
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 25 August 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.929576
 Yan Yang1,2
Yan Yang1,2 Yawei Wang2,3
Yawei Wang2,3 Zhengbin Wang4*
Zhengbin Wang4*
Aim: This study aims to construct a new staging system for colorectal cancer (CRC) based on the lymph node ratio (LNR) as a supplement to the American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system for predicting the prognosis of CRC patients with <12 lymph nodes.
Methods: The data of 26,695 CRC patients with <12 lymph nodes were extracted from the Surveillance, Epidemiology, and End Results (SEER) database as a training set. A total of 635 CRC patients were also enrolled from Northern Jiangsu People's Hospital affiliated with Yangzhou University as an independent validation set. Classification and regression tree analysis was used to obtain the LNR cutoff value. Survival curves were estimated using the Kaplan–Meier method, and the log-rank test was used for comparisons of differences among the survival curves. The monotonic decreasing trend of the overall survival curve in the staging system was expressed by the linear correlation degree R.
Results: The 5-year survival rates of patients in the training set based on the AJCC staging system from stage I to stage IV were 75.6% (95%CI: 74.4–76.8), 59.8% (95%CI: 58.6–61.0), 42.1% (95%CI: 34.5–49.7), 33.2% (95%CI: 24.6–41.8), 72.0% (95%CI: 69.1–74.9), 48.8% (95%CI: 47.4–50.2), 26.5% (95%CI: 23.0–30.0), and 11.3% (95%CI: 10.3–12.3). The 5-year survival rates of patients in the training set from stage I to stage IIIC were 80.4%, 72.9%, 59.8%, 48.4%, 32.5%, and 15.0%, according to the TNM + LNR (TNRM) staging system. According to the AJCC staging system, the 5-year survival rates of patients in the validation set from stage I to stage IIIC were 91.3%, 90.8%, 72.6%, 61.3%, 72.4%, 58.1%, and 32.8%. Based on the TNRM staging system, the 5-year survival rates of patients in the validation set from stage I to stage IIIC were 99.2%, 90.5%, 81.4%, 78.6%, 60.2%, and 35.8%.
Conclusion: The TNRM staging system successfully eliminated “survival paradox” in the AJCC staging system, which might be superior to the AJCC staging system.
Colorectal cancer (CRC) is the third most common malignancy, with the third highest mortality rate all over the world (1). The global burden of CRC has been estimated to increase by 60%, with more than 2.2 million new cases and 1.1 million cancer deaths by 2030 (2). Although the diagnosis and treatment of CRC have improved in the previous years, the 5-year survival rate for CRC was only 50% (3). Currently, the American Joint Committee on Cancer (AJCC) 8th version tumor node metastasis (TNM) staging system is the most widely used tool for predicting the survival of CRC patients (4). In general, higher-stage malignant tumors were associated with a lower survival rate (5). It is strange that a survival contradiction between stage IIIA and stage II of CRC existed in the current TNM staging system, which refers to the “survival paradox” (6, 7). Edge and Compton reported that the 5-year survival rate of CRC patients at stage IIB/C was about 46%–61%, which was lower than that of patients at stage IIIA (70%) (5). Several previous studies have shown that the prognosis of CRC patients at stage IIIA was better than patients at stages IIB and IIC (8, 9). Additionally, the existing AJCC N staging system requires at least 12 lymph nodes to be excised and examined histopathologically to ensure a reliable result (10). However, the total number of retrieved lymph nodes for CRC patients in actual clinical practice is often <12 (11). Constructing a new clinical staging system for predicting the prognosis of CRC patients with insufficient lymph nodes is of great value.
Previously, researchers have established several new staging systems combining the AJCC staging system with other biomarkers to evaluate the prognosis of CRC patients. Liu et al. combined carcinoembryonic antigen (CEA) levels with the AJCC staging system to assess the prognosis of CRC patients (12). Another study modified the pathological N stage of the AJCC, including the status of tumor deposits, to evaluate the prognosis and survival of CRC patients, especially those with positive regional lymph nodes (13). In a study by Liu et al., the prognostic score was supplemented with the AJCC TNM staging system to improve the prognostic accuracy and clinical management of colon cancer (14). These studies aimed to measure the prognosis of all CRC patients despite the number of retrieved lymph nodes. The clinical staging system for improving the prognostic accuracy of CRC patients with insufficient lymph nodes was still lacking.
Lymph node ratio (LNR), calculated as the ratio of the number of metastatic lymph nodes to the total number of lymph nodes examined, is now being proposed to address problems related to staging deviation caused by the lack of the total number of lymph nodes (15). LNR is reported to be an essential prognosis indicator of CRC patients (16). Previous studies also indicated that LNR has an advantage over the AJCC N staging system in predicting the prognosis of CRC patients with inadequate retrieved lymph nodes (17, 18). In light of these considerations, based on the data from the Surveillance, Epidemiology, and End Results (SEER) database, our study aimed to construct a new clinical staging system including LNR to supplement the existing AJCC TNM staging system (TNRM staging system) in predicting the prognosis of CRC patients with limited lymph nodes. We also used the data of CRC patients from our hospital to validate the efficiency of the TNRM staging system for predicting the prognosis of CRC patients with limited lymph nodes.
This study extracted the data of 135,980 CRC patients between 2004 and 2016 with <12 lymph nodes from the SEER database [SEER 18 Regs Custom Data (with additional treatment field), Nov 2018 Sub (1973–2016 varying)]. SEER is a representative program that collects demographic, clinical, and outcome information about all cancers, which covers 18 geographically diverse populations with 30% of the US population (19). The diagnosis of CRC was based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), histology codes 8020/3, 8032/3, 8070/3, 8140/3, 8201/3, 8213/3, 8480/3, 8490/3, 8510/3, 8560/3, “Site recode ICD-O-3/WHO 2008.” After excluding patients who had nonprimary tumors and participants with unclear pathological diagnosis, invalid follow-up, no surgery, appendiceal tumor and unclear tumor location, a history of radiotherapy before and/or after operation, unclear pathological grading, unclear tumor size, unclear number of positive lymph nodes, and unclear tumor (T) and metastasis (M) stages of the AJCC 8th version, a total of 26,695 patients were eventually included in our study.
