radiologists from our center considered the initial diagnosis of neurogenic tumors such as schwannoma. Schwannoma is a benign peripheral nerve sheath tumor (PNST), which may also present as a hypoechoic cystic mass on US. On MRI, schwannoma shows hypo- or iso-intensity, compared with adjacent muscles on T1WI and high intensity on T2WI, especially on the periphery. Cystic degeneration may also exist on CT/MRI. However, typical characteristics, such as nerve entering or existing the mass, “fusiform” shape, “split-fat” sign, and “target” sign, were absent in our case (58, 59). Moreover, imaging signs, including predominant solid components, rich blood supply, perilesional effusions, and a heterogeneous enhancement pattern implied the possibility of a soft tissue sarcoma, for example, malignant PNST. Thus, pathological examination seemed necessary for the diagnosis.
There exist controversial opinions on the optimal way to acquire pathological specimens in this circumstance. According to a literature review, all genitourinary AH cases underwent surgery, while some non-genitourinary cases chose relatively conservative treatment (Supplementary Figure S2E). Image-guided percutaneous biopsy provides a less traumatic choice (36), and the outcome could help decide subsequent therapies. However, the limited histologic materials acquired from a percutaneous biopsy may be challenging to make an accurate diagnosis, especially when the mass shows heterogeneous entities. Results from surgically acquired specimens might be more reliable, while the extent of resection remains to be discussed. A literature review on the surgical management of spermatic cord sarcoma (60) and an MDT discussion were conducted. There were several factors in favor of radical inguinal orchiectomy: First, the mass had no clear border with adjacent spermatic cord vessels and was rich in blood supply, which made sole dissection rather difficult. Second, considering that the mass might be malignant and that no metastatic sites were noted, surgical margin status would be maximally guaranteed and the possibility of reoperation as well as local recurrence might be reduced. Last but not least, our patient was an 84-year-old and did not have fertile demands. The pursuit of R0 resection of a susceptible malignant inguinal mass was acceptable. In consideration of the patient's condition and preference, a radical inguinal right orchiectomy at the level of the deep inguinal ring was performed.
According to the literature, the majority of AHs have a clear margin but lack a definite capsule and usually present a hemorrhagic “mahogany brown” (47) or “red-tan spongy” (1) gross appearance. Microscopically, the tumors are composed of “anastomosing” capillary-sized vessels resembling the splenic sinusoids. The vascular channels within the tumor are capillary-sized, lined by a single layer of endothelial cells that frequently shows a hobnail morphology (1, 11, 39, 42). Edematous or myxoid components may exist in the surrounding stroma (25, 61). Extramedullary hematopoiesis and intraluminal thrombi are occasionally observed (10, 32, 45). Some uncommon features, such as focally infiltrative patterns (6, 46) and hyaline globules (18, 28), might raise concerns about some aggressive malignant tumors, such as angiosarcoma and Kaposi sarcoma. Differential diagnosis of well-differentiated low-grade angiosarcoma required the most careful recognition (39). However, AHs lack malignant signs like significant endothelial tufting, nuclear atypia, pleomorphism, mitosis, and multilayers of endothelial cells. These may help morphological discrimination through careful observation (13, 39). In IHC staining, AHs are typically positive for endothelial markers like CD31, CD34, Factor VIII, and FLI1, while they usually lack the immunoactivity of lymphatic endothelial cell marker D2-40, splenic sinusoidal cell marker CD8, and immunosuppressive-related tumor marker HHV8 (62).
Our patient presented a gross gray-white appearance, which was distinct from the reported AHs. Rather than a neurogenic tumor, the microscopic histological features revealed typical features of an endothelial vascular tumor reminiscent of spleen sinusoids, and malignant morphologic signs of angiosarcoma were absent. CD31, CD34, and ERG were positive in IHC staining. ERG is also an endothelial marker, but it is more specific and sensitive than CD31 or CD34. ERG (+) has also been reported previously in AH patients (15, 16, 30, 40, 44, 53). As spindle stromal cell hyperplasia was observed within the spermatic cord, we stained the markers of spindle cell sarcoma (e.g., rhabdomyosarcoma, leiomyosarcoma, regressed germ cell tumors, and MPNST), such as desmin, inhibin-α, S-100, and NSE. They all showed negative results. Thus, the diagnosis of a rare spermatic cord AH was eventually confirmed.
