
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 22 June 2022
Sec. Orthopedic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.930036
This article is part of the Research Topic New Perspectives and Innovative Techniques in Contemporary Spine Surgery View all 17 articles
Introduction: This study aimed to demonstrate the safety and effectiveness of modified percutaneous endoscopic transforaminal discectomy (PETD) in the surgical management of single-segment lumbar disc herniation (LDH) gluteal pain and to determine whether it provides a better clinical outcome than open lumbar discectomy (OD).
Methods: A retrospective analysis of patients treated with modified PETD and OD for gluteal pain in LDH from January 2015 to December 2020 was conducted. Sample size was determined using a priori power analysis. Demographic information, surgical outcomes including procedure time (minutes), intraoperative blood loss (mL), hospital days, costs (RMB), fluoroscopy shots, recurrence and complications, etc., were recorded and analyzed. Prognostic outcomes were assessed using the visual analog scale (VAS), the Oswestry Disability Index (ODI), the Japanese Orthopedic Association Score (JOA) and modified MacNab criteria. The preoperative and postoperative VAS, ODI and JOA scores were recorded by two assistants. When the results were inconsistent, the scores were recorded again by the lead professor until all scores were consistently recorded in the data. MRI was used to assess radiological improvement and all patients received follow-ups for at least one year.
Results: The sample size required for the study was calculated by a priori analysis, and a total of 72 participants were required for the study to achieve 95% statistical test power. A total of 93 patients were included, 47 of whom underwent modified PETD, and 46 of whom underwent OD. In the modified PETD intragroup comparison, VAS scores ranged from 7.14 ± 0.89 preoperatively to 2.00 ± 0.58, 2.68 ± 0.70, 2.55 ± 0.69, 2.23 ± 0.81, and 1.85 ± 0.72 at 7 days, 1 month, 3 months, 6 months, and 12 months postoperatively. Patients showed significant pain relief postoperatively (P < 0.01). According to the modified MacNab score, the excellent rate in the PETD group was 89.36%. There was no significant difference compared to the OD group (89.13%, P > 0.05). Complication rates were lower (P > 0.05) but recurrence rates were higher (P > 0.05) in the modified PETD group than in the OD group. The modified PETD group had a faster operative time (P < 0.01), shorter hospital stay (P < 0.01), less intraoperative bleeding (P < 0.01), and less financial burden to the patient (P < 0.01) than the OD group. At 7 days postoperatively, the VAS score for low back pain was higher in the OD group than in the modified PETD group (P < 0.01). The VAS and JOA scores at 1, 3, 6, and 12 months postoperatively were not significantly different between the modified PETD and OD groups (P > 0.05), and the ODI was significantly different at 3 months postoperatively (P < 0.05).
Conclusion: Modified PETD treatment is safe and effective for gluteal pain due to L4/5 disc herniation and has the advantages of a lower complication rate, faster postoperative recovery, shorter length of stay, fewer anesthesia risks and lower cost of the procedure compared with OD. However, modified PETD has a higher recurrence rate.
Lumbar disc herniation (LDH) is one of the most common degenerative diseases of the lumbar spine, typically causing lower back pain and sciatica (1, 2). Gluteal pain has often been a clinical manifestation, and sometimes the only manifestation, of patients with LDH (3).
In a retrospective study reported by Fang et al. (3), 94.64% of patients with gluteal pain had responsible L4/5 segments (P < 0.001), and 5.36% had L5/S1. Wang et al. (4) subsequently described the mechanism of gluteal pain in LDH and suggested that it may be related to the superior and inferior gluteal nerves. All the fibers of the anterior branch of the L5 nerve root form the lumbosacral trunk, which forms part of the sacral plexus and branches distally into the superior gluteal nerve (L4, L5, S1) and the inferior gluteal nerve (L5, S1, S2), innervating the sensory muscles of the gluteal region, respectively (5, 6). In addition, compression of the posterior branch of the spinal nerve may contribute to gluteal pain, as the anterior and posterior roots merge at the intervertebral foramen to form the spinal nerve, which immediately divides into the posterior branch, creating a thicker nerve trunk that includes the superior cluneal nerves. Previous studies (7, 8) have shown that in addition to L1, L2, and L3, the posterior branches of the L4 and L5 spinal nerves are also involved in the formation of the superior cluneal nerves. Further autopsies have confirmed that approximately 10% of the superior cluneal nerves originate from L5 (9), a group of purely sensory nerve fibers controlling the gluteal region (10, 11). This reveals a strong correlation between gluteal pain and L4/5 disc herniation.
In terms of surgical treatment, OD remains the standard of care for pain secondary to LDH (12, 13), which is performed via a posterior approach, where the epidural space is exposed in the posterior midline by separating the paravertebral muscles as well as excising the lamina and ligamentum flavum. The herniated disc is removed after excision of a section of the facet joint on the symptomatic side while protecting the spinal cord and nerve roots (14). Although OD is effective, it can also cause considerable tissue damage (15).
With the development of minimally invasive methods, PETD is rapidly replacing OD in procedures requiring discectomy and decompression (16). Experienced surgeons can reach the lesion directly through Kambin’s triangle bypass (17). PETD avoids extensive damage to the skin, muscles, laminae, and synapses (18), and more significantly, excessive strain on the dural sac is avoided (19). Li et al. (14) also demonstrated that PETD achieved satisfactory results in the treatment of LDH with a reduced incidence of iatrogenic injury and minimal activity restrictions compared to OD, thus accelerating rapid recovery.
However, PETD focuses on the surgical approach and removal of the nucleus pulposus. The annulus fibrous and posterior longitudinal ligament, which may cause gluteal pain, are not treated or described in detail (20). Therefore, this study investigated a modified PETD that hypothesized that resection of the annulus fibrosus and posterior longitudinal ligament might significantly reduce pain in patients. The purpose of this study was to assess the safety and efficacy of a modified PETD compared with OD for treating L4/5 single-segment disc herniation.
The clinical study was approved by the Chinese Ethics Committee (No. 2021001). We recruited patients who underwent either the modified PETD technique or OD patients for LDH at our institution from January 2015 to November 2020 and were followed up for at least one year. Telephone follow-ups were carried out at each follow-up time, and basic information about all patients was reviewed. In addition, patients were invited to undergo reexamination to observe their most recent clinical and radiological results.
