
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 17 August 2022
Sec. Reconstructive and Plastic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.926936
This article is part of the Research TopicBreast Reconstruction After Cancer Treatment: What is New?View all 4 articles
 Yang Hu1,†
Yang Hu1,† Xuan Zhou2,†
Xuan Zhou2,† Xiaofei Tong1
Xiaofei Tong1 Xiangyu Chen1
Xiangyu Chen1 Mingzhu Wang1
Mingzhu Wang1 Xianrui Wu1
Xianrui Wu1 Peiting Li1
Peiting Li1 Fengjie Tang1
Fengjie Tang1 Jianda Zhou1*
Jianda Zhou1* Ping Li1*
Ping Li1*
Purpose: Infection is the most common complication following breast implant surgery. Nevertheless, the systematic administration of antibiotics after breast implant surgery has been subjected to controversial debate. In this study, we sought to elucidate the association between infection and the use of antibiotics as an aftermath of breast implantation surgical procedures.
Methods: Relevant studies were identified from PubMed, Web of Science, and EMBASE search mining. The extracted data included study type, basic characteristics, administrated antibiotic information, and clinical outcomes. Random-effects models were utilized to estimate outcomes, while study quality, statistical bias, and heterogeneity were also analyzed.
Results: A total of 7 studies involving a total of 9,147 subjects were included. The results demonstrated that the use of antibiotics after breast implantation reduced the incidence of infection (risk ratio [RR]: 0.65, 95% CI, 0.46–0.90). Nevertheless, smoking, obesity and diabetes type II are risk factors for postoperative infections. Sensitivity analysis verified the robustness of the results.
Conclusions: Our study identified the administration of antibiotics after breast implantation as an intervention that decreased the incidence of infection. Smoking, obesity, and diabetes type II are risk factors for postoperative infections. These findings strongly suggest that timely and effective antibiotic interventions will be crucial in future clinical practice, which may reduce the risk of postoperative infection following breast implantation.
Breast reconstruction surgery approaches after breast cancer and/or mastectomy can provide physiological and psychological comfort to breast patients by surgically restoring the shape of the breast (1). To this extent, an increasing case of patients are opting for breast reconstruction after breast cancer surgery, which robustly magnifies the clinical importance of breast reconstruction. Implant-based breast reconstruction is the most popular option, accounting for approximately 80% of post-operative breast cancer reconstructions (2, 3). Despite the growing popularity of the approach, infection is a major complication after breast implant surgery. In particular, breast reconstruction after mastectomy and cancer radiotherapy is associated with a higher risk of infection (4).
Previous study showed that the infection rate associated with breast implant surgery can be as high as 35.40% (5). Nonetheless, according to the Centers for Disease Control (CDC) guidelines, breast surgery is considered as an aseptic-field procedure and therefore a maximum of 24 h of perioperative antibiotics is currently recommended (6). In fact, breasts are not sterile. What's more, endogenous skin flora colonizing the nipple can spread to deeper breast tissue through the milk ducts or during surgical procedures, which can lead to infection. Coagulase-negative staphylococci can be isolated from the breast in more than half of women undergoing breast augmentation or breast reduction. Other skin flora frequently isolated from the breast including diphtheria-like, Lactobacillus, Bacillus, beta-hemolytic streptococci, and Propionibacterium acnes (7). In particular, the incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections is increasing (8, 9). Other common Gram-positive bacteria including Streptococcus, although recent reports showed an increased incidence of Gram-negative bacterial infections, such as Pseudomonas (10). Therefore, in the long term, bacteriological treatment with prophylactic antibiotics in patients with breast implants is necessary (11, 12).
The American Society of Plastic Surgery has developed guidelines for implant reconstruction, but in terms of infection prevention, they only cover the use of perioperative antibiotics and do not address the use of postoperative antibiotics. However, in a survey of members of the American Society of Plastic Surgeons (ASPS), roughly 72 percent of physicians prefer to continue using antibiotics following breast reconstruction surgery for a period of time, usually longer than 24 h (13). In the following years after this milestone study, several physicians have tried a plethora of therapeutic approaches, without achieving universal guidelines and concordance. On the contrary, other studies demonstrated that postoperative antibiotics failed to reduce the incidence of infection (14–16), while Townley et al. suggested that only preoperative antibiotics and postoperative antibiotics were equally effective on reducing the infection rates (17). Nevertheless, some cohort prospective studies have indicated that postoperative antibiotic use was effective in reducing the incidence of infection (18–21). Meanwhile, a previous meta-analysis on the use of extended prophylactic antibiotics in breast reconstruction which only focused on immediate breast reconstruction, fail to reach on a definite conclusion on the causal relationship between antibiotic use and infection after breast implantation (22). Therefore, antibiotics usage after breast implantation remains highly controversial, which introduces a significant clinical dilemma regarding the risk of infection and antibiotic use after breast implantation.