The data of 635 CRC patients were also collected from the Gastrointestinal Center of Northern Jiangsu People's Hospital affiliated with Yangzhou University between 2012 and 2016 as an independent validation set. The study was approved by the Ethics Committee of the Northern Jiangsu People's Hospital affiliated with Yangzhou University (2019081). All patients were identified by pathological diagnosis, and patients with missing data on T, node (N), or M staging or survival were excluded. The detailed screening process of all participants from the SEER database and our own cases is shown in Figure 1.
The clinical data of 26,695 CRC patients from the SEER database and 635 CRC patients from Northern Jiangsu People's Hospital affiliated with Yangzhou University were collected, including the mean age at diagnosis (≥60 or <60 years), marital status (married or other), sex, race (White or not White), tumor site (colon or rectum), pathological grade (I/II or III/IV), tumor size (≤5 or >5 cm), AJCC T stage (T1, T2, T3, T4a, or T4b), AJCC N stage (N0, N1a, N1b/c, N2a, or N2b), AJCC M stage (M0 or M1), CEA (elevated or normal/unknown), perineural invasion (positive or negative/unknown), and chemotherapy (yes or no/unknown).
In terms of the comparison of the AJCC 6th and 7th versions, the main substaging remained unchanged in the 7th version. Some N2 diseases were reclassified from stage IIIC to stage IIIA/IIIB, and stage IIB (T4N0) disease was further subdivided into stages IIB (T4aN0) and IIC (T4bN0). Subdivision of N stages was made in the 7th version of AJCC, including N1 into N1a and N1b, N2 into N2a, N2b, and a new definition of N1c for tumor deposits. M stage was changed, including M1a for single-site metastasis and M1b for multiple-site or peritoneal metastasis.
The AJCC 8th version added stage IVC, which referred to patients at a stage involving peritoneal metastasis with or without metastasis of other organs. The 8th version of AJCC continues to use vascular lymphatic vessel infiltration and tumor deposition as prognostic predictors, while microsatellite instability status and BRAF status are applied to evaluate prognostic risk, and BRAF, KRAS, and the degeneration of the NRAS were utilized as efficacy predictors.
M1 patients with long-term metastasis (n = 4,694) were grouped into IV stage in accordance with the AJCC staging system as those patients could be at any N stage and thus were excluded from the LNR grouping. LNR grouping was performed based on the data of 22,001 patients with no distant metastasis. Patients with metastatic lymph nodes and no long-term metastasis (n = 7,404) in the training set were grouped according to the cutoff value of classification and regression tree analysis (17). Patients with metastatic lymph nodes and no long-term metastasis were included in the classified regression tree analysis to identify the optimal LNR cutoff value: LNR1: 0 < LNR ≤ 0.12; LNR2: 0.12 < LNR ≤ 0.35; LNR3: 0.35 < LNR ≤ 0.71; LNR4: 0.71 < LNR ≤ 1 (Figure 2).
Lymph node-negative patients with ≥6 lymph nodes were classified as LNR0, and the 5-year survival rate of these patients (n = 10,674) was significantly better than that of lymph node-negative patients with ≤5 lymph nodes (n = 3,747) (67.2% vs. 62.4%, P < 0.001). The 5-year survival rate of lymph node-negative patients with ≤5 lymph nodes was not statistically different from LNR1 patients with positive lymph nodes (62.4% vs. 59.9%, P = 0.203), and these two groups were combined into LNR1. The 5-year survival rates of patients at the N1c stage (n = 176) with tumor deposits were not statistically different with lymph node-positive LNR3 (48.0% vs. 43.7%, P = 0.712), and the two groups were merged into LNR3.
The data of 135,980 CRC patients from the SEER database were regarded as the training set for constructing the TNRM staging system. LNR grouping was conducted based on the cutoff value of LNR. Univariate and multivariate Cox regression analyses were applied to identify factors associated with the prognosis of CRC. A total of 635 cases of CRC from the Gastrointestinal Center of Northern Jiangsu People's Hospital affiliated with Yangzhou University were applied for external validation of the efficacy of the TNRM staging system.
Survival curves were estimated using the Kaplan–Meier method, and the log-rank test was used for comparisons of differences among the survival curves. Factors associated with the prognosis of CRC patients were investigated using univariate and multivariate Cox regression analyses. The monotonic decreasing trend of the overall survival (OS) curve in the staging system was expressed by the linear correlation degree R to assess and compare the prediction ability of the TNRM staging system for prognosis of CRC patients. R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria), r-part, and map-tree software packages were applied to construct the model. SPSS version 24.0 was employed for data analyses, and the entire test was two-sided. P < 0.05 was considered statistically significant.
In total, 135,980 CRC patients with <12 lymph nodes from the SEER database were included in the current study. Among them, patients who had nonprimary tumor as well as those with unclear pathological diagnoses, invalid follow-up, and no surgery were excluded (n = 85,104). Patients with appendiceal tumor and unclear tumor location and history of radiotherapy before and/or after operation were also excluded (n = 13,039). After excluding patients with unclear pathological grading, unclear tumor size, and unclear number of positive lymph nodes (n = 6,300) and patients with unclear T and M stages (n = 4,842), 26,695 patients were finally included in our study as the training set. A total of 635 CRC patients were recruited from Northern Jiangsu People's Hospital affiliated with Yangzhou University between 2012 and 2016 and included as the validation set.