The inclusion criteria were adult patients with single-segment L4/5 disc herniation with only symptoms of gluteal pain. Patients chose to be treated with either a modified PETD technique or OD. The exclusion criteria were as follows: a previous history of lumbar operation; missed visits within one year or recurrence within the follow-up period; multisegment lumbar degenerative disease; and severe peripheral nerve disease (Figure 1). Recurrence was defined as a recurrence of the same level of disc herniation, and reoperation was performed.
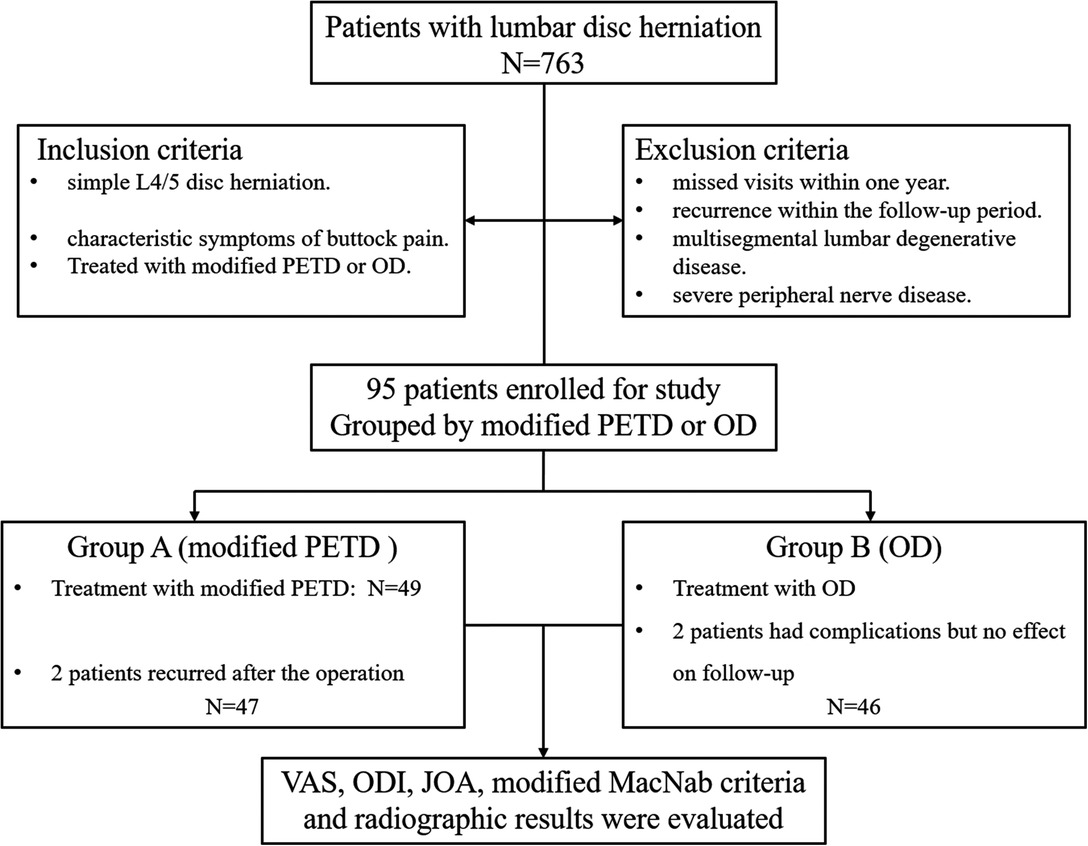
Figure 1. Flow chart of patient inclusion and stratification. Modified PETD, modified percutaneous endoscopic transforaminal discectomy; OD, open lumbar discectomy.
All patients underwent preoperative magnetic resonance imaging (MRI) of the lumbar spine, computed tomography (CT), and lumbar X-ray plain radiographs (anterior and lateral views). The same physician treated all patients. The modified PETD technique was performed using local anesthesia, and patients were informed of the potential for intraoperative discomfort and pain. A transilluminated surgical bed and C-arm were used for intraoperative positioning. Normal saline (3,000 mL) was used for continuous irrigation via the endoscope.
The routine procedure was as described in a previous study (21). In brief, the patient was operated on in a lateral position with the affected side facing upward and a soft cushion on the lumbar area. The skin entry point was above the iliac crest, 12–14 cm from the midline. After local anesthesia, the superior articular eminence of the external L5 was fixed under C-arm guidance and infiltrated locally with additional anesthetic. A guidewire was inserted through an 18-gauge needle, and an incision of approximately 0.7 cm was made at the edge of the guidewire. A stepwise dilating catheter was placed along the guidewire to bluntly separate the surrounding muscle tissue, place a working channel and connect to the endoscopic system. Physiological saline was continuously irrigated to ensure a clear view, and the protruding nucleus pulposus was removed using endoscopic forceps.
After visualization of the symptomatic lateral annulus fibrous in endoscopic view, denervation of the annulus fibrosus was performed starting from the posterior longitudinal ligament at the posterior edge of the vertebral body up to the pediculus arcus vertebrae, with emphasis on radiofrequency ablation of the ruptured end of the annulus fibrosus. The proliferating nerves and vessels were eliminated (Figure 2).
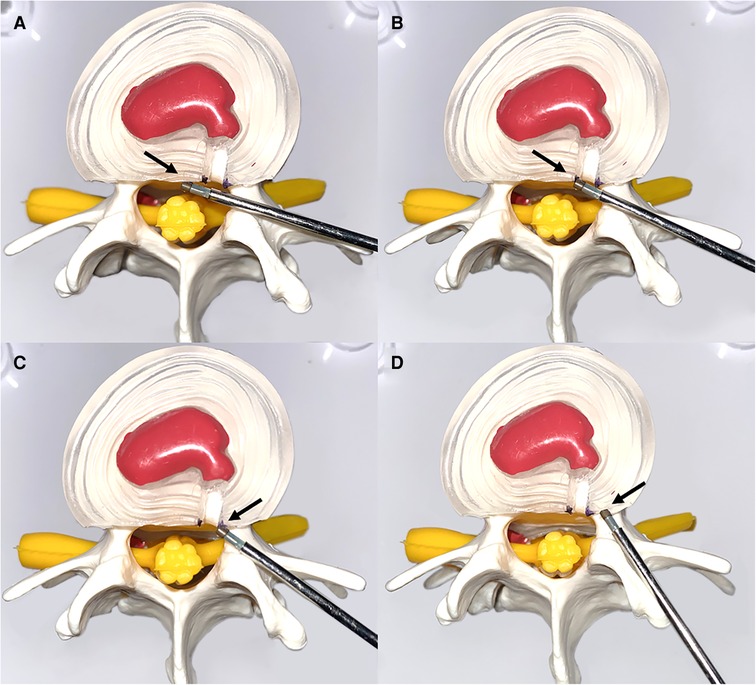
Figure 2. Schematic representation of the denervation of an annulus fibrosus. (A) Denervation from the posterior longitudinal ligament (black arrow). (B,C) Focused treatment of radiofrequency ablation of an annulus fibrosus dissection (black arrow). (D) Final denervation of the annulus fibrosus superior to the vertebral arch (black arrow).