The purpose of this article is to elucidate the effectiveness and feasibility of postoperative antibiotics on controlling the incidence of infection after breast implantation, in an effort to optimize the clinical decisions for the management of these patients.
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic research strategy was constructed based on the following question, whether the postoperative antibiotic usage can reduce the incidence of infectious outcomes in breast implant surgery. To address this question, two authors independently identified studies published in English as of September 30th, 2021, through the following online databases: PubMed, Embase, and Web of Science. Search terms were used in different combinations to identify the maximum number of relevant studies in these databases. Where possible, subject headings, web banners, and other forms of indexing were utilized. The detailed search strategy used in this study is outlined in Figure 1. In addition, we collected relevant articles that were not included in the search but satisfied the search requirements.
In our study, exposure is the administration of antibiotics after breast implantation. Specifically, we defined the postoperative antibiotics uniformly as the use of antibiotics after the operation, usually for no more than 24 h. The outcome is the occurrence of topical infection, which was defined by the CDC guidelines as septic drainage, positive bacterial cultures, erythema around the incision, surgical cleaning of the surgical incision site, and physician diagnosis.
While screening articles titles and abstracts, we intentionally expanded the inclusion criteria to identify any relevant studies. As a first inclusion criterion, studies were included if they reported an association between antibiotics and breast implant surgical infection and were published in English. Secondly, the full text of the selected studies was reviewed. Studies were included if they met the following study design criteria: the design was observational; the study population had at least two groups (one with and one without postoperative antibiotic use); or demonstrated sufficient information to allow accurate risk estimation, subsequent statistical analyses and calculation of the corresponding 95% CI. Studies were excluded if they were review articles, case reports, letters to the editor, or conference abstracts, were not controlled, provided unclear or incomplete data or were redundant publications. In the case one of a study from the same population, only the most comprehensive or most recently published study was included.
Using self-designed tables, two researchers independently extracted the following data and demographics from each study: first author, year of publication, geographic region, study design, type of reconstruction, antibiotic application regimen, duration of postoperative antibiotic, follow-up time, infection rate, route of administration, time to infection diagnosis, sample size, quality score, control confounders and risk estimates, and the corresponding 95% CIs (adjusted CIs was extracted, if available). Any discrepancies were handled through conversation or using an independent third reviewer, to increase the methodology soundness of the study.
Two researchers (Y.H. and X.Z.) independently assessed the methodological quality of studies using the Newcastle-Ottawa Scale (NOS) for quality evaluation of cohort studies. There were eight items in the NOS. Each study received an objective rating of 1–9 stars based on three distinct criteria: selection, comparability, and result (cohort studies). A final median score of 6 and above was considered as excellent.
Risk ratio (RR) was used as a reference value to measure the association between the use of antibiotics after breast implant surgery and infection. Random effects model meta-analysis was used as a tool to calculate combined RRs and 95% CIs (23). Heterogeneity was assessed by the Chi-square test for categorical variables (p < 0.05 represented statistically significant heterogeneity) and I2 statistic (I2 > 75% indicated an extremely high level of heterogeneity, 51%–75% showed a high level of heterogeneity, 26%–50% demonstrated a moderate-level of heterogeneity, and ≤25% signified a low level of heterogeneity) (24, 25). The Chi-square test was used to estimate whether the variance between studies was ascribed to chance and the I2 statistic was used to estimate the proportion of total variation in prevalence evaluations owing to statistical heterogeneity instead of sampling error. Publication bias of included articles was assessed by using the Egger test (p < 0.05 indicated statistically significant differences). Subgroup analysis included geographic region (e.g., Canada, USA), duration of postoperative antibiotics use (e.g., >48 h, until drainage removal or unknown), confounders controlled (e.g., none of the confounder controlled, one or more confounders controlled), sample size (e.g., ≤500, >500) publication years (e.g., before 2018, 2018 or after) and quality assessment (e.g., ≤7, >7). To assess the robustness of the meta-analysis results, sensitivity analyses were performed, which involved performing meta-analyses after eliminating one included research at a time and comparing the results before and after removal. RevMan version 5.4.1 (The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) and R version 4.0.5 were used for all statistical analyses (The R Foundation for Statistical Computing).