Overall, AHs have a benign course. In the literature review, follow-up time ranges from 0.5 to 156 months. Few instances of recurrence or metastasis were reported, except for two unique cases. One was an intracranial AH (10), and the other one had multifocal, recurrent AHs located at different sites (12). Three patients died from unrelated disease. No disease-related death was reported. These results imply that there might be no need for further therapies when the diagnosis of AH is confirmed. If the patient was diagnosed with surgical resection, negative margin status could minimize the risk of local recurrence. Regular follow-up is still recommended, especially for those diagnosed with percutaneous biopsy or for those choosing active surveillance. As for the pathogenesis of AH, recent studies have found that recurrent GNA11, GNA14, and GNAQ gene mutation occurs in some AH cases, which is different from that of angiosarcoma (7, 8, 28). Mutational analysis may serve as a tool of differential diagnosis in the future.
There exist some limitations in the current study. IHC staining of some markers like D2–40, GLUT-1, and HHV-8, which may help to exclude lymphatic tumors and other vascular tumors, was absent. The follow-up time is not so long to evaluate recurrence and tumor-specific survival.
Spermatic cord AH is quite rare and could be misdiagnosed as a malignant inguinal tumor. Pathological examination is necessary when the diagnosis cannot be made through multi-modal radiology assessment. The optimal choice of treatment should be determined through a comprehensive assessment. If the mass has a high risk of malignancy and the patient may not benefit from percutaneous biopsy, a meticulously tailored surgery might be a reasonable choice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Peking University Third Hospital. The patients/participants provided their written informed consent to participate in this study.
LLM, SDZ, XJT, PH, and ZYZ conceptualized and designed the study; LLM and SDZ provided administrative support; XJT and HYH provided study materials or patients; ZYZ, SHD, SYT, ZL, and HYH were in charge of collection and assembly of literature; ZYZ, SHD, SYT, and ZL performed data analysis and interpretation; all authors were involved in manuscript writing; all authors gave final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Clinical Projects of Peking University Third Hospital [BYSYZD2019032] and the National Nature Science Foundation of China [No. 81972381].
The authors thank the entire staff of the Department of Urology, Peking University Third Hospital.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.930160/full#supplementary-material.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. (2009) 33(9):1364–9. doi: 10.1097/PAS.0b013e3181ad30a7
2. Kryvenko ON, Gupta NS, Meier FA, Lee MW, Epstein JI. Anastomosing hemangioma of the genitourinary system: eight cases in the kidney and ovary with immunohistochemical and ultrastructural analysis. Am J Clin Pathol. (2011) 136(3):450–7. doi: 10.1309/AJCPJPW34QCQYTMT
