- Shanghai Key Laboratory of Orthopaedic Implants, Department of Orthopaedic Surgery, Shanghai Ninth People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Background: Bone defects in revision total hip arthroplasties (rTHAs) caused by osteolysis are routinely treated with autografts or allografts, despite their various disadvantages. Currently, little is known about the prognosis of ungrafted cavities with complete debridement following prosthetic revision in rTHAs with component loosening, as few reports have focused on the application of debridement without bone grafting in osteolytic lesions that do not compromise structural stability in revision THAs with revised components.
Methods: In this study, 48 patients receiving rTHAs with components revised for aseptic loosening with osteolysis between 2015 and 2019 were included. Anteroposterior and lateral radiographs of hips before and after revision surgery and last follow-up were compared to measure whether the size of the debrided osteolytic cavity without bone graft had changed.
Results: In total, 48 patients with 59 osteolytic lesions were enrolled. The mean follow-up period was 3.33 years (range 2–6 years). None of the 59 cavities had progressed at the last follow-up, and 11 (18.6%) regressed. Two patients underwent re-revision according to dislocation during follow-up.
Conclusion: In rTHAs with revised components, osteolytic lesions that do not influence structural stability could be debrided without grafting to avoid the disadvantages of grafting. Debridement and component revision are sufficient to prevent the progression of osteolytic lesions during surgery, without having adverse effects on the short-to mid-term prognosis.
Introduction
The number of revision total hip arthroplasties (rTHAs) is increasing year by year (1, 2). The reasons for rTHAs include aseptic loosening, infection, and dislocation, among which aseptic loosening caused by osteolysis is one of the most common (3, 4). RTHA is a challenging procedure; if performed improperly, patients may need to undergo a second revision (5). Correcting the osteolytic cavity during surgery is of great significance to the success of the operation and long-term prognosis (6). Owing to factors such as chronic wear, uneven alignment, and outdated design, the interface friction between the femoral head prosthesis and acetabulum prosthesis can produce worn polyethylene or metal particles (7, 8). These particles are scattered throughout the effective joint cavity by the flow of the joint fluid, resulting in the release of cytokines and triggering a series of inflammatory immune cascade reactions following their phagocytosis by macrophages (9–11). The activation of osteoclasts leads to bone absorption and granuloma formation (12). Research has shown that osteolysis is still one of the main reasons patients undergo revision (4, 13). At present, related research has mostly focused on the effectiveness and long-term survival of grafting in the treatment of osteolytic lesions with well-fixed prosthesis retention, which has good clinical outcomes (14). However, some studies (15) have shown unsatisfactory incorporation between the grafts and the host bone.
Not all lesions affect prosthesis stability. Small cavities that exert insignificant influence on the osteointegration interface between the host bone and prosthesis will not affect prosthesis stability following correct intraoperative trial fit verification (16). During complete rTHA, lesions that affect prosthesis stability are reconstructed with bone grafts, augments, or jumbo cups. However, few studies have focused on the treatment of bone defects that do not affect the stability of the prosthesis and do not require reconstruction. Grafting is routinely performed in clinical practice. However, bone grafting has some inherent disadvantages. Some studies have pointed out that the implanted graft may not integrate with the original bone tissue, and may even become a new source of wear particles, thus further accelerating the occurrence and progression of osteolysis (17). In addition, allogeneic bone also faces problems such as high costs and shortage in supply (18, 19). Currently, there are few stable allogeneic bone sources that patients can afford in clinical practice (19). More importantly, bone grafts also carry the risk of infection and immune rejection when adopting allogeneic bone, which delays bone healing and increases the risk of re-revision (17, 20). Similarly, autogenous bone grafts also face problems such as limited bone quantity, numbness, and pain after bone extraction (17).
Consequently, the prognosis of mere debridement without grafting in small osteolytic lesions is significant as this alternative treatment can avoid the disadvantages and economic burden of bone grafting if osteolysis is blocked. However, to the best of our knowledge, no studies have yet reported such a prognosis; thus, we conducted retrospective study to explore this topic.
This study aimed to discover (1) how debridement alone will affect the size of osteolysis lesions that do not need reconstruction in revision THAs with revised components; (2) whether simple debridement could block the cascade reaction of osteolysis; (3) whether debridement without bone graft affects the integration of prosthesis and bone; and (4) whether this method would affect the stability of prosthesis due to the progression of osteolysis.
This study was reported in accordance with the TREND reporting checklist.