A total of 1,296 articles were identified by system search and manual search. Among them, 1,040 unique articles were included after the exclusion of duplicate publications, 169 articles were retained after title and abstract screening, falling to 82 articles that were included following full-text screening. After the inclusion criteria procedure, 7 articles were ultimately included in the meta-analysis (see Figure 1) (14–20). These articles were all published between 2012 and 2021, and all these articles were cohort studies, involving a total of 9,147 subjects. Details of the included articles are shown in Table 1.
The types of procedures in the McCullough et al. (14) and Ranganathan et al. (15) studies were all immediate breast reconstruction approaches. In addition to this, subjects in three studies involved expander placement, which was performed in both the anterior thoracic and submuscular planes (14, 16, 20). Moreover, five studies addressed the duration of antibiotic application after breast implant reconstruction, including >48 h and until drainage removal (16–20). For the specific application of antibiotics, four of the included studies noted the use of cephalosporin antibiotics for general patients and clindamycin or vancomycin for allergic patients (14, 17–19). In one study, the majority of subjects chose methomyl/sulfamethoxazole. For patients with sulfonamides allergy, doxycycline and clindamycin were used (20). Another study grouped the duration of postoperative antibiotic application as 1–5 days, 1–5 days and >10 days, however, the risk of infection for all three group was not statistically significant compared to patients who did not use postoperative antibiotics (15). The most common bacteria found in bacterial cultures of infected tissues were methicillin-sensitive Staphylococcus aureus (MSSA), followed by methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus epidermidis (14, 20).
The included studies reported RRs ranging from 0.21 to 0.93 (see Figure 2). A pooled meta-analysis of these risk estimates yielded an RR of 0.65 (95% CI, 0.46–0.90), with acceptable heterogeneity (I2 = 71%, p < 0.05), which means that postoperative antibiotics is a protective factor for infection after breast implant reconstruction. Notably, the Egger test showed no potential publication bias (z = −2.47, p = 0.0564). Sensitivity analyses were repeated as outlined in the methods, after excluding each included study in the meta-analyses. The results of the sensitivity analysis showed that excluding any of the studies did not substantially alter the risk estimates for postoperative antibiotic use (RRs between 0.57 and 0.73; see Table 2).
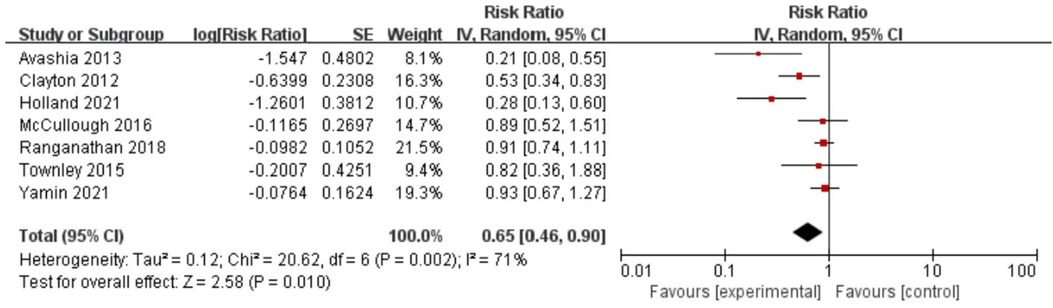
Figure 2. Forest plot for the relationship between antibiotic use and infection after breast implantation.

Table 2. Sensitivity analysis for the association between antibiotic use and infection after breast implantation.
Table 3 showed the results of the subgroup analysis between postoperative antibiotics and infection rates after breast implant surgery. Following subgroup analysis, variables involving geographic region (test for subgroup differences [TSD]: I2 = 0%), duration of postoperative antibiotics use (TSD: I2 = 69.80%), sample size (TSD: I2 = 80.30%), publication years (TSD: I2 = 0%), quality assessment (TSD: I2 = 0%) and confounders controlled (TSD: I2 = 33.30%) were determined as potential heterogeneity moderators. When stratified by duration of postoperative antibiotic use, antibiotic use until drainage removal remains an effective measure to prevent infection after breast reconstruction (RR: 0.49, 95% CI, 0.30–0.82) (see Figure 3).