After denervation, the hypertrophied annulus fibrous and posterior longitudinal ligament at the superior margin of the symptomatic inferior vertebral body was removed. The posterior longitudinal ligament was removed with endoscopic forceps (Figure 3A).
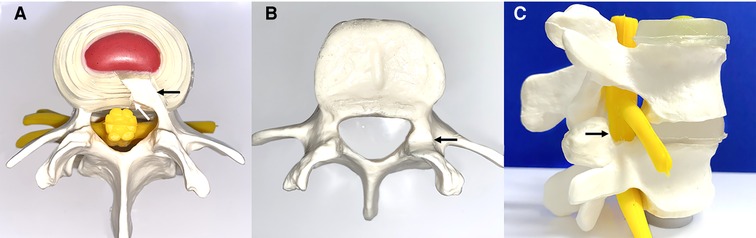
Figure 3. Schematic diagram of annulus fibrosus excision and lateral recess decompression. (A) Excision of the hypertrophic annulus fibrosus (black arrow) and posterior longitudinal ligament (white arrow). (B,C) Transverse and sagittal demonstration of lateral recess decompression with partial resection of the superior facet joint (black arrow).
An endoscopic circular saw and osteotome were used to remove the hyperplastic superior facet joint up to the superior edge of the vertebral arch. A portion of the ligamentum flavum was removed to completely decompress the “peripheral recess” (Figure 3B). The endoscopic view showed good nerve root pulsation and complete decompression. Fluid gelatin was injected before removing the working cannula to prevent hematoma, and finally, the wound was sutured. A preoperative and postoperative MRI comparison showed complete removal of the nucleus pulposus and decompression of the lateral saphenous fossa (Figure 4).
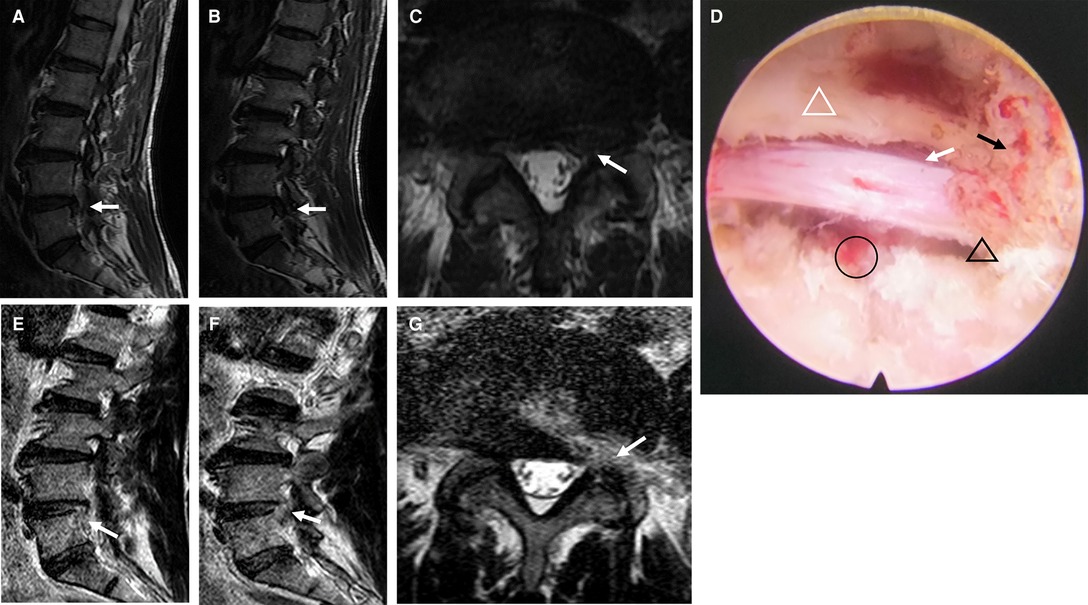
Figure 4. Pre- and postoperative images and intraoperative microscopic images of modified PETD. (A–C) Preoperative MRI demonstrated lumbar disc herniation (white arrows). (D) A completely decompressed nerve root is visible endoscopically (white arrow), with removal of the fibrous annulus (black circle), decompression of the lateral recess (black triangle), facet joint resection (black arrow), and partial ligamentum flavum resection (white triangle). After modified PETD, (E,F), the fibrous ring at the superior margin of the inferior conus was removed, and lateral saphenous fossa decompression was performed (G) (white arrow).
Demographic information included age, sex, body mass index (BMI), smoking habit, alcohol consumption, hypertension, diabetes, duration of symptoms, side of symptoms, and follow-up time. Surgical outcomes included the duration of surgery, intraoperative blood loss, length of hospital stay, cost of surgery, number of radiation sessions, recurrence, and complications. Recurrence was defined as a reherniation of the disc at the same segment and on the same side with a VAS score >4. The prognostic outcome was assessed by the outcome values and improvement rates of VAS, ODI, JOA, and the modified MacNab criteria, where the primary outcomes are the outcome values and the improvement rates of VAS. The improvement rates for VAS and ODI were calculated using the (preoperative-postoperative)/preoperative formula for the results and the (postoperative-preoperative)/(29-preoperation) formula for JOA. The VAS is a subjective numerical pain scale that assesses the gluteal pain experienced by the patient in the last 24 h. The ODI, JOA and modified MacNab criteria are used to measure the degree of disability and treatment for the life of patients with gluteal pain, reflecting the recovery of function and the ability of patients to manage daily life after surgery (22).