3. Zhao M, Li C, Zheng J, Sun K. Anastomosing hemangioma of the kidney: a case report of a rare subtype of hemangioma mimicking angiosarcoma and review of the literature. Int J Clin Exp Pathol. (2013) 6(4):757–65. PMID: 23573324; PMCID: PMC3606867
4. Lo CH, Cheng SY. Case report on anastomosing haemangioma: an unusual vascular tumor in kidney. Case Rep Nephrol. (2021) 2021:8847998. doi: 10.1155/2021/8847998
5. Abboudi H, Tschobotko B, Carr C, DasGupta R. Bilateral renal anastomosing hemangiomas: a tale of two kidneys. J EndourolCase Rep. (2017) 3(1):176–8. doi: 10.1089/cren.2017.0018
6. Al-Maghrabi HA, Al Rashed AS. Challenging pitfalls and mimickers in diagnosing anastomosing capillary hemangioma of the kidney: case report and literature review. Am J Case Rep. (2017) 18:255–62. doi: 10.12659/AJCR.902939
7. Bean GR, Joseph NM, Folpe AL, Horvai AE, Umetsu SE. Recurrent GNA14 mutations in anastomosing haemangiomas. Histopathology. (2018) 73(2):354–7. doi: 10.1111/his.13519
8. Bean GR, Joseph NM, Gill RM, Folpe AL, Horvai AE, Umetsu SE. Recurrent GNAQ mutations in anastomosing hemangiomas. Mod Pathol. (2017) 30(5):722–7. doi: 10.1038/modpathol.2016.234
9. Berker NK, Bayram A, Tas S, Bakir B, Caliskan Y, Ozcan F, et al. Comparison of renal anastomosing hemangiomas in end-stage and non-end-stage kidneys: a meta-analysis with a report of 2 cases. Int J Surg Pathol. (2017) 25(6):488–96. doi: 10.1177/1066896917706025
10. Bodman A, Goodman A, Olson JJ. Intracranial thrombosed anastomosing hemangioma: case report. Neuropathology. (2020) 40(2):206–10. doi: 10.1111/neup.12624
11. Brown JG, Folpe AL, Rao P, Lazar AJ, Paner GP, Gupta R, et al. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol. (2010) 34(7):942–9. doi: 10.1097/PAS.0b013e3181e4f32a
12. Burton KR, Jakate K, Pace KT, Vlachou PA. A case of recurrent, multifocal anastomosing haemangiomas. BMJ Case Rep. (2017) 2017. doi: 10.1136/bcr-2017-220076
13. Büttner M, Kufer V, Brunner K, Hartmann A, Amann K, Agaimy A. Benign mesenchymal tumours and tumour-like lesions in end-stage renal disease. Histopathology. (2013) 62(2):229–36. doi: 10.1111/j.1365-2559.2012.04349.x
14. Caballes AB, Abelardo AD, Farolan MJ, Veloso JAD. Pediatric anastomosing hemangioma: case report and review of renal vascular tumors in children. Pediatr Dev Pathol. (2019) 22(3):269–75. doi: 10.1177/1093526618809230
15. Cheon PM, Rebello R, Naqvi A, Popovic S, Bonert M, Kapoor A. Anastomosing hemangioma of the kidney: radiologic and pathologic distinctions of a kidney cancer mimic. Curr Oncol. (2018) 25(3):e220–e3. doi: 10.3747/co.25.3927
16. Chou S, Subramanian V, Lau HM, Achan A. Renal anastomosing hemangiomas with a diverse morphologic spectrum: report of two cases and review of literature. Int J Surg Pathol. (2014) 22(4):369–73. doi: 10.1177/1066896913492850
17. Dundr P, Němejcová K, Laco J, Skálová H, Bauerová L, Matěj R, et al. Anastomosing hemangioma of the ovary: a clinicopathological study of six cases with stromal luteinization. Pathol Oncol Res. (2017) 23(4):717–22. doi: 10.1007/s12253-016-0186-y
18. Dutta R, Kakkar A, Sakthivel P, Kumar R. Anastomosing hemangioma of the larynx: a unicorn among head and neck tumors. Ann Otol Rhinol Laryngol. (2020) 130(3):298–303. doi: 10.1177/0003489420943640