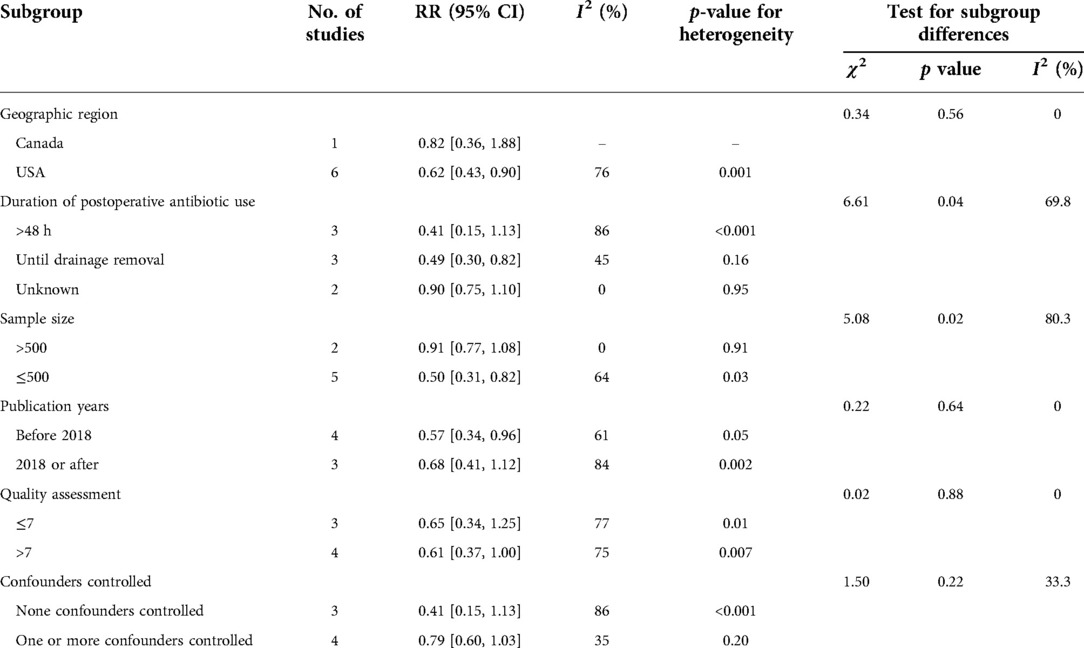
Table 3. Subgroup analyses for the association between antibiotic use and infection after breast implantation.
During quantitative analysis, smoking, obesity, and diabetes type II were clearly identified as risk factors for infection after breast implant surgery. Specifically, non-smokers were 1.53 times less likely to have an infection than smokers (RR: 1.53, 95% CI, 1.08–2.16), and with a small heterogeneity (I2 = 0%, p < 0.05) (see Figure 4A). Obesity and postoperative infection analysis showed that the risk of postoperative infection in obese patients was 1.78 times higher than that in non-obese patients (RR: 1.78, 95%CI, 1.20–2.63, I2 = 0%, p < 0.005) (see Figure 4B). In the analysis of diabetes for occurrence of infection, patients with diabetes were 1.53 times more likely to have an infection compared to patients without diabetes (RR: 1.53, 95%CI, 1.14–2.06, I2 = 0%, p = 0.005) (see Figure 4C). However, in the analysis of infection and other associated factors, including adjuvant radiation, adjuvant chemotherapy and neoadjuvant chemotherapy, the RRs were 1.53 (95% CI, 0.79–2.98, p > 0.05) (see Figure 4D), 1.40 (95%CI, 0.92–2.12, p > 0.05) (see Figure 4E) and 0.87 (95%CI, 0.59–1.26, p > 0.05) (see Figure 4F), respectively, while neither of which factors reached statistical significance.