In the cohort of patients with <12 lymph nodes from the SEER database, 20,350 (76.2%) patients were ≥60 years. A total of 22,355 (83.7%) people with primary tumors in the colon, and 4,340 (16.3%) patients had tumors located in the rectum. A total of 20,520 (76.9%) patients were associated with tumors ≤5 cm. A total of 21,907 (82.1%) patients were at pathological grade I/II. A total of 17,313 (64.9%) patients were classified as postoperative stage T3, and 7,744 (29.0%) patients received chemotherapy. As for patients from Northern Jiangsu People's Hospital affiliated with Yangzhou University, 461 (72.6%) were ≥60 years. A total of 390 (61.4%) patients were male. There were 246 (38.7%) patients with the tumor site in the colon, and 389 (61.3%) patients had tumors located in the rectum. A total of 382 (60.2%) patients were at pathological grade I/II. There were 479 (75.4%) patients with tumor size >5 cm. A total of 311 (49.0%) patients received chemotherapy (Table 1).
As exhibited in Table 2, the 5-year survival rates in patients were 75.6% (95%CI: 74.4–76.8) at stage I, 59.8% (95%CI: 58.6–61.0) at stage IIA, 42.1% (95%CI: 34.5–49.7) at stage IIB, 33.2% (95%CI: 24.6–41.8) at stage IIC, 72.0% (95%CI: 69.1–74.9) at stage IIIA, 48.8% (95%CI: 47.4–50.2) at stage IIIB, 26.5% (95%CI: 23.0–30.0) at stage IIIC, and 11.3% (95%CI: 10.3–12.3) at stage IV. As shown in the Kaplan–Meier survival curve, the 5-year survival rate of stage IIIA was significantly higher than that of stages IIB (72.0% vs. 42.1%) and IIC (72.0% vs. 33.2%) and even better than that of stage IIA (72.0% vs. 59.8%) (Figure 3). The results illustrated that CRC patients with insufficient lymphoid examination were associated with a “survival paradox” based on AJCC staging.
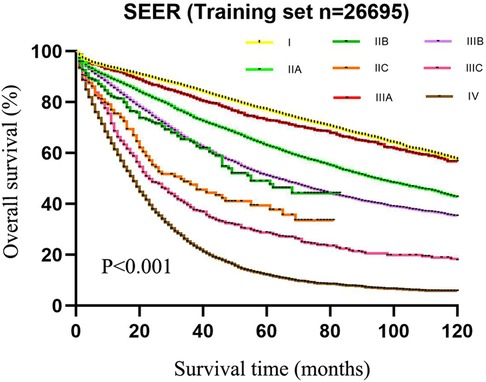
Figure 3. The 5-year survival rate of patients in the training set using CRC patients from the SEER database based on the AJCC TNM staging system.

Table 2. The 5-year survival rate of CRC patients in the training set from the SEER database based on AJCC staging.
Based on the LNR grouping, the 5-year survival rates of the five groups from LNR0 to LNR4 were 67.2% (n = 10,674), 61.8% (n = 5,044), 55.2% (n = 3,233), 43.9% (n = 2,215), and 30.6% (n = 835), respectively. Statistical differences were obtained among the groups (χ2 = 834.572, P < 0.001) (Table 3).
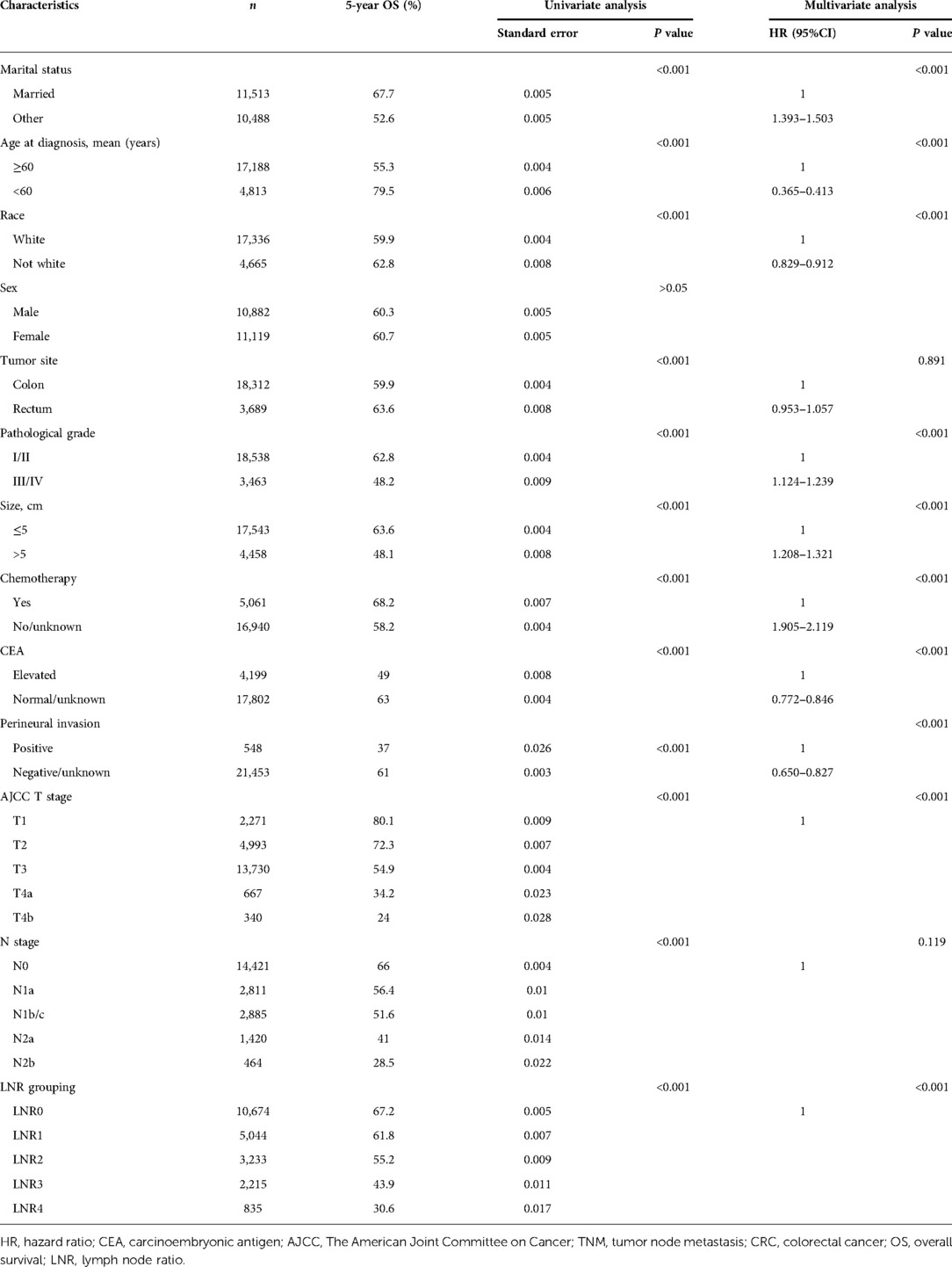
Table 3. Univariate and multivariate analyses identifying factors associated with the prognosis of patients with CRC.