We used G-POWER Analysis (Version 3.1.9.7) (23, 24) to obtain the minimum sample size required to achieve a medium effect (effect size, d = 0.25), a power of 95%, and a statistical significance level of 0.05. To achieve statistical significance, we found that at least 72 samples were required. IBM SPSS Statistics (version 23.0) was used for data analysis. The data are expressed as the mean ± standard deviation (SD) and frequency (percentage). The two groups were compared using Pearson chi-square tests or Fisher exact tests for categorical variables and independent samples t tests or Mann–Whitney tests for continuous variables. Outcome values and improvement rates for VAS, ODI, JOA and excellent rates for modified MacNab criteria were compared between groups using multivariate analysis. Modified PETD intragroup comparisons were performed using two-way repeated-measures ANOVA. P values <0.05 were considered statistically significant. All graphs were constructed with GraphPad Prism (version 8.0.2).
The results of the a priori power analysis indicated that the study required at least 72 subjects. A total of 93 participants eventually met the inclusion criteria, of whom 49 opted for the modified PETD technique and 46 patients for OD. All participants had unilateral gluteal pain, and the type of LDH was paramedian. The mean follow-up times were 15.98 ± 4.23 and 16.11 ± 4.32 months for the modified PETD and OD groups, respectively. Demographic information, including age, sex, BMI smoking, alcohol, hypertension, diabetes, duration of symptoms, side of symptoms and follow-up time, were not significantly different between the two groups (P > 0.05). Compared to the OD group, the modified PETD group had a significantly shorter operative time (P < 0.01), less intraoperative bleeding (P < 0.01), and a shorter hospital stay (P < 0.01). In addition, the modified PETD technique imposed a smaller financial burden on the patient (P < 0.01). However, there were fewer fluoroscopic shots in the OD group (P < 0.01) (Table 1).
All patients were interviewed by telephone at 1 month, 3 months, 6 months, and 12 months after the operation. The results showed that the VAS score outcome values decreased from 7.14 preoperatively to 2.00, 2.68, 2.55, 2.23, and 1.85 at 7 days, 1 month, 3 months, 6 months and 12 months postoperatively in the modified PETD group, with significant differences at each follow-up time compared with preoperatively (P < 0.01). Comparing between groups, the VAS score outcome value of 1.61 for gluteal pain at 7 days postoperatively in the OD group was better than that of 2.00 in the modified PETD group (P < 0.05) (Figure 5A), but the VAS score outcome value of 1.53 for low back pain (caused by surgical incision) at 7 days postoperatively in the modified PETD group was less severe compared to 2.70 in the OD group (P < 0.05) (Figure 5B). The improvement rates of VAS scores in the modified PETD group were 61.77%, 63.41%, 67.85%, and 73.47% at 1, 3, 6, and 12 months postoperatively, respectively. There was no significant difference compared to the OD group (P > 0.05) (Figure 5C). Within-group comparisons of the modified PETD group, the preoperative and postoperative outcome values for ODI (Figure 6A) and JOA (Figure 6C) showed dramatic improvements in both symptoms and function (P < 0.05). Compared with the OD group, the improvement rate of the ODI was statistically significant (P < 0.05) at 3 months postoperatively (Figure 6B), and there was no statistically significant (P > 0.05) improvement of the JOA during the follow-up period (Figure 6D).
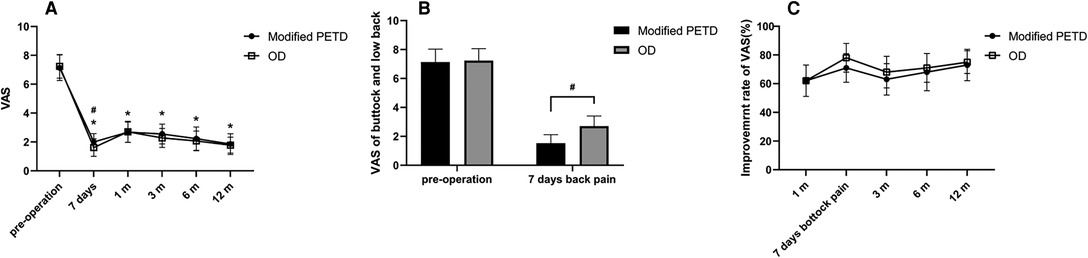
Figure 5. VAS score outcome values (A), VAS score outcome values for low back pain at 7 days postoperatively (B), and VAS score improvement rate (C). * indicates statistical significance compared within groups (P < 0.01), # indicates statistical significance compared between groups (P < 0.05).
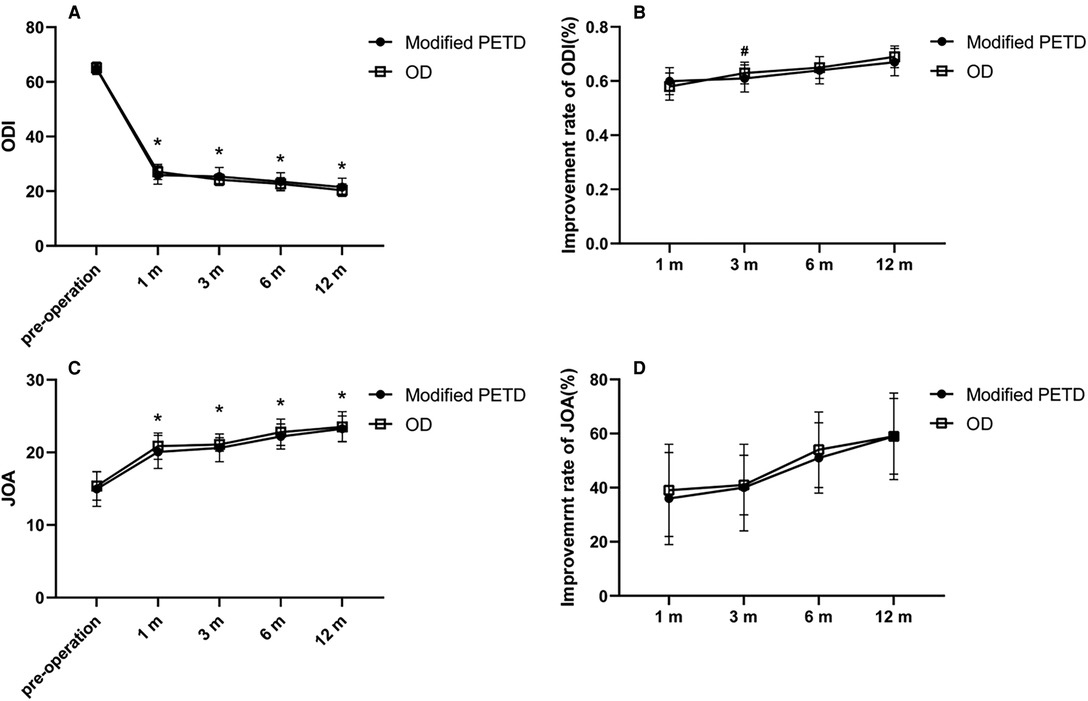
Figure 6. Outcome values (A) and improvement rates (B) for the ODI. Outcome values (C) and improvement rates (D) for the JOA. * indicates statistical significance compared within groups (P < 0.01). # indicates statistical significance compared between groups (P < 0.05).