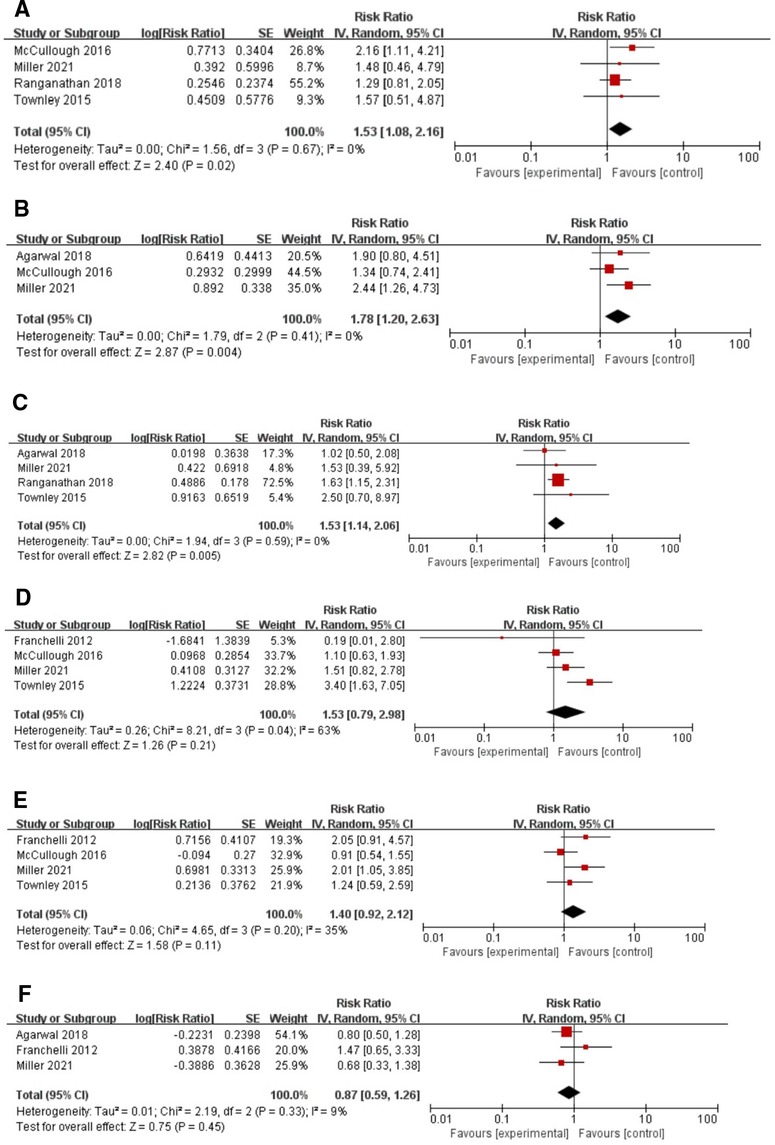
Figure 4. Forest plots for the meta-analyses. (A) Smoking; (B) Obesity; (C) Diabetes; (D) Adjuvant radiation; (E) Adjuvant chemotherapy; (F) Neoadjuvant chemotherapy.
Does postoperative antibiotics application reduce the chance of infection after breast implant reconstruction? Based on the importance of this research topic, a growing number of researchers have investigated the association between antibiotic use and infection after breast implantation in recent years, but with no concordant results. The aim of our study was to elucidate the relationship between antibiotic use and infection after breast implantation.
After vigorous selection criteria and statistical analysis, we demonstrated that the use of antibiotics after breast implantation can reduce the incidence of infection (RR: 0.65, 95% CI, 0.46–0.90). More importantly, our study discovered that antibiotic use until drainage removal was beneficial in lowering infection rates after breast implantation (RR: 0.49, 95% CI, 0.30–0.82). Furthermore, the study quantified smoking, obesity, and diabetes as risk factors for infection during breast implantation. Therefore, we can provide robust evidence to the highly controversial issue of antibiotic utilization after breast implant reconstruction surgery. These results will provide great support to clinicians for the use of antibiotics in their clinical practice.
Infection following breast implant restoration is considered to be caused by a combination of causes. Firstly, numerous studies have shown that infection and implant failure are associated with endogenous skin flora colonizing the nipple, including Staphylococcus aureus, Streptococcus, and Lactobacillus, which is a considerable bottleneck in the patients' quality of life and an economical burden on the healthcare system (18, 19, 26–29). Secondly, residual cavity after mastectomy was considered as a risk factor for infection. Surgical complications and techniques, such as damage to surrounding blood vessels and removal of lymph nodes may ultimately result in the formation of localized hematomas and poor lymphatic drainage in the breast, leading to bacterial growth and reproduction, increasing the chance of local advanced infection (19). Thirdly, incorrect placement of breast implants can cause excessive tension, which can lead to skin necrosis, fat necrosis, flap necrosis, nipple areola necrosis, or glandular necrosis, as well as infection. In most cases, the infection is caused by local tissue ischemia (30). Additionally, several studies have discovered that utilizing surgical drains raises the risk of breast implant infection by up to fivefold (31). These factors can increase the chances of infection and even aggravate it, causing the emergence of more resistant bacteria, which ultimately affects the efficacy of antibiotic use (32).