19. Gonzalez SP, Wachtel MS, Onkendi EO. Operative management of T1b gallbladder carcinoma with concurrent hepatic anastomosing hemangioma. Cureus. (2019) 11(7):e5081. doi: 10.7759/cureus.5081
20. Gunduz M, Hurdogan O, Onder S, Yavuz E. Cystic anastomosing hemangioma of the ovary: a case report with immunohistochemical and ultrastructural analysis. Int J Surg Pathol. (2019) 27(4):437–40. doi: 10.1177/1066896918817148
21. Heidegger I, Pichler R, Schäfer G, Zelger B, Zelger B, Aigner F, et al. Long-term follow up of renal anastomosing hemangioma mimicking renal angiosarcoma. Int J Urol. (2014) 21(8):836–8. doi: 10.1111/iju.12433
22. Huang ZY, Chen CC, Thingujam B. Anastomosing hemangioma of the nasal cavity. Laryngoscope. (2020) 130(2):354–7. doi: 10.1002/lary.27998
23. Jayaram A, Manipadam MT, Jacob PM. Anastomosing hemangioma with extensive fatty stroma in the retroperitoneum. Indian J Pathol Microbiol. (2018) 61(1):120–2. doi: 10.4103/IJPM.IJPM_259_16
24. Jin LU, Liu J, Li Y, Sun S, Mao X, Yang S, et al. Anastomosing hemangioma: the first case report in the bladder. Mol Clin Oncol. (2016) 4(2):310–2. doi: 10.3892/mco.2015.699
25. John I, Folpe AL. Anastomosing hemangiomas arising in unusual locations: a clinicopathologic study of 17 soft tissue cases showing a predilection for the paraspinal region. Am J Surg Pathol. (2016) 40(8):1084–9. doi: 10.1097/PAS.0000000000000627
26. Kishida N, Sentani K, Terada H, Honda Y, Goto K, Hatanaka Y, et al. Anastomosing haemangioma with fatty changes in the perirenal space: a lesion mimicking liposarcoma. BJR Case Rep. (2018) 4(2):20170022. doi: 10.1259/bjrcr.20170022
27. Kryvenko ON, Haley SL, Smith SC, Shen SS, Paluru S, Gupta NS, et al. Haemangiomas in kidneys with end-stage renal disease: a novel clinicopathological association. Histopathology. (2014) 65(3):309–18. doi: 10.1111/his.12394
28. Liau JY, Tsai JH, Lan J, Chen CC, Wang YH, Lee JC, et al. GNA11 joins GNAQ and GNA14 as a recurrently mutated gene in anastomosing hemangioma. Virchows Arch. (2020) 476(3):475–81. doi: 10.1007/s00428-019-02673-y
29. Lin J, Bigge J, Ulbright TM, Montgomery E. Anastomosing hemangioma of the liver and gastrointestinal tract: an unusual variant histologically mimicking angiosarcoma. Am J Surg Pathol. (2013) 37(11):1761–5. doi: 10.1097/PAS.0b013e3182967e6c
30. Lin MS, Ngo T, Schwartz MR, Mehta RR, Ayala AG, Ro JY. Anastomosing hemangioma of the breast: an unusual case at an unusual site. J Breast Cancer. (2020) 23(3):326–30. doi: 10.4048/jbc.2020.23.e15
31. Lunn B, Yasir S, Lam-Himlin D, Menias CO, Torbenson MS, Venkatesh SK. Anastomosing hemangioma of the liver: a case series. Abdom Radiol. (2019) 44(8):2781–7. doi: 10.1007/s00261-019-02043-x
32. Manohar V, Krishnamurthy S, Ranganathan J, Pai VD. A case of giant anastomosing hemangioma of the kidney with extramedullary hematopoiesis: a great mimicker. Indian J Pathol Microbiol. (2020) 63(2):292–4. doi: 10.4103/IJPM.IJPM_434_18
33. Mehta V, Ananthanarayanan V, Antic T, Krausz T, Milner J, Venkataraman G, et al. Primary benign vascular tumors and tumorlike lesions of the kidney: a clinicopathologic analysis of 15 cases. Virchows Arch. (2012) 461(6):669–76. doi: 10.1007/s00428-012-1333-9