According to the modified MacNab criteria, 33 (70.21%) and 9 patients (19.15%) in the modified PETD group were considered “excellent” and “good” at 12 months postoperatively (Figure 7A), respectively; similarly, 33 (71.74%) and 8 patients (17.39%) in the OD group were considered “excellent” and “good,”, respectively (Figure 7B). Comparisons between groups were not statistically significant (P > 0.05).
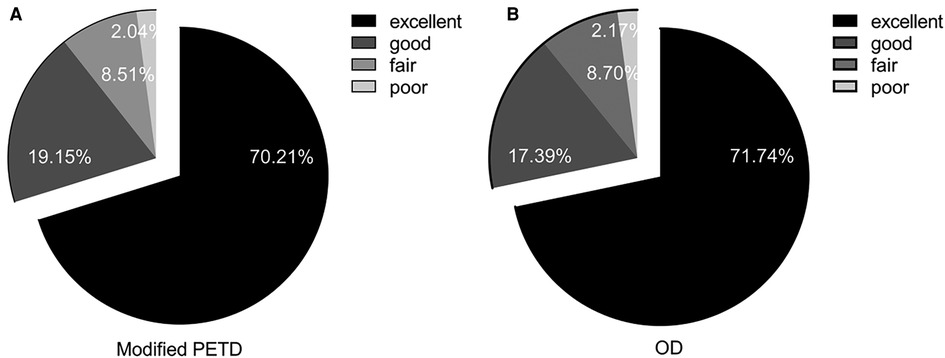
Figure 7. Modified MacNab scores for the modified PETD group (A) and for the OD group (B) at the postoperative follow-up at 12 months.
In the modified PETD group, two patients presented with recurrence at 15 days and 21 days postoperatively. We then treated them with OD, and the prognosis was favorable. Two patients in the OD group developed complications, a cerebrospinal fluid (CSF) leak and a hematoma. The former underwent intraoperative dural suturing and returned to the ward in a decubitus position for 12 h, where the headache caused by the CSF leak was relieved 5 days postoperatively. The latter presented with neurological compression due to a hematoma and recovered well after emergency debridement (Table 2).
With the development of endoscopic techniques, surgeons have become more experienced, and patients prefer minimally invasive surgery, resulting in the rapid development of minimally invasive procedures for the spine (25). PETD has become the most used minimally invasive technique in recent decades due to its small incision, minimal damage to muscle and soft tissue structures, and minimal postoperative epidural fibrosis (20, 26).
It is generally accepted that PETD appears to be indicated for all types of LDH (27, 28). However, an RCT by Chen et al. (29) showed that PETD is more suitable for treating paracentral herniations, where a transforaminal approach facilitates visualization of the lesion. For median-type herniations, the limitations of the intervertebral foramen and dura lead to poorer clinical outcomes. This provides theoretical support for our study. All patients with gluteal pain had unilateral nerve root compression in the present study. Furthermore, the absence of iliac crest obstruction at the L4/5 level makes PETD a great advantage in the treatment of gluteal pain. However, PETD focuses on the surgical approach and removal of the nucleus pulposus and does not address or describe the annulus fibrous and posterior longitudinal ligament (20).
During clinical procedures, we found that stimulation of the patient’s fibrous annulus and posterior longitudinal ligament induced symptoms of gluteal pain. The patient showed considerable relief after denervation and removal of the fibrous annulus and posterior longitudinal ligament on the symptomatic side. Li et al. (30) showed that the sinus vertebral nerve (SVN) was divided into two types, the SVN deputy branch (type I) and the SVN main trunk (type II), with the SVN deputy branch entering the posterior lateral border of the disc and the SVN main trunk originating from the spinal ganglion and connecting to the sympathetic nerve via a traffic branch. Seventy (22.44%) SVN deputy branches and 23 (21.74%) SVN main trunks were found in the L4/5 intervertebral foramen. According to R et al. (31), part of the ascending branch of the SVN originates in the posterior longitudinal ligament, and microscopic observation of the sensory fibers of the posterior longitudinal ligament revealed that it receives a large number of traffic fibers of the SVN and forms a fiber network (32). When LDH is present, the production of inflammatory mediators leads to the transmission of inflammatory cytokines that hypersensitize SVN terminal receptors (33), which reduces the pain threshold and triggers buttock pain (34). Therefore, we hypothesized that gluteal pain might be associated with both and made improvements to the original.
The modified PETD is safe and effective for treating gluteal pain caused by L4/5 disc herniation. The results showed that patients treated with the modified PETD showed a significant improvement postoperatively compared to preoperatively (P < 0.01). According to the modified MacNab score, 89.36% of patients were satisfied with the outcome 12 months after the procedure. There were no statistically significant differences in the VAS and JOA assessments of patients at 1, 3, 6, and 12 months after the modified PETD compared to those of the OD group (P > 0.05). The ODI was statistically significant only at 3 months postoperatively (P < 0.05), which we believe may be related to the subjective nature of the rating scale. In addition, there were no complications after treatment in the modified PETD group, although two patients experienced recurrence (4%), which we speculate may be related to the removal of the annulus fibrous tissue. Further studies are needed to determine whether the removal of the nucleus pulposus should be expanded. In summary, the modified PETD provides direct access to the lesion, relieves compression, and provides effective radiofrequency ablation of the SVN on the annulus fibrosus and posterior longitudinal ligament, relieving the patient’s symptoms. In addition, decompression of the lateral recess can also be accomplished with good results with modified PETD using a circular saw and a high-speed drill (35). The potential of the modified PETD for the treatment of LDH for gluteal pain was revealed.