The 5-year survival rates of patients based on the AJCC N staging system were 66.0% (n = 14,421) at N0, 56.4% (n = 2811) at N1a, 51.9% (n = 2709) at N1b, 48.0% (n = 176) at N1c, 41.0% (n = 1420) at N2a, and 28.5% (n = 464) at N2b (χ2 = 698.650, P < 0.001). The survival rates between N1b and N1c were not statistically different (χ2 = 2.586, P = 0.108), and patients with N1c and N1b were combined into one group. The 5-year overall survival rates of patients were 66.0% (n = 14,421) at N0, 56.4% (n = 2811) at N1a, 51.6% (n = 2885) at N1b/c, 41.0% (n = 1420) at N2a, and 28.5% (n = 464) at N2b. The difference in the survival rates among the five groups was statistically different (χ2 = 694.258, P < 0.001) (Table 3).
The results of the univariate analysis revealed that AJCC N stage (P < 0.001) and LNR grouping (P < 0.001), age (P < 0.001), tumor location (P < 0.001), tumor size (P < 0.001), tumor pathological grade (P < 0.001), tumor T stage (P < 0.001), and chemotherapy (P < 0.001) were associated with the prognosis of patients. Factors with a statistical difference were included in the multivariate analysis, which showed that the AJCC N stage (P > 0.05) was not statistically associated with the prognosis of patients, while the LNR grouping (P < 0.001) remained as an independent factor associated with the survival rate of patients (Table 3).
The new staging system was established based on the combination of the AJCC T stage and LNR grouping. The training set was divided into a total of 25 different groups obtained according to five T stages and five LNR groupings (Table 4). The 5-year survival rate of each stage was calculated, and the survival curves were compared pairwise. The new groups were sorted according to the 5-year survival rate. Neighborhood survival curves with no statistical difference were combined, and three new stage groups were formed:
1. good prognosis stage with a 5-year survival rate ≥75% was classified as stage I;
2. moderate prognosis stage with a 5-year survival rate of 55%–75% was classified as stage II; and
3. poor prognosis stage with a 5-year survival rate <55% was classified as stage III.
Stage III was further classified into IIIA with a 5-year survival rate of 40%–55%, IIIB with a 5-year survival rate of 25%–40%, and IIIC with a 5-year survival rate <25% (Table 4). Finally, six new TNRM staging were obtained (Table 5).
As displayed in Figure 4, the 5-year survival rates of patients from stage I to IIIC were 80.4%, 72.9%, 59.8%, 48.4%, 32.5%, and 15.0%, according to the TNRM staging system (χ2 = 1765.947, P < 0.001).
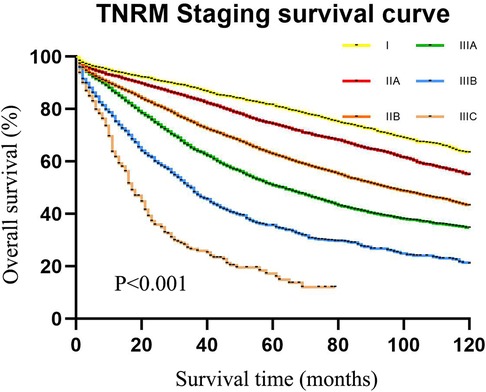
Figure 4. The 5-year survival rate of CRC patients in the training set using CRC patients from the SEER database according to TNRM staging.
The results of the chi-square test displayed that the TNRM staging system monotonously decreased in the whole interval with an R value of 1215.621, while the R value was 653.858 for the AJCC staging system, which revealed that the new TNRM staging system was also superior to the AJCC system in the monotonic decreasing gradient of each substage.
The AJCC TNM stages were restratified by TNRM stages to compare the homogeneity of the TNRM and AJCC TNM staging systems. As shown in Table 6, the AJCC IIIC stage was found to be the most heterogeneous in respect of survival; it contained three new TNRM stages as follows: IIIA (n = 85), IIIB (n = 363), and IIIC (n = 230) with the 5-year OS ranging from 10.3% to 45.0% (range = 34.7%). The survival span of the AJCC IIIB stage (n = 5836) containing three TNRM subgroups also showed poor homogeneity with a 5-year OS of 18.0%–58.9% (range = 30.9%). When TNRM stages (stages I–IIIC) were restratified according to AJCC stages, the most heterogeneity was found in the TNRM IIIC stage with a survival span of 10.3%–27.6% (range = 17.3%), and the sample size in this stage was very small (n = 290).