Methods
Study participants
This study was conducted in accordance with the Declaration of Helsinki (revised in 2012), and was approved by the Ethics Committee of the Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine. Informed consent was obtained from all participants. All 310 subjects who underwent revision hip arthroplasty between January 2015 and June 2019 were retrospectively reviewed. The inclusion criteria were as follows: (1) The patients were diagnosed with periprosthetic osteolysis according to the standards of DeLee and Charnley (21). (2) Patients underwent revision THAs for aseptic loosening. (3) Debridement without bone grafts was applied to small lesions, and all related components were revised during surgery. The exclusion criteria were as follows: (1) Patients who did not agree to participate in the study. (2) Patients who received revision THAs for infection, periprosthetic fractures, or other reasons. (3) Patients who were lost to follow-up or who had a follow-up of less than 2 years.
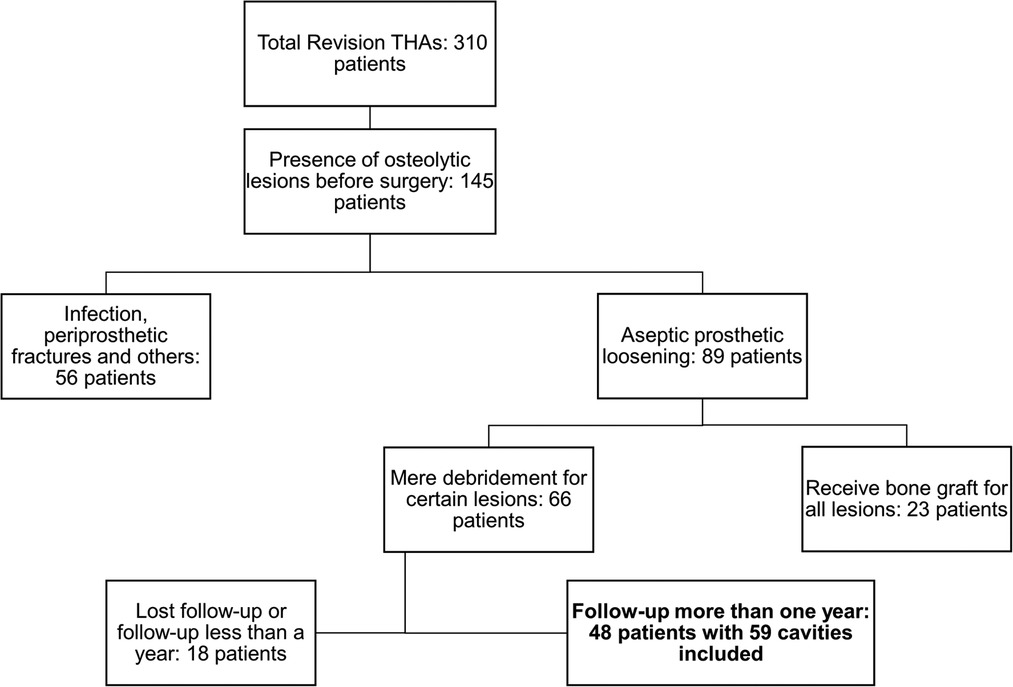
Of the 310 revision cases, 145 were classified as having osteolytic lesions before revision based on preoperative radiographs. Among them, 89 patients were diagnosed with aseptic loosening, while 56 underwent surgery for infection, periprosthetic fractures, and other reasons. Among the 89 candidates, 23 patients received bone grafts of all detectable osteolytic lesions during revision surgery, and 18 were lost to follow-up or had a follow-up period of less than 1 year. Finally, 48 patients with 59 osteolytic cavities treated using debridement alone with a follow-up period of at least 2 years were included in our study. The strategy of study participant selection is shown in Figure 1.