34. Merritt B, Behr S, Umetsu SE, Roberts J, Kolli KP. Anastomosing hemangioma of liver. J Radiol Case Rep. (2019) 13(6):32–9. doi: 10.3941/jrcr.v13i6.3644
35. Omiyale AO, Golash A, Mann A, Kyriakidis D, Kalyanasundaram K. Anastomosing haemangioma of the kidney involving a segmental branch of the renal vein. Case Rep Surg. (2015) 2015:927286. doi: 10.1155/2015/927286
36. O’Neill AC, Craig JW, Silverman SG, Alencar RO. Anastomosing hemangiomas: locations of occurrence, imaging features, and diagnosis with percutaneous biopsy. Abdom Radiol. (2016) 41(7):1325–32. doi: 10.1007/s00261-016-0690-2
37. Patel SR, Abimbola O, Bhamber T, Weida C, Roy O. Incidental finding of bilateral renal and adrenal anastomosing hemangiomas: a rare case report. Urol Case Rep. (2019) 27:100912. doi: 10.1016/j.eucr.2019.100912
38. Peng X, Li J, Liang Z. Anastomosing haemangioma of liver: a case report. Mol Clin Oncol. (2017) 7(3):507–9. doi: 10.3892/mco.2017.1341
39. Perdiki M, Datseri G, Liapis G, Chondros N, Anastasiou I, Tzardi M, et al. Anastomosing hemangioma: report of two renal cases and analysis of the literature. Diagn Pathol. (2017) 12(1):14. doi: 10.1186/s13000-017-0597-4
40. Rathore K, Yussouf R, Teh M, Jindal S, Wong D, Newman M. Left atrial anastomosing hemangioma causing recurrent pericardial effusion. Ann Thorac Surg. (2020) 109(3):e157–e9. doi: 10.1016/j.athoracsur.2019.06.082
41. Rezk A, Richards S, Patricia Castillo R, Schlumbrecht M. Anastomosing hemangioma of the ovary mimics metastatic ovarian cancer. Gynecol Oncol Rep. (2020) 34:100647. doi: 10.1016/j.gore.2020.100647
42. Ross M, Polcari A, Picken M, Sankary H, Milner J. Anastomosing hemangioma arising from the adrenal gland. Urology. (2012) 80(3):e27–8. doi: 10.1016/j.urology.2012.05.032
43. Silva MA, Fonseca E, Yamauchi FI, Baroni RH. Anastomosing hemangioma simulating renal cell carcinoma. Int Braz J Urol. (2017) 43(5):987–9. doi: 10.1590/s1677-5538.ibju.2016.0653
44. Stewart CJR, Salfinger SG. Anastomosing haemangioma of the ovary with hilus cell hyperplasia. Pathology. (2020) 52(3):392–4. doi: 10.1016/j.pathol.2019.11.010
45. Subbarayan D, Devaraji A, Senthilnayagam B, Ramanujam S, Nandagopalradha R. Anastomosing hemangioma of the ovary clinically masquerading as epithelial malignancy: a rare case report. J Midlife Health. (2019) 10(1):48–50. doi: 10.4103/jmh.JMH_121_18
46. Tahir M, Folwell A. Anastomosing haemangioma of kidney: a rare subtype of vascular tumour of the kidney mimicking angiosarcoma. ANZ J Surg. (2016) 86(10):838–9. doi: 10.1111/ans.12779
47. Tao LL, Dai Y, Yin W, Chen J. A case report of a renal anastomosing hemangioma and a literature review: an unusual variant histologically mimicking angiosarcoma. Diagn Pathol. (2014) 9:159. doi: 10.1186/s13000-014-0159-y
48. Tran TA, Pernicone P. Anastomosing hemangioma with fatty changes of the genitourinary tract: a lesion mimicking angiomyolipoma. Cent European J Urol. (2012) 65(1):40–2. doi: 10.5173/ceju.2012.01.art13
49. Tran TAN, Linos K, Carlson JA, Bridge JA. A primary cutaneous vascular neoplasm with histologic features of anastomosing hemangioma. J Cutan Pathol. (2019) 46(5):353–7. doi: 10.1111/cup.13426