Cefazolin is the antibiotic of choice for antibiotic prophylaxis after prosthetic joint and prosthetic cardiovascular material implantation based on the CDC guidelines. Nevertheless, there are no updated recommendations for breast implant surgery (6). Although cefadroxil is the empirical drug for the treatment of infections in breast surgery, some studies have shown a higher failure rate of cefadroxil for skin and soft tissue infections. An additional concern is the increasing resistance of staphylococci to cephalosporin and other β-lactam antibiotics (33). As a result, the Association for Breast Surgery (ABS) recommendations for implant reconstruction recommended a single intravenous dose of antibiotics at the time of anesthesia induction, especially in cases where antibiotic use is debatable. Antibiotics that are commonly prescribed include intravenous first- or second-generation cephalosporins. Non-β-lactam antibiotics with a sufficiently broad spectrum, such as clindamycin and vancomycin, are advised for patients who are allergic to β-lactam antibiotics (34).
According to our knowledge, this is the first study to demonstrate that postoperative antibiotic treatment is a protective factor for breast implant infections. In addition, this is the first study to independently identify risk factors for postoperative infection, through robust quantitative meta-analysis. Notably, we included the most recent literature to elaborate and support our results. Moreover, our study compensates for the deficiencies of the previous meta-analysis (RR: 0.80, 95%CI, 0.60–1.07), which did not clarify the protective effect of postoperative antibiotics against infection after breast implantation. In terms of antibiotic duration of action, administering antibiotics until drainage removal after breast implant reconstruction is obviously useful in controlling infection (RR: 0.49, 95% CI, 0.30–0.82).
However, our article still has some limitations. Firstly, our review was confined to English-language literature, which may result in linguistic bias. Besides, possible publication bias, such as the under-publishing of unfavorable statistical results, may have an impact on the findings and should not be overlooked. Secondly, we identified significant variability in our research study. Although we conducted several subgroup analyses, including geographic region, sample size, publication years, duration of postoperative antibiotic use, quality assessment and confounders controlled, the objective heterogeneity observed cannot be ignored. Thirdly, in the included studies, data sources were only provided by a single institution, and sample size was small in most of the studies. To enhance the confidence of the findings of the study, future studies should be performed on a large scale and on a multicenter clinical verification-manner. Moreover, antibiotic regimens are not uniform, including the type of antibiotic, duration of administration and dose. The choice of antibiotic regimen may vary from one clinician to another, which makes it impossible to ensure the robustness of the study. Fifthly, prior to the administration of antibiotics, physicians were neither given indicators of infection nor the results of bacterial cultures, and treated patients based on experience alone, which negatively impacted the results of the study. Last but not least, we focused on the antibiotic regimen in the initial data extraction, but due to the lack of data, we only analyzed the association between antibiotic duration and infection and were unable to perform a statistical analysis of the relationship between the specific antibiotic types and infection, which was also a deficiency of this study.
The results of this study provide scientific evidence that postoperative antibiotics significantly reduce the incidence of infection after breast implantation (RR: 0.65, 95% CI, 0.46–0.90). However, there is a lack of universal consensus in the plastic surgery literature regarding the optimal timing and duration of antibiotic regiments after breast reconstruction (34). In the future, more high-quality multi-center randomized controlled studies are essential. In the meantime, surgeons should concentrate on more detailed protocols for antibiotic application on the present foundation. From our perspective, researchers should also focus on the stratification of infections or other related complications, which would lead to a more precise utilization of our medical resources. More standardized and universal definitions of infection and antibiotic regimens are also required, which will require a concerted effort by the scientific and medical community.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Study concept and design: PL and JZ. Acquisition, analysis, or interpretation of data: YH, XZ, XT, XC, MW, XW. Statistical analysis: YH and XZ. Drafting of the manuscript: YH and XZ. Critical revision of the manuscript for important intellectual content: YH, XZ and XT. Obtained funding: PL. Administrative, technical, or material support: PL, XW and FT. Study supervision: JZ and PL. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation Program of China (81872219). Hunan Provincial Key Research and Development Program (2018SK2084).