Radiographic measurement and evaluation
After the patient's visit to the hospital, radiographs of the anteroposterior and lateral views were first obtained for the affected hip. In addition, anteroposterior and lateral radiographs were obtained immediately after surgery and at each follow-up at 3 weeks, 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Three doctors identified and evaluated the borders of the osteolytic lesions in a blinded manner. The size of the osteolytic lesions was measured in the anteroposterior x-ray as the longest diameter of the lesion in the horizontal axis multiplied by that in the vertical axe (22) (Figure 2). To determine whether the osteolytic process was blocked, we compared the latest follow-up lesion size to the initial postoperative lesion size. According to the evaluation standard of Min et al. (23), if the lesion size increased by more than 50%, or was greater than 1 cm2, the osteolytic process was defined as having progressed. If lesion size decreased by more than 50% or was greater than 1 cm2, it was defined as regressed. Osteolysis with a size change of <50% and less than 1 cm2 was defined as stabilized (23). The locations of the femoral and acetabular osteolytic lesions were identified according to the Gruen zone classification (24) and DeLee and Charnley zone classification (21), respectively (Figure 3). All the lesions around the femoral component were located at zone 1 and zone 7. Cup loosening was defined as described in a previous study (25). The migration of prostheses was determined by comparing radiographs taken at the last follow-up with those taken immediately postoperatively. Definite loosening was defined as an acetabular migration of ≥2 mm, with implant rotation or screw breakage. Probable loosening was defined as a radiolucent line >1 mm in all three acetabular zones without any signs of migration, rotation, or screw breakage. Osteointegration was evaluated using the criteria described by Moore et al. (26).
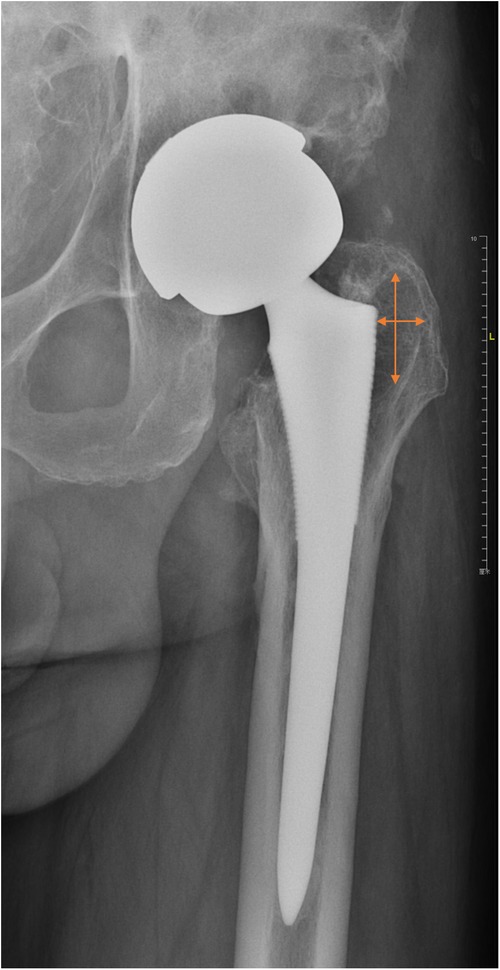
Figure 2. An example to illustrate our measuring approach. Two lines with arrows represent two longest diameters in the horizontal and vertical axes, respectively. Lesion size (cm2) equals the product of two diameters.
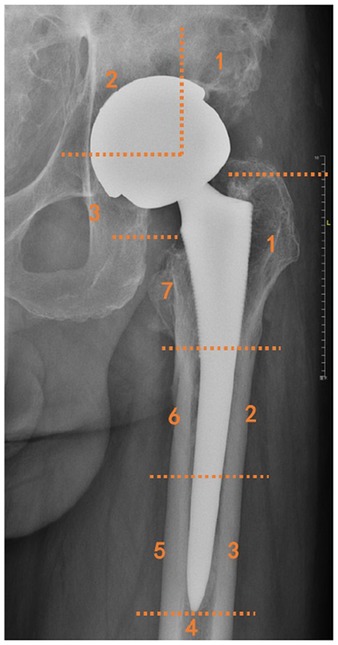
Figure 3. Illustration of femoral and acetabular zone classification by Gruen and by DeLee and Charnley.
Statistical analysis
Statistical analysis was conducted using SPSS version 19.0 for Windows (Inc., Chicago, IL, United States). Quantitative data with normal distribution are presented as means with ranges, and categorical variables are presented as percentages.
Results
Demographic information of patients and their osteolytic lesions
We enrolled 48 revision patients with 59 osteolytic lesions in this study. The patients had a mean follow-up time of 3.33 years, and 30 (62.5%) received revision surgery on the right side. Among the 59 ungrafted osteolytic cavities, 39 (66.1%) were located in zone 1 of the femur according to the Gruen zone classification. Other detailed demographic information of the patients and lesions, such as age at surgery and sex, are shown in Tables 1, 2.

Table 2. Locations of osteolytic cavities according to Gruen classification (femur) and DeLee and Charnley classification (acetabulum).