For patients with LDH with severe ossification or severe lumbar spinal stenosis, OD is an excellent treatment option. Complete extraction of the nucleus pulposus considerably reduces the possibility of recurrence. Furthermore, the adverse effects of recurrence should also be considered. A study by K et al. (36) showed that the reoperation rate of minimally invasive surgery patients was 3.1% higher than that of open surgery patients and that reoperation not only has negative psychological and physical impacts on the patients but also increases their financial burden. Nevertheless, due to the greater invasiveness, the patient has a longer recovery time and must endure the pain of a large incision (37), which can fail to heal in some diabetic patients. In addition, fixation of the nail bar system accelerates the degeneration of adjacent segments (38, 39). More importantly, when open surgery is performed, in addition to the removal of the lamina, the medial articular processes may be removed, and the surrounding ligament system and muscles may be destroyed. Extensive disruption of the posterior column may increase the risk of lumbar kyphosis (40) and lumbar spondylolisthesis (41). PETD avoids damage to the vertebral plates and spinous processes and greatly reduces the incidence of retroflection deformities (42). G et al. (43) showed no difference between PETD and OD for medium- to long-term pain and functional status. This is consistent with the results of our study. Similarly, this suggests that the two surgical strategies have the same efficacy. In addition, the modified PETD group had less postoperative low back pain (P < 0.01) and fewer complications than the OD group. For elderly patients with comorbidities, we should avoid the risks associated with general anesthesia and opt for safer local anesthesia (44). More importantly, for single-segment LDH, the modified PETD procedure appears to offer more benefit to patients than OD.
Buttock pain is often not a typical symptom of LDH. In clinical practice, it is often difficult for physicians to connect them, resulting in misdiagnosis and a delay in optimal treatment. Conditions that can cause buttock pain include deep gluteal syndrome and pain caused by the facet joint or sacroiliac joint (4, 45, 46). Deep gluteal syndromes are sciatica of nondiscogenic origin (47), including piriformis syndrome, gemelli-obturator internus syndrome, and ischiofemoral impingement syndrome (48). According to the literature by H et al. (49), the most common clinical feature of deep gluteal syndrome is pain in the buttocks, which is aggravated by prolonged sitting. In some patients, the straight leg raising test may be positive. These symptoms can be easily confused with the symptoms of buttock pain caused by LDH. Therefore, an accurate diagnosis of the disease before the operation is essential. In addition to a careful physical examination and empirical diagnosis, the surgeon should determine whether the patient’s symptoms are related to the lumbar spine by visualizing the MRI with the support of a radiological examination.
While this was a good retrospective study, there are still some limitations of which to be aware. A significant limitation is the retrospective nature of the study. The decision on surgical strategy was based on patient preferences. Second, the study population included only 93 patients from one hospital, which may have biased the results to some extent. Another is that this study reviewed patients with single-segment L4/5 disc herniations, and further research is still needed to determine the applicability of the modified PETD technique in patients with other segmental disc herniations. Third, further research is still required to explore the necessity of extended disc removal and preventing postoperative recurrence. Finally, when calculating the cost, we only counted the cost of minimally invasive surgery and not the cost of other procedures due to recurrence, which may lead to bias.
The symptoms of gluteal pain due to L4/5 disc herniation should be highlighted in clinical practice. Modified PETD treatment is safe and effective and has the advantages of a lower complication rate, faster postoperative recovery, shorter length of stay, fewer anesthesia risks and lower cost of the procedure compared with OD. However, modified PETD has a higher recurrence rate, and reoperation caused by recurrence may increase the financial burden.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the second hospital of Jilin university. The patients/participants provided their written informed consent to participate in this study.
JYA, JPW, TH, ZHY, RL, WX and LQ collected data. JYA prepared the manuscript with important intellectual input from all authors. JZ and TY verified the analytical methods and performed statistical analysis. QYL and YZL performed a final review of the manuscript. All authors approved the final manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Natural Science Foundation of the Science and Technology Department of Jilin Province (20200201536JC), the Department of Science and Technology of Jilin Province (20210101265JC), and the Department of Finance of Jilin Province (ZXWSTZXEY041).