We thank all our colleagues working in Department of Plastic Surgery of Third Xiangya Hospital, Central South University, and Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rozen WM, Ashton MW, Taylor GI. Defining the role for autologous breast reconstruction after mastectomy: social and oncologic implications. Clin Breast Cancer. (2008) 8(2):134–42. doi: 10.3816/CBC.2008.n.013
2. Hanna KR, Tilt A, Holland M, Colen D, Bowen B, Stovall M, et al. Reducing infectious complications in implant based breast reconstruction: impact of early expansion and prolonged drain use. Ann Plast Surg. (2016) 76(Suppl 4):S312–5. doi: 10.1097/SAP.0000000000000760
3. Platt J, Baxter N, Zhong T. Breast reconstruction after mastectomy for breast cancer. CMAJ. (2011) 183(18):2109–16. doi: 10.1503/cmaj.110513
4. Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. (2005) 5(2):94–106. doi: 10.1016/S1473-3099(05)01281-8
5. Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. (2002) 109(7):2265–74. doi: 10.1097/00006534-200206000-00015
6. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for disease control and prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. (1999) 27(2):97–132, 133–4, 96. doi: 10.1016/S0196-6553(99)70088-X
7. Thornton JW, Argenta LC, McClatchey KD, Marks MW. Studies on the endogenous flora of the human breast. Ann Plast Surg. (1988) 20(1):39–42. doi: 10.1097/00000637-198801000-00008
8. Reish RG, Damjanovic B, Austen WJ, Winograd J, Liao EC, Cetrulo CL, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. (2013) 131(6):1223–30. doi: 10.1097/PRS.0b013e31828bd377
9. Feldman EM, Kontoyiannis DP, Sharabi SE, Lee E, Kaufman Y, Heller L. Breast implant infections: is cefazolin enough? Plast Reconstr Surg. (2010) 126(3):779–85. doi: 10.1097/PRS.0b013e3181e5f7ff
10. Franchelli S, Vassallo F, Porzio C, Mannucci M, Priano V, Schenone E, et al. Breast implant infections after surgical reconstruction in patients with breast cancer: assessment of risk factors and pathogens over extended post-operative observation. Surg Infect. (2012) 13(3):154–8. doi: 10.1089/sur.2011.004.
11. Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. (2003) 111(5):1605–11. doi: 10.1097/01.PRS.0000054768.14922.44
12. Hunter JG, Padilla M, Cooper-Vastola S. Late Clostridium perfringens breast implant infection after dental treatment. Ann Plast Surg. (1996) 36(3):309–12. doi: 10.1097/00000637-199603000-00014
13. Phillips BT, Wang ED, Mirrer J, Lanier ST, Khan SU, Dagum AB, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: experience, evidence, and implications for postoperative care. Ann Plast Surg. (2011) 66(5):460–5. doi: 10.1097/SAP.0b013e31820c0593
14. McCullough MC, Chu CK, Duggal CS, Losken A, Carlson GW. Antibiotic prophylaxis and resistance in surgical site infection after immediate tissue expander reconstruction of the breast. Ann Plast Surg. (2016) 77(5):501–5. doi: 10.1097/SAP.0000000000000275
15. Ranganathan K, Sears ED, Zhong L, Chung TT, Chung KC, Kozlow JH, et al. Antibiotic prophylaxis after immediate breast reconstruction: the reality of its efficacy. Plast Reconstr Surg. (2018) 141(4):865–77. doi: 10.1097/PRS.0000000000004204
16. Yamin F, Nouri A, McAuliffe P, Vasilakis V, Ganz J, Khan S, et al. Routine postoperative antibiotics after tissue expander placement postmastectomy does not improve outcome. Ann Plast Surg. (2021) 87(1s Suppl 1):S28–S30. doi: 10.1097/SAP.0000000000002826
17. Townley WA, Baluch N, Bagher S, Maass SW, O’Neill A, Zhong T, et al. A single pre-operative antibiotic dose is as effective as continued antibiotic prophylaxis in implant-based breast reconstruction: a matched cohort study. J Plast Reconstr Aesthet Surg. (2015) 68(5):673–8. doi: 10.1016/j.bjps.2014.12.041