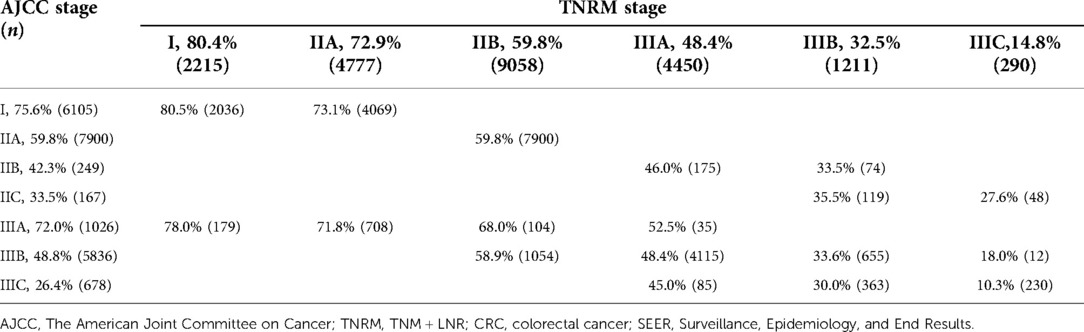
Table 6. Five-year overall survival by AJCC staging and TNRM staging for predicting the survival of CRC patients in the training set from SEER.
The data of our CRC cases from Northern Jiangsu Hospital affiliated with Yangzhou University were applied for the external validation of the performance of the TNRM staging system for predicting the 5-year survival rate of CRC patients. The survival curves of patients based on the AJCC staging system (χ2 = 156.223, P < 0.001) and the TNRM staging system (χ2 = 150.648, P < 0.001) are shown in Figures 5 and 6, respectively.

Figure 5. The 5-year survival rate of patients in the validation set using our cases of CRC based on the AJCC TNM staging system.
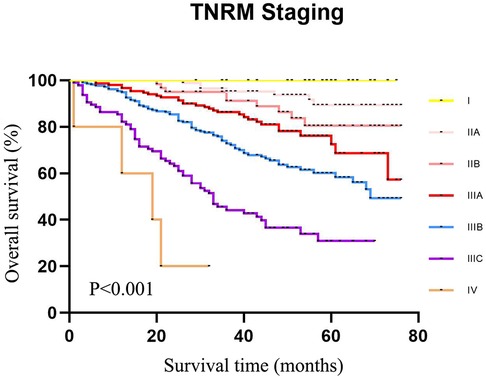
Figure 6. The 5-year survival rate of CRC patients in the validation set using our cases of CRC according to TNRM staging.
According to the AJCC staging system, the 5-year survival rates of patients from stage I to stage IIIC were 91.3%, 90.8%, 72.6%, 61.3%, 72.4%, 58.1%, and 32.8%,. The survival rate of patients at stage IIIA was not significantly different between IIB and IIIA (72.6% vs. 72.4%, χ2 = 0.135, P = 0.713). No significant difference was observed between IIC and IIIBC (61.3% vs. 58.1%, χ2 = 0.010, P = 0.920). Although the sample size of AJCC stage IIIA was small, an obvious “survival paradox” was observed from the survival curves (Figure 5). Based on the TNRM staging system, the 5-year survival rates of patients from stage I to stage IIIC were 99.2%, 90.5%, 81.4%, 78.6%, 60.2%, and 35.8%. Although there was no significant difference between the survival rates at TNRM stage IIB and stage IIIA (81.4% vs. 78.6%, χ2 = 2.026, P = 0.155), there was a significant difference between the survival rates at stage IIIA and other staging curves (P < 0.05) (Figure 6). In particular, the staging curves of the high-risk stages IIIB and IIIC were both clearly differentiated from all other stages (P < 0.01). In addition, the monotonic decreasing linear correlation in the TNRM staging system (R = 107.083) was better than that in the AJCC staging system (R = 99.188) even with one fewer staging curve (7 vs. 8).
The present study collected the data of 26,695 CRC patients to construct a novel clinical staging system for predicting the survival rates of CRC patients with insufficient lymph nodes via combining LNR ratio and AJCC T stages and validated the results in 635 of our cases of CRC from Northern Jiangsu People's Hospital affiliated with Yangzhou University. The results depicted that the 5-year survival rates of patients from stage I to stage IIIC were 80.4%, 72.9%, 59.8%, 48.4%, 32.5%, and 15.0%in the training set and 99.2%, 90.5%, 81.4%, 78.6%, 60.2%, and 35.8% in the validation set according to the TNRM staging system, which eliminated the “survival paradox” in survival rates of CRC patients estimated by the AJCC staging system. The findings of our study might provide a new tool for predicting the survival of CRC patients with <12 lymph nodes.
Previously, a study identified that the overall survival rate of CRC patients at the IIB/C stage was lower than that at the IIIA stage based on the AJCC staging system (2). In our study, we also found that for CRC patients with <12 lymph nodes examined, the overall prognosis of stage IIIA patients was better than that of stage II patients based on the AJCC staging system. Even the 5-year survival rate of CRC patients at stage IIIB was better than those at stage IIC. The results of our study indicated that the “survival paradox” of CRC patients existed in both patients with ≥12 and <12 lymph nodes examined. Some studies demonstrated that the “survival paradox” of CRC patients based on the AJCC staging system might result from stage migration or lack of systematic treatment (9). Other studies also revealed that advances in adjuvant chemotherapy and radiotherapy have improved the survival rate of CRC patients at stage III, and the benefits of adjuvant therapy may narrow the survival gap between patients at stage II and stage III (20, 21). However, for rectal cancer patients at stage II or stage III with the same adjuvant chemotherapy and radiotherapy, the prognosis of patients at stage IIIA was still better than that of patients at stage II (8). These results suggested that the “survival paradox” of CRC was not caused by adjuvant chemotherapy (22).