Change in lesion size during the follow-up period
Based on the previously listed criteria, during the 2-year-minimum follow-up, 11 cavities (18.6%) regressed, while the other cavities remained stable. The mean lesion size of the 39 cavities in zone 1 of the femur reduced from 2.82 cm2 immediately after operation to 2.31 cm2 at the last follow-up (Table 3). The time-dependent change in lesion size of the 11 regressed cavities is illustrated in Figure 4.
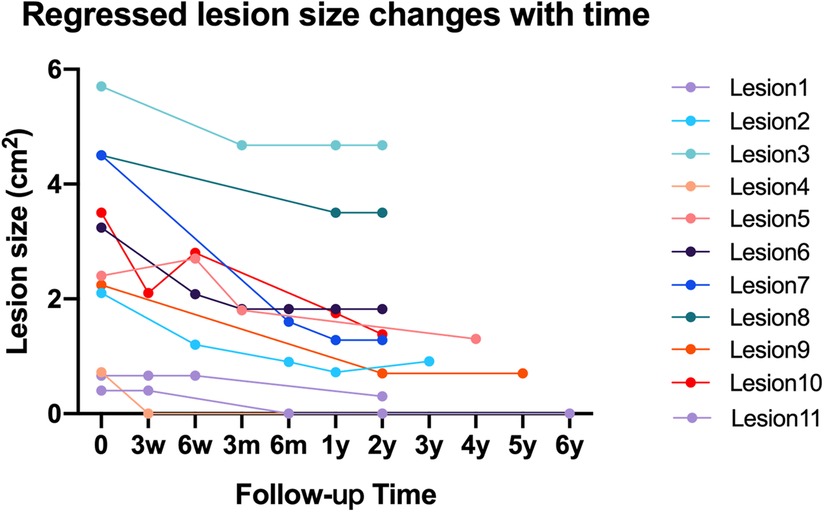
Figure 4. Line chart to show time-dependent change of lesion size in 11 regressed osteolytic lesions.

Table 3. Comparison of lesion sizes between immediate postoperation and last follow-up and the proportion of regressed lesions and stable lesions, respectively.
Short- to mid-term prognosis of ungrafted osteolytic lesions in revision THAs
In addition to the lesion size change, we also focused on the short-term prosthesis stability and integration between the bone and prosthesis. At the last follow-up, all the components remained stable without prosthesis migration. None had new circumferential radiolucent lines or lesions at the last follow-up. Two patients underwent re-revision for dislocation, and the overall survival of our revision THAs was 95.8% (46/48).
Discussion
The incidence of osteolytic cavities is high in revision patients (3), and it is generally accepted that bone graft or metal reinforcement implantation is required to reconstruct the acetabulum for large-scale osteolysis and bone defects that affect structural stability (27–29). Furthermore, whether debridement without bone grafting can be applied in osteolytic cavities that do not affect prosthesis installation and initial stability after related prosthesis revision remains controversial.
Bone grafting is a common surgical method. In a prior study, Verspeek et al. (30) followed 86 hip revision patients for 15 years and found that bone grafts could significantly reduce the risk of re-revision and osteolysis. However, they have inherent disadvantages. Gamradt and Lieberman (17) stated in their research that extravasation during operation may be a source of third-party wear particles, which could have negative effects on bone grafts. In addition, there are still several limitations, such as limited sources and the high cost of bone grafts. According to Beswick and Blom (31), the cost of 1 g of allogeneic cancellous bone is 78–86 US dollars, and even the cost of calcium phosphate cement is 26 US dollars per gram. Furthermore, applied bone grafts are prone to infection. Lee et al. (32) previously reported that in the follow-up of 140 patients with bone grafts, 7.8% developed bone graft infection. Allogeneic bone also has the possibility of self-rejection, and the osteoinduction ability of allogeneic bone itself is damaged by irradiation.
On the other hand, there are also disadvantages to not receiving a bone graft. In theory, osteolysis is a cascading process. Macrophages release inflammatory factors and activate the downstream NF-κB signaling pathway following phagocytosis of foreign particles (such as those released by grafts), and other extensively verified pathways are activated to amplify this signal (33). Whether osteolysis continues to occur if this pathway cannot be completely blocked remains unclear. Owing to the complex shape of bone defects, in some revision surgeries, some osteolytic areas may not be completely cleaned. This is particularly true when the components are retained. In contrast, more complete debridement is performed when components are revised during revision THAs. Few studies have reported the prognosis of ungrafted lesions with prostheses that do not affect structural stability, which is one reason why this study was conducted.