18. Clayton JL, Bazakas A, Lee CN, Hultman CS, Halvorson EG. Once is not enough: withholding postoperative prophylactic antibiotics in prosthetic breast reconstruction is associated with an increased risk of infection. Plast Reconstr Surg. (2012) 130(3):495–502. doi: 10.1097/PRS.0b013e31825dbefe
19. Avashia YJ, Mohan R, Berhane C, Oeltjen JC. Postoperative antibiotic prophylaxis for implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. (2013) 131(3):453–61. doi: 10.1097/PRS.0b013e31827c6d90
20. Holland M, Lentz R, Sbitany H. Utility of postoperative prophylactic antibiotics in prepectoral breast reconstruction: a single-surgeon experience. Ann Plast Surg. (2021) 86(1):24–8. doi: 10.1097/SAP.0000000000002407
21. Dassoulas KR, Wang J, Thuman J, Ndem I, Schaeffer C, Stovall M, et al. Reducing infection rates in implant-based breast reconstruction: impact of an evidence-based protocol. Ann Plast Surg. (2018) 80(5):493–9. doi: 10.1097/SAP.0000000000001407
22. Hai Y, Chong W, Lazar MA. Extended prophylactic antibiotics for mastectomy with immediate breast reconstruction: a meta-analysis. Plast Reconstr Surg Glob Open. (2020) 8(1):e2613. doi: 10.1097/GOX.0000000000002613
23. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1(2):97–111. doi: 10.1002/jrsm.12
24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
26. Weichman KE, Levine SM, Wilson SC, Choi M, Karp NS. Antibiotic selection for the treatment of infectious complications of implant-based breast reconstruction. Ann Plast Surg. (2013) 71(2):140–3. doi: 10.1097/SAP.0b013e3182590924
27. Mukhtar RA, Throckmorton AD, Alvarado MD, Ewing CA, Esserman LJ, Chiu C, et al. Bacteriologic features of surgical site infections following breast surgery. Am J Surg. (2009) 198(4):529–31. doi: 10.1016/j.amjsurg.2009.06.006
28. Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg. (2009) 124(2):387–94. doi: 10.1097/PRS.0b013e3181aee95b
29. Throckmorton AD, Baddour LM, Hoskin TL, Boughey JC, Degnim AC. Microbiology of surgical site infections complicating breast surgery. Surg Infect. (2010) 11(4):355–9. doi: 10.1089/sur.2009.029.
30. Persoff MM. Vertical mastopexy with expansion augmentation. Aesthetic Plast Surg. (2003) 27(1):13–9. doi: 10.1007/s00266-002-0072-8
31. Araco A, Gravante G, Araco F, Delogu D, Cervelli V, Walgenbach K. Infections of breast implants in aesthetic breast augmentations: a single-center review of 3,002 patients. Aesthetic Plast Surg. (2007) 31(4):325–9. doi: 10.1007/s00266-006-0156-y
32. Reiffel AJ, Barie PS, Spector JA. A multi-disciplinary review of the potential association between closed-suction drains and surgical site infection. Surg Infect. (2013) 14(3):244–69. doi: 10.1089/sur.2011.126
33. Madaras-Kelly KJ, Arbogast R, Jue S. Increased therapeutic failure for cephalexin versus comparator antibiotics in the treatment of uncomplicated outpatient cellulitis. Pharmacotherapy. (2000) 20(2):199–205. doi: 10.1592/phco.20.3.199.34780
Keywords: meta-analysis, postoperative antibiotics, infection, breast reconstruction, implant-based breast surgery
Citation: Hu Y, Zhou X, Tong X, Chen X, Wang M, Wu X, Li P, Tang F, Zhou J and Li P (2022) Postoperative antibiotics and infection rates after implant-based breast reconstruction: A systematic review and meta-analysis. Front. Surg. 9:926936. doi: 10.3389/fsurg.2022.926936
Received: 23 April 2022; Accepted: 1 August 2022;
Published: 17 August 2022.
Edited by:
Frontiers in Surgery Editorial Office, Frontiers Media SA, Switzerland© 2022 Hu, Zhou, Tong, Chen, Wang, Wu, Li, Tang, Zhou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianda Zhou emhvdWppYW5kYUBjc3UuZWR1LmNuOw== Ping Li OTkzODUwMzQ3QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.