50. Wetherell DR, Skene A, Manya K, Manecksha RP, Chan Y, Bolton DM. Anastomosing haemangioma of the kidney: a rare morphological variant of haemangioma characteristic of genitourinary tract location. Pathology. (2013) 45(2):193–6. doi: 10.1097/PAT.0b013e32835c782b
51. Zhang W, Wang Q, Liu YL, Yu WJ, Liu Y, Zhao H, et al. Anastomosing hemangioma arising from the kidney: a case of slow progression in four years and review of literature. Int J Clin Exp Pathol. (2015) 8(2):2208–13. PMID: 25973131
52. Zheng LP, Shen WA, Wang CH, Hu CD, Chen XJ, Shen YY, et al. Anastomosing hemangioma arising from the left renal vein: a casereport. World J Clin Cases. (2020) 8(20):4986–92. doi: 10.12998/wjcc.v8.i20.4986
53. Zhou J, Yang X, Zhou L, Zhao M, Wang C. Anastomosing hemangioma incidentally found in kidney or adrenal gland: study of 10 cases and review of literature. Urol J. (2020) 17(6):650–6. doi: 10.22037/uj.v0i0.5514
54. Vagnoni V, Brunocilla E, Schiavina R, Borghesi M, Passaretti G, Gentile G, et al. Inguinal canal tumors of adulthood. Anticancer Res. (2013) 33(6):2361–8. PMID: 23749883
55. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. (1987) 205(4):349–59. doi: 10.1097/00000658-198704000-00003
56. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71(1):7–33. doi: 10.3322/caac.21654
57. Lee YJ, Ha WS, Park ST, Choi SK, Hong SC, Park JW. Which biopsy method is more suitable between a basin dissection and pick-up biopsy for sentinel nodes in laparoscopic sentinel-node navigation surgery (LSNNS) for gastric cancer? J Laparoendosc Adv Surg Tech A. (2008) 18(3):357–63. doi: 10.1089/lap.2007.0024
58. Tagliafico AS, Isaac A, Bignotti B, Rossi F, Zaottini F, Martinoli C. Nerve tumors: what the MSK radiologist should know. Semin Musculoskelet Radiol. (2019) 23(1):76–84. doi: 10.1055/s-0038-1676290
59. Abreu E, Aubert S, Wavreille G, Gheno R, Canella C, Cotten A. Peripheral tumor and tumor-like neurogenic lesions. Eur J Radiol. (2013) 82(1):38–50. doi: 10.1016/j.ejrad.2011.04.036
60. Goldberg H, Wong LM, Dickson B, Catton C, Yap SA, Alkasab T, et al. Long-term oncological outcomes of patients with paratesticular sarcoma. BJU Int. (2019) 124(5):801–10. doi: 10.1111/bju.14775
61. Kryvenko ON, Epstein JI. Testicular hemangioma: a series of 8 case. Am J Surg Pathol. (2013) 37(6):860–6. doi: 10.1097/PAS.0b013e318278817f
Keywords: hemangioma, spermatic cord, urogenital neoplasms, hemangiosarcoma, nerve sheath neoplasms
Citation: Zhang Z, Hong P, Deng S, Tang S, Liu Z, He H, Ma L, Zhang S and Tian X (2022) Spermatic cord anastomosing hemangioma mimicking a malignant inguinal tumor: A case report and literature review. Front. Surg. 9:930160. doi: 10.3389/fsurg.2022.930160
Received: 27 April 2022; Accepted: 16 June 2022;
Published: 22 July 2022.
Edited by:
Petros Sountoulides, Aristotle University of Thessaloniki, GreeceReviewed by:
Surasak Sangkhathat, Prince of Songkla University, Thailand© 2022 Zhang, Hong, Deng, Tang, Liu, He, Ma, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lulin Ma bWFsdWxpbnBrdUAxNjMuY29t Shudong Zhang c2hvb3RvbmdAMTYzLmNvbQ== Xiaojun Tian MTM1MTEwMjkwMDNAMTYzLmNvbQ==
†These authors have contributed equally to this work.
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.