Thanks to Elsevier for the language editing service.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Stevens S, Agten A, Timmermans A, Vandenabeele F. Unilateral changes of the multifidus in persons with lumbar disc herniation: a systematic review and meta-analysis. Spine J. (2020) 20(10):1573–85. doi: 10.1016/j.spinee.2020.04.007
2. Awad JN, Moskovich R. Lumbar disc herniations: surgical versus nonsurgical treatment. Clin Orthop Relat Res. (2006) 443:183–97. doi: 10.1097/01.blo.0000198724.54891.3a
3. Fang G, Zhou J, Liu Y, Sang H, Xu X, Ding Z. Which level is responsible for gluteal pain in lumbar disc hernia? BMC Musculoskelet Disord. (2016) 17(1):356. doi: 10.1186/s12891-016-1204-7
4. Wang Y, Yang J, Yan Y, Zhang L, Guo C, Peng Z, et al. Possible pathogenic mechanism of gluteal pain in lumbar disc hernia. BMC Musculoskelet Disord. (2018) 19(1):214. doi: 10.1186/s12891-018-2147-y
5. Ling ZX, Kumar VP. The course of the inferior gluteal nerve in the posterior approach to the hip. J Bone Joint Surg Br. (2006) 88(12):1580–3. doi: 10.1302/0301-620X.88B12.18182
6. Akita K, Sakamoto H, Sato T. Origin, course and distribution of the superior gluteal nerve. Acta Anat (Basel). (1994) 149(3):225–30. doi: 10.1159/000147581
7. Konno T, Aota Y, Kuniya H, Saito T, Qu N, Hayashi S, et al. Anatomical etiology of “Pseudo-Sciatica” from superior cluneal nerve entrapment: a laboratory investigation. J Pain Res. (2017) 10:2539–45. doi: 10.2147/JPR.S142115
8. Akbas M, Yegin A, Karsli B. Superior cluneal nerve entrapment eight years after decubitus surgery. Pain Pract. (2005) 5(4):364–6. doi: 10.1111/j.1533-2500.2005.00040.x
9. Iwanaga J, Simonds E, Schumacher M, Oskouian RJ, Tubbs RS. Anatomic study of superior cluneal nerves: revisiting the contribution of lumbar spinal nerves. World Neurosurg. (2019) 128:e12–5. doi: 10.1016/j.wneu.2019.02.159
10. Kikuta S, Iwanaga J, Watanabe K, Tubbs RS. Revisiting the middle cluneal nerves: an anatomic study with application to pain syndromes and invasive procedures around the sacrum. World Neurosurg. (2019) 127:e1228–31. doi: 10.1016/j.wneu.2019.04.109
11. Tubbs RS, Levin MR, Loukas M, Potts EA, Cohen-Gadol AA. Anatomy and landmarks for the superior and middle cluneal nerves: application to posterior iliac crest harvest and entrapment syndromes. J Neurosurg Spine. (2010) 13(3):356–9. doi: 10.3171/2010.3.SPINE09747
12. Kim M, Lee S, Kim HS, Park S, Shim SY, Lim DJ. A comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for lumbar disc herniation in the Korean: a meta-analysis. Biomed Res Int. (2018) 2018:9073460. doi: 10.1155/2018/9073460
13. Ong D, Chua NH, Vissers K. Percutaneous disc decompression for lumbar radicular pain: a review article. Pain Pract. (2016) 16(1):111–26. doi: 10.1111/papr.12250
14. Li Z, Zhang C, Chen W, Li S, Yu B, Zhao H, et al. Percutaneous endoscopic transforaminal discectomy versus conventional open lumbar discectomy for upper lumbar disc herniation: a comparative cohort study. Biomed Res Int. (2020) 2020:1852070. doi: 10.1155/2020/1852070
15. Zhang Y, Pan Z, Yu Y, Zhang D, Ha Y, Yi S, et al. The modified transforaminal endoscopic technique in treating intracanalicular combining foraminal and/or extraforaminal lumbar disc herniations. Quant Imaging Med Surg. (2018) 8(9):936–45. doi: 10.21037/qims.2018.10.02
16. Singh V, Derby R. Percutaneous lumbar disc decompression. Pain Physician. (2006) 9(2):139–46. https://pubmed.ncbi.nlm.nih.gov/16703975/16703975
17. Xu Z, Lin G, Zhang H, Xu S, Zhang M. Three-dimensional architecture of the neurovascular and adipose zones of the upper and lower lumbar intervertebral foramina: an epoxy sheet plastination study. J Neurosurg Spine. (2020) 32(5):722–32. doi: 10.3171/2019.10.Spine191164
18. Ahn Y, Lee SG, Son S, Keum HJ. Transforaminal endoscopic lumbar discectomy versus open lumbar microdiscectomy: a comparative cohort study with a 5-year follow-up. Pain Physician. (2019) 22(3):295–304. https://pubmed.ncbi.nlm.nih.gov/31151337/31151337
19. Shin MH, Bae JS, Cho HL, Jang IT. Extradiscal epiduroscopic percutaneous endoscopic discectomy for upper lumbar disc herniation a technical note. Clin Spine Surg. (2019) 32(3):98–103. doi: 10.1097/bsd.0000000000000755
20. Kanno H, Aizawa T, Hahimoto K, Itoi E. Minimally invasive discectomy for lumbar disc herniation: current concepts, surgical techniques, and outcomes. Int Orthop. (2019) 43(4):917–22. doi: 10.1007/s00264-018-4256-5
21. Pan Z, Ha Y, Yi S, Cao K. Efficacy of transforaminal endoscopic spine system (Tessys) technique in treating lumbar disc herniation. Med Sci Monit. (2016) 22:530–9. doi: 10.12659/msm.894870
22. Wu J, Du Z, Lv Y, Zhang J, Xiong W, Wang R, et al. A new technique for the treatment of lumbar facet joint syndrome using intra-articular injection with autologous platelet rich plasma. Pain Physician. (2016) 19(8):617–25. https://pubmed.ncbi.nlm.nih.gov/27906940/27906940
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39(2):175–91. doi: 10.3758/bf03193146
24. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41(4):1149–60. doi: 10.3758/brm.41.4.1149
25. Telfeian AE, Veeravagu A, Oyelese AA, Gokaslan ZL. A brief history of endoscopic spine surgery. Neurosurg Focus. (2016) 40(2):E2. doi: 10.3171/2015.11.FOCUS15429
26. Vaishnav AS, Othman YA, Virk SS, Gang CH, Qureshi SA. Current state of minimally invasive spine surgery. J Spine Surg. (2019) 5(Suppl 1):S2–S10. doi: 10.21037/jss.2019.05.02
27. Hu QF, Pan H, Fang YY, Jia GY. Percutaneous endoscopic lumbar discectomy for high-grade down-migrated disc using a trans-facet process and pedicle-complex approach: a technical case series. Eur Spine J. (2018) 27(Suppl 3):393–402. doi: 10.1007/s00586-017-5365-3