Li et al. suggested that the possible reason for the “survival paradox” of CRC was due to the excessive predictive weight of the N stage, and they proposed a new system by strengthening the weight of the T stage and modifying the corresponding relationship between the T stage and TN score (the T and N stage relative weights) (23). They reclassified T4bN0 into colon cancer stage IIIA and rectal cancer stage IIIB, while the AJCC stage IIIA (T1-2N1 and T1N2a) was reclassified into stage I or stage II. However, this classification also had limitations. Increasing T stages’ weight had a direct impact on the TN score. As a result, T2N2a patients with higher TN scores were classified into stage IIIA, but the 5-year overall survival rate was 66.6%, which was significantly higher than the rest of the patients in the same IIIA group. On the other hand, T1N2a patients with lower TN scores were classified into stage II, and their 5-year overall survival rate (57.4%) was significantly lower than that of the rest of stage II patients. The author explained that the inconsistency might be due to the application of linear regression in this study, but the impact of T stage and N stage combination on survival may be nonlinear. Additionally, whether the accuracy of N stage weight was affected by the number of lymph nodes examined was not investigated in the study.
In the past three decades, the AJCC TNM staging system has updated many versions. The AJCC 7th edition improved the N staging via dividing N1 into N1a (1 lymph node metastasis), N1b (2–3 lymph nodes metastasis), and N1c (0 lymph node metastasis) and N2 into N2a (4–6 lymph nodes metastasis) and N2b (>7 lymph nodes metastasis), and these were included in the AJCC 8th edition (24). The AJCC N stage recommends a minimum of 12 lymph nodes dissection, but the extent of lymph node dissection and the number of lymph nodes pathologically examined vary in different institutions and regions (25). At present, there are still a large number of CRC patients with <12 lymph nodes dissection (26). In our study, the median number of lymph nodes dissection was eight in patients from the training set and seven in patients from the validation set. In previous studies, the staging bias caused by insufficient lymph node dissection has been largely reported (27, 28). LNR was reported as an important prognostic predictor of CRC patients, which was not affected by the total number of lymph nodes examined (29). Currently, the stratification method and the cutoff value of LNR are still not unified, and some studies stratified LNR via average, quartile, receiver operator characteristic curve method, or arbitrary classification (30, 31). Different stratification methods may result in different results of the predictive value of LNR in the prognosis of CRC patients (32). Therefore, the optimal grouping standard or the cutoff value of LNR may need to be adjusted accordingly. In the present study, the LNR was grouped using classification and regression tree analysis based on Rosenberg et al. (33), who divided 3,026 patients into five groups with LNR cutoff values of 0.17, 0.41, and 0.69. They demonstrated that the cutoff values had the highest survival impact and LNR could be used as an independent prognostic factor in predicting the survival rate of CRC patients. In this study, the cutoff values of LNR were 0.12, 0.35, and 0.71 in the training set, which were close to the results from Rosenberg et al. Additionally, the lymph node-negative group was further refined. Significant differences were found in the 5-year survival rate between lymph node-negative patients with ≥6 and <6 lymph nodes. Lymph node-negative patients with ≥6 lymph nodes were included in LNR0 to optimize intrastage homogeneity (34).
Accurate cancer staging is essential for clinicians to predict the prognosis of patients, provide appropriate interventions for those patients, and choose the most effective treatment (35). Patients at stage I or stage II with a low risk of recurrence do not require adjuvant chemotherapy, while stage III patients are strongly recommended to receive adjuvant chemotherapy after surgery (36). The “survival paradox” may result in an overestimation of the prognostic risk of stage IIIA and an underestimation of the risk of stage II, which may lead to inappropriate treatments for those patients. In the current AJCC staging system, the relatively high weight of the N stage over the T stage may lead to poor monotonicity of the gradient from early stage tumor to advanced stage tumor (37). This supported the findings of our study, which revealed that the 5-year survival rates from stage I to stage IIIC in the training set were 75.6%, 59.8%, 42.1%, 33.2%, 72.0%, 48.8%, and 26.5% and in the testing set were 91.3%, 90.8%, 72.6%, 61.3%, 72.4%, 58.1%, and 32.8%. The results indicated that the “survival paradox” also existed in our study. Based on TNRM staging system, the 5-year survival rates of the training set from stage I to stage IIIC were 80.4%, 72.9%, 59.8%, 48.4%, 32.5%, and 15.0% and in the testing set were 99.2%, 90.5%, 81.4%, 78.6%, 60.2%, and 35.8%. The results demonstrated that the “survival paradox” was successfully avoided.