In revision THAs, the bone defect with a small scope, which does not affect prosthesis installation or initial stability during operation, was treated by complete debridement following component removal without bone graft in our hospital. The follow-up results showed that the dissolution area would not continue to expand if complete debridement was performed. Our results provide convincing evidence that complete debridement is an effective alternative to handle small lesions. In addition, our study included patients whose acetabular or femoral components were revised during revision to expand the scope of our conclusion, as complete debridement minimizes the number of particles and further decreases the occurrence of recurrent osteolysis.
This further shows that even if in cases with osteolytic tissue that is invisible to the naked eye or cannot be thoroughly cleaned due to other reasons, when the prosthesis with a large number of wear particles is replaced, osteolysis is blocked after the majority of osteolytic tissue is cleaned. Mochida et al. (34) showed that the concentration of wear particles around the prosthesis is proportional to the degree of osteolysis. In addition, Kobayashi (35) quantitatively analyzed the diameter and number of wear particles around the prosthesis in 18 patients following joint replacement and found that osteolysis was more likely to occur when the number of wear particles per gram of tissue was greater than 1010. Based on the above research, we believe that the residual wear particles were insufficient to initiate the osteolysis process after debridement of the main osteolytic tissue during the operation. In our study, no osteolysis progressed, which indicates that complete debridement following prosthesis removal blocked the recurrence of osteolysis because it minimized the number of particles.
Our research reports the short-to mid-term follow-up of non-grafted patients, showing a similar failure rate compared with that reported in grafted patients in a prior study by Villatte et al., indicating that non-grafting exerts no adverse effect on the short-to mid-term prognosis of patients (36). Moreover, the two re-revision patients in our study underwent surgery for dislocation of the prosthesis, which has little to do with the remaining cavities in the femur or acetabulum.
Nevertheless, this study has several limitations that should be considered. First, we did not compare the lesion size between the patients without bone graft and those with bone graft after component removal. This setting is due to the fact that in recent years, our hospital has rarely performed bone graft treatment for small cavities. It is obviously inappropriate to compare the prognosis of a huge grafted cavity with the small cavity discussed in this paper, and the follow-up time is relatively short. If osteolysis progression is not blocked after revision and continues to worsen, 2 years is sufficient to observe significant changes. Furthermore, if the osteolysis cavity is enlarged during long-term follow-up, it is difficult to judge whether this may be due to the continuous progression of residual osteolysis during the last operation or the new osteolytic lesions caused by the generation of new wear particles. We will continue to follow-up these patients and update our study at an appropriate time point. Finally, the number of patients included in the study was limited. We will continue to pay attention to, and include, such in future, larger patients, and will update the research results in a timely manner.
Conclusion
For some osteolytic cavities that do not affect structural integrity after prosthesis revision, debridement can be performed without bone grafting. This treatment can not only avoid the defects of allogeneic bone and autogenous bone grafts but also block the occurrence and progression of osteolysis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design: HL and JZ. Administrative support: HL. Provision of study materials or patients: JZ and HL. Collection and assembly of data: KK, FL, YC, and HQ. Data analysis and interpretation: KK, FL, and YH. All authors contributed to the article and approved the submitted version.