28. Kim HS, Paudel B, Jang JS, Lee K, Oh SH, Jang IT. Percutaneous endoscopic lumbar discectomy for all types of lumbar disc herniations (Ldh) including severely difficult and extremely difficult Ldh cases. Pain Physician. (2018) 21(4):E401–8. https://pubmed.ncbi.nlm.nih.gov/30045606/30045606
29. Chen Z, Zhang L, Dong J, Xie P, Liu B, Wang Q, et al. Percutaneous transforaminal endoscopic discectomy versus microendoscopic discectomy for lumbar disc herniation: two-year results of a randomized controlled trial. Spine (Phila Pa 1976). (2020) 45(8):493–503. doi: 10.1097/BRS.0000000000003314
30. Zhao Q, Cheng L, Yan H, Deng S, Zhao J, Liu Z, et al. The anatomical study and clinical significance of the sinuvertebral nerves at the lumbar levels. Spine (Phila Pa 1976). (2020) 45(2):E61–6. doi: 10.1097/BRS.0000000000003190
31. Raoul S, Faure A, Robert R, Rogez JM, Hamel O, Cuillere P, et al. Role of the sinu-vertebral nerve in low back pain and anatomical basis of therapeutic implications. Surg Radiol Anat. (2003) 24(6):366–71. doi: 10.1007/s00276-002-0084-8
32. Imai S, Konttinen YT, Tokunaga Y, Maeda T, Hukuda S, Santavirta S. Tyrosine hydroxylase-immunoreactive nerve fibres in rat posterior longitudinal ligament. J Auton Nerv Syst. (1997) 63(1-2):51–60. doi: 10.1016/s0165-1838(96)00130-0
33. Amin RM, Andrade NS, Neuman BJ. Lumbar disc herniation. Curr Rev Musculoskelet Med. (2017) 10(4):507–16. doi: 10.1007/s12178-017-9441-4
34. McNally DS, Naish C, Halliwell M. Intervertebral disc structure: observation by a novel use of ultrasound imaging. Ultrasound Med Biol. (2000) 26(5):751–8. doi: 10.1016/s0301-5629(00)00209-x
35. Li Y, Wang B, Wang S, Li P, Jiang B. Full-endoscopic decompression for lumbar lateral recess stenosis via an interlaminar approach versus a transforaminal approach. World Neurosurg. (2019) 128:e632–8. doi: 10.1016/j.wneu.2019.04.221
36. Kang TW, Park SY, Oh H, Lee SH, Park JH, Suh SW. Risk of reoperation and infection after percutaneous endoscopic lumbar discectomy and open lumbar discectomy : a nationwide population-based study. Bone Joint J. (2021) 103-b(8):1392–9. doi: 10.1302/0301-620x.103b8.Bjj-2020-2541.R2
37. Shi R, Wang F, Hong X, Wang YT, Bao JP, Liu L, et al. Comparison of percutaneous endoscopic lumbar discectomy versus microendoscopic discectomy for the treatment of lumbar disc herniation: a meta-analysis. Int Orthop. (2019) 43(4):923–37. doi: 10.1007/s00264-018-4253-8
38. Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). (2004) 29(7):726–33; discussion 33–4. doi: 10.1097/01.brs.0000119398.22620.92
39. Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). (1988) 13(3):375–7. doi: 10.1097/00007632-198803000-00029
40. McGirt MJ, Garcés-Ambrossi GL, Parker SL, Sciubba DM, Bydon A, Wolinksy JP, et al. Short-term progressive spinal deformity following laminoplasty versus laminectomy for resection of intradural spinal tumors: analysis of 238 patients. Neurosurgery. (2010) 66(5):1005–12. doi: 10.1227/01.Neu.0000367721.73220.C9
41. Yun DJ, Park SJ, Lee SH. Open lumbar microdiscectomy and posterolateral endoscopic lumbar discectomy for antero- and retrospondylolisthesis. Pain Physician. (2020) 23(4):393–404. https://pubmed.ncbi.nlm.nih.gov/32709174/32709174
42. Bai J, Zhang W, Liu X, Cheng J, Wang X, Ding W, et al. Percutaneous transforaminal endoscopic discectomy in the treatment of senior patients with lumbar degenerative disc disease. Exp Ther Med. (2019) 17(1):874–82. doi: 10.3892/etm.2018.6996
43. Gadjradj PS, Harhangi BS, Amelink J, van Susante J, Kamper S, van Tulder M, et al. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: a systematic review and meta-analysis. Spine (Phila Pa 1976). (2021) 46(8):538–49. doi: 10.1097/BRS.0000000000003843
44. Shin SH, Bae JS, Lee SH, Keum HJ, Kim HJ, Jang WS. Transforaminal endoscopic decompression for lumbar spinal stenosis: a novel surgical technique and clinical outcomes. World Neurosurg. (2018) 114:e873–82. doi: 10.1016/j.wneu.2018.03.107
45. Kurosawa D, Murakami E, Aizawa T. Referred pain location depends on the affected section of the sacroiliac joint. Eur Spine J. (2015) 24(3):521–7. doi: 10.1007/s00586-014-3604-4
46. Bartynski WS. Clinical, anatomic, and imaging correlation in spine-related pain: the essential elements. Tech Vasc Interv Radiol. (2009) 12(1):2–10. doi: 10.1053/j.tvir.2009.06.002
47. Kizaki K, Uchida S, Shanmugaraj A, Aquino CC, Duong A, Simunovic N, et al. Deep gluteal syndrome is defined as a non-discogenic sciatic nerve disorder with entrapment in the deep gluteal space: a systematic review. Knee Surg Sports Traumatol Arthrosc. (2020) 28(10):3354–64. doi: 10.1007/s00167-020-05966-x
48. Park JW, Lee YK, Lee YJ, Shin S, Kang Y, Koo KH. Deep gluteal syndrome as a cause of posterior hip pain and sciatica-like pain. Bone Joint J. (2020) 102-B(5):556–67. doi: 10.1302/0301-620x.102b5.Bjj-2019-1212.R1
Keywords: lumbar disc herniation, gluteal pain, percutaneous endoscopic transforaminal discectomy, open lumbar discectomy, minimally invasive surgery
Citation: An J, Zhang J, Yu T, Wu J, Nie X, He T, Yun Z, Liu R, Xue W, Qi L, Li Y and Liu Q (2022) A Retrospective Comparative Study of Modified Percutaneous Endoscopic Transforaminal Discectomy and Open Lumbar Discectomy for Gluteal Pain Caused by Lumbar Disc Herniation. Front. Surg. 9:930036. doi: 10.3389/fsurg.2022.930036
Received: 27 April 2022; Accepted: 1 June 2022;
Published: 22 June 2022.
Edited by:
Luca Ambrosio, Campus Bio-Medico University, ItalyReviewed by:
Sergio De Salvatore, Policlinico Universitario Campus Bio-Medico, ItalyCopyright © 2022 An, Zhang, Yu, Wu, Nie, He, Yun, Liu, Xue, Qi, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinyi Liu cWlueWlAamx1LmVkdS5jbg== Yingzhi Li ZGx5ejIwMDVAMTYzLmNvbQ==
Specialty section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.