At present, several new staging systems combining the AJCC with other biomarkers including carcinoembryonic antigen levels, pathological N stage, and the prognostic score for evaluating the prognosis of CRC patients were established (12–14). These staging systems were applied for all CRC patients rather than CRC patients with <12 lymph nodes. No special attention was paid to those with <12 lymph nodes. The combined biomarkers such as the prognostic score were not comprehensive, and some important prognostic factors were not included (12). In addition, these studies were conducted based on the data from the SEER database with a large sample size, but no external data were applied to verify their results. This study initially constructed a TNRM staging system by combining LNR with the AJCC T stage for predicting the prognosis of CRC patients with <12 lymph nodes. The system successfully eliminated the “survival paradox” of the AJCC staging system. External validation in the validation set using our own cases of CRC also showed a better monotonic decreasing trend between each stage under the TNRM staging system. The finding of our study might help improve the prediction accuracy of the survival rate in CRC patients with <12 lymph nodes. Our study had several limitations. First, the details of surgical resection and adjuvant chemotherapy in patients could not be obtained from the SEER database. Second, the sample size in the validation set was small and from a single center, which might decrease the statistical power of our analysis. To enable a broader application of the TNRM staging system, optimization is required in the future. First, the TNRM staging system can be improved by better T stage, LNR, or M stage definitions or by adding other factors, such as tumor location. Second, whether the TNRM staging system was suitable for predicting CRC patients ≥12 lymph node dissection still requires validation in more studies as broader lymph node dissection and more lymph nodes analysis always lead to a higher staging accuracy (38). In the future, we will focus on exploring the prediction value of the TNRM system on the survival of CRC patients with ≥12 lymph nodes. More data will be collected from multiple centers to verify the findings of the current study.
In the current study, the data of 26,695 CRC patients with <12 lymph nodes were collected to construct a new staging system for CRC patients with <12 lymph nodes and the data of 635 of our cases were applied for the validation of the new staging system. The results depicted that the TNRM staging system successfully eliminated “survival paradox” of the AJCC staging system, which might be superior to the AJCC staging system. The results of this study might highlight a new staging system for CRC patients with <12 lymph nodes, which might help improve the accuracy of the staging of CRC patients with <12 lymph nodes and give a reference for clinicians to provide appropriate treatment for those patients.
Publicly available datasets were analyzed in this study. These data can be found here: https://seer.cancer.gov/.
Ethical approval was not provided for this study on human participants from a public database. The patients/participants provided their written informed consent to participate in this study. The analysis of data from Northern Jiangsu People's Hospital affiliated with Yangzhou University was approved by the Ethics Committee of the Northern Jiangsu People's Hospital affiliated with Yangzhou University (2019081).
YY and ZW designed the study. YY wrote the manuscript. YW collected, analyzed, and interpreted the data. ZW critically reviewed, edited, and approved the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912
3. Feng LH, Su T, Bu KP, Ren S, Yang Z, Deng CE, et al. A clinical prediction nomogram to assess risk of colorectal cancer among patients with type 2 diabetes. Sci Rep. (2020) 10(1):14359. doi: 10.1038/s41598-020-71456-2
4. Weiser MR. AJCC 8th Edition: colorectal cancer. Ann Surg Oncol. (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
5. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
6. Kim MJ, Jeong SY, Choi SJ, Ryoo SB, Park JW, Park KJ, et al. Survival paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon cancer. Ann Surg Oncol. (2015) 22(2):505–12. doi: 10.1245/s10434-014-3982-1
7. Huang B, Mo S, Zhu L, Xu T, Cai G. The survival and clinicopathological differences between patients with stage IIIA and stage II rectal cancer: an analysis of 12,036 patients in the SEER database. Oncotarget. (2016) 7(48):79787–96. doi: 10.18632/oncotarget.12970
8. Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. (2010) 28(2):256–63. doi: 10.1200/jco.2009.23.9194
9. Hari DM, Leung AM, Lee JH, Sim MS, Vuong B, Chiu CG, et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg. (2013) 217(2):181–90. doi: 10.1016/j.jamcollsurg.2013.04.018
10. Kang JS, Higuchi R, He J, Yamamoto M, Wolfgang CL, Cameron JL, et al. Proposal of the minimal number of retrieved regional lymph nodes for accurate staging of distal bile duct cancer and clinical validation of the three-tier lymph node staging system (AJCC 8th edition). J Hepatobiliary Pancreat Sci. (2020) 27(2):75–83. doi: 10.1002/jhbp.690
11. Ng SK, Lu CT, Pakneshan S, Leung M, Siu S, Lam AK. Harvest of lymph nodes in colorectal cancer depends on demographic and clinical characteristics of the patients. Int J Colorectal Dis. (2018) 33(1):19–22. doi: 10.1007/s00384-017-2927-0
12. Liu Q, Lian P, Luo D, Cai S, Li Q, Li X. Combination of carcinoembryonic antigen with the American Joint Committee on Cancer TNM staging system in rectal cancer: a real-world and large population-based study. Onco Targets Ther. (2018) 2018(11):5827–34. doi: 10.2147/OTT.S171433
13. Pei JP, Zhang CD, Liang Y, Zhang C, Wu KZ, Li YZ, et al. A modified pathological N stage including status of tumor deposits in colorectal cancer with nodal metastasis. Front Oncol. (2020) 2020(10):548692. doi: 10.3389/fonc.2020.548692
14. Liu Q, Luo D, Cai S, Li Q, Li X. P-TNM staging system for colon cancer: combination of P-stage and AJCC TNM staging system for improving prognostic prediction and clinical management. Cancer Manag Res. (2018) 2018(10):2303–14. doi: 10.2147/CMAR.S165188
15. Shen F, Cui J, Cai K, Pan H, Bu H, Yu F. Prognostic accuracy of different lymph node staging systems in rectal adenocarcinoma with or without preoperative radiation therapy. Jpn J Clin Oncol. (2018) 48(7):625–32. doi: 10.1093/jjco/hyy070
16. Prassas D, Verde PE, Pavljak C, Rehders A, Krieg S, Luedde T, et al. Prognostic discrimination of alternative lymph node classification systems for patients with radically resected non-metastatic colorectal cancer: a cohort study from a single tertiary referral center. Cancers. (2021) 13(15):3898. doi: 10.3390/cancers13153898
17. Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. (2010) 251(6):1070–8. doi: 10.1097/SLA.0b013e3181d7789d
18. Vergara-Fernandez O, Navarro-Navarro A, Rangel-Ríos HA, Salgado-Nesme N, Reyes-Monroy JA, Velázquez-Fernández D. Oncological implications of lymph nodes retrieval and perineural invasion in colorectal cancer: outcomes from a referral center. Rev Invest Clin. (2018) 70(6):291–300. doi: 10.24875/ric.18002505
19. Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The surveillance, epidemiology, and End results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol. (2016) 40(12):e94–e102. doi: 10.1097/pas.0000000000000749
20. Tsuruta A, Yamashita K, Tanioka H, Tsuji A, Inukai M, Yamakawa T, et al. Feasibility of sequential adjuvant chemotherapy with a 3-month oxaliplatin-based regimen followed by 3 months of capecitabine in patients with stage III and high-risk stage II colorectal cancer: JSWOG-C2 study. Drug Des Devel Ther. (2016) 10:3827–35. doi: 10.2147/dddt.S112322
21. Simillis C, Singh H, Afxentiou T, Mills S, Warren OJ, Smith JJ, et al. Postoperative chemotherapy improves survival in patients with resected high-risk stage II colorectal cancer: results of a systematic review and meta-analysis. Colorectal Dis. (2020) 22(10):1231–44. doi: 10.1111/codi.14994
22. Yamanaka T, Oki E, Yamazaki K, Yamaguchi K, Muro K, Uetake H, et al. 12-Gene recurrence score assay stratifies the recurrence risk in stage II/III colon cancer with surgery alone: the SUNRISE study. J Clin Oncol. (2016) 34(24):2906–13. doi: 10.1200/jco.2016.67.0414
23. Li J, Guo BC, Sun LR, Wang JW, Fu XH, Zhang SZ, et al. TNM Staging of colorectal cancer should be reconsidered by T stage weighting. World J Gastroenterol. (2014) 20(17):5104–12. doi: 10.3748/wjg.v20.i17.5104
24. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67(2):93–9. doi: 10.3322/caac.21388
25. Pellino G, Warren O, Mills S, Rasheed S, Tekkis PP, Kontovounisios C. Comparison of Western and Asian guidelines concerning the management of colon cancer. Dis Colon Rectum. (2018) 61(2):250–9. doi: 10.1097/dcr.0000000000001012
26. Zhang QW, Zhang CH, Pan YB, Biondi A, Fico V, Persiani R, et al. Prognosis of colorectal cancer patients is associated with the novel log odds of positive lymph nodes scheme: derivation and external validation. J Cancer. (2020) 11(7):1702–11. doi: 10.7150/jca.38180
27. Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. (2010) 17(11):2847–55. doi: 10.1245/s10434-010-1158-1
28. Moccia F, Tolone S, Allaria A, Napolitano V, Rosa D, Ilaria F, et al. Lymph node ratio versus TNM system as prognostic factor in colorectal cancer staging. A single center experience. Open Med (Wars). (2019) 14:523–31. doi: 10.1515/med-2019-0058
29. Lee CHA, Wilkins S, Oliva K, Staples MP, McMurrick PJ. Role of lymph node yield and lymph node ratio in predicting outcomes in non-metastatic colorectal cancer. BJS Open. (2019) 3(1):95–105. doi: 10.1002/bjs5.96
30. Li Destri G, La Greca G, Pesce A, Conti E, Puleo S, Portale TR, et al. Lymph node ratio and liver metachronous metastases in colorectal cancer. Ann Ital Chir. (2019) 90:275–80. doi: 10.9738/intsurg-d-15-00274.1
31. Jung W, Kim K, Kim J, Shim SJ. Prognostic impact of lymph node ratio in patients undergoing preoperative chemoradiotherapy followed by curative resection for locally advanced rectal cancer. In Vivo. (2020) 34(3):1247–53. doi: 10.21873/invivo.11898
32. Jakob MO, Guller U, Ochsner A, Oertli D, Zuber M, Viehl CT. Lymph node ratio is inferior to pN-stage in predicting outcome in colon cancer patients with high numbers of analyzed lymph nodes. BMC Surg. (2018) 18(1):81. doi: 10.1186/s12893-018-0417-0
33. Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. (2008) 248(6):968–78. doi: 10.1097/SLA.0b013e318190eddc
34. Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. (2007) 99(6):433–41. doi: 10.1093/jnci/djk092
35. Mahar AL, Compton C, Halabi S, Hess KR, Weiser MR, Groome PA. Personalizing prognosis in colorectal cancer: a systematic review of the quality and nature of clinical prognostic tools for survival outcomes. J Surg Oncol. (2017) 116(8):969–82. doi: 10.1002/jso.24774
36. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. (2014) 383(9927):1490–502. doi: 10.1016/s0140-6736(13)61649-9
37. Chen P, Zuo ZL, Feng LB, Chen XL, Hu XY, Liu Q, et al. Questioning the staging of tumor deposits of colorectal cancer in the eighth edition of the TNM classification: validation by prognosis. Int J Clin Exp Pathol. (2019) 12(12):4309–18.31933832
Keywords: lymph node ratio, AJCC staging system, colorectal cancer, survival, CRC
Citation: Yang Y, Wang Y and Wang Z (2022) Construction of a new clinical staging system for colorectal cancer based on the lymph node ratio: A validation study. Front. Surg. 9:929576. doi: 10.3389/fsurg.2022.929576
Received: 27 April 2022; Accepted: 11 July 2022;
Published: 25 August 2022.
Edited by:
Gaetano Luglio, University of Naples Federico II, ItalyReviewed by:
Weiqi Rong, Chinese Academy of Medical Sciences and Peking Union Medical College, China© 2022 Yang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengbin Wang emhlbmdiaW5nd2FuZzEyMDJAMTYzLmNvbQ==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.