Funding
This study was sponsored by National Nature Science Foundation of China (Grant Nos. 31900941 and 82072397), Shanghai Municipal Education Commission Two-Hundred Talent, Project of Biobank (YBKA201911), Shanghai Ninth People's Hospital and Interdisciplinary Program of Shanghai Jiao Tong University (Project No. ZH2018QNA06), and Shanghai JiaoTong University Medical and Engineering Cross Fund (Grant No. YG2017MS09).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schwartz BE, Piponov HI, Helder CW, Mayers WF, Gonzalez MH. Revision total hip arthroplasty in the United States: national trends and in-hospital outcomes. Int Orthop. (2016) 40(9):1793–802. doi: 10.1007/s00264-016-3121-7
2. Gwam CU, Mistry JB, Mohamed NS, Thomas M, Bigart KC, Mont MA, et al. Current epidemiology of revision total hip arthroplasty in the United States: national inpatient sample 2009 to 2013. J Arthroplasty. (2017) 32(7):2088–92. doi: 10.1016/j.arth.2017.02.046
3. Kahlenberg CA, Swarup I, Krell EC, Heinz N, Figgie MP. Causes of revision in young patients undergoing total hip arthroplasty. J Arthroplasty. (2019) 34(7):1435–40. doi: 10.1016/j.arth.2019.03.014
4. Kelmer G, Stone AH, Turcotte J, King PJ. Reasons for revision: primary total hip arthroplasty mechanisms of failure. J Am Acad Orthop Surg. (2021) 29(2):78–87. doi: 10.5435/jaaos-d-19-00860
5. Yu S, Saleh H, Bolz N, Buza J, Iorio R, Rathod PA, et al. Re-revision total hip arthroplasty: epidemiology and factors associated with outcomes. J Clin Orthop Trauma. (2020) 11(1):43–6. doi: 10.1016/j.jcot.2018.08.021
6. Sheth NP, Nelson CL, Paprosky WG. Acetabular bone loss in revision total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. (2013) 21(10):601. doi: 10.5435/JAAOS-21-10-601
7. Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. (2007) 454(454):251–61. doi: 10.1097/01.blo.0000238813.95035.1b
8. Firkins PJ, Tipper JL, Saadatzadeh MR, Ingham E, Stone MH, Farrar R, et al. Quantitative analysis of wear and wear debris from metal-on-metal hip prostheses tested in a physiological hip joint simulator. Biomed Mater Eng. (2001) 11(2):143–57.11352113
9. Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. (1992) 74(6):849–63. doi: 10.2106/00004623-199274060-00006
10. Jiang YP, Jia TH, Wooley PH, Yang SY. Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris. Acta Orthop Belg. (2013) 79(1):1–9.23547507
11. Howie DW, Manthey B, Hay S, Vernon-Roberts B. The synovial response to intraarticular injection in rats of polyethylene wear particles. Clin Orthop Relat Res. (1993) (292):352–7. doi: 10.1097/00003086-199307000-00046
12. Urban RM, Hall DJ, Della Valle C, Wimmer MA, Jacobs JJ, Galante JO. Successful long-term fixation and progression of osteolysis associated with first-generation cementless acetabular components retrieved post mortem. J Bone Joint Surg Am. (2012) 94A(20):1877–85. doi: 10.2106/jbjs.J.01507
13. Hall A, Eilers M, Hansen R, Robinson BS, Maloney WJ, Paprosky WG, et al. Advances in acetabular reconstruction in revision total hip arthroplasty. J Bone Joint Surg Am. (2013) 95(18):1709–18. doi: 10.2106/JBJS.9518icl
14. Petis SM, Kubista B, Hartzler RU, Abdel MP, Berry DJ. Polyethylene liner and femoral head exchange in total hip arthroplasty: factors associated with long-term success and failure. J Bone Joint Surg. (2019) 101(5):421–8. doi: 10.2106/JBJS.18.00522
15. Callaghan JJ, Liu SS, Phruetthiphat OA. The revision acetabulum—allograft and bone substitutes vestigial organs for bone deficiency. Bone Joint J. (2014) 96B:70–2. doi: 10.1302/0301-620x.96b11.34452
16. Zhang J, Hu Y, Ying H, Mao Y, Zhu Z, Li H. Reliability and validity test of a novel three-dimensional acetabular bone defect classification system aided with additive manufacturing. BMC Musculoskelet Disord. (2022) 23(1):432. doi: 10.1186/s12891-022-05365-y
17. Gamradt SC, Lieberman JR. Bone graft for revision hip arthroplasty: biology and future applications. Clin Orthop Relat Res. (2003) 417(417):183–94. doi: 10.1097/01.blo.0000096814.78689.77
18. Leung H-B, Fok MW-M, Chow LC-Y, Yen C-H. Cost comparison of femoral head banking versus bone substitutes. J Orthop Surg. (2010) 18(1):50–4. doi: 10.1177/230949901001800111
19. Floeren M, Kappe T, Reichel H. Analysis of the effectiveness of an internal hospital bone bank. Orthopade. (2007) 36(7):667–72. doi: 10.1007/s00132-007-1093-4
20. Stevenson S, Horowitz M. The response to bone allografts. JBJS. (1992) 74:939–50. doi: 10.2106/00004623-199274060-00017
21. DeLee J, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin. Orthop. (1976) 121:20–32. doi: 10.1097/00003086-197611000-00003
22. Maloney WJ, Herzwurm P, Paprosky W, Rubash HE, Engh CA. Treatment of pelvic osteolysis associated with a stable acetabular component inserted without cement as part of a total hip replacement. J Bone Joint Surg Am. (1997) 79(11):1628. doi: 10.2106/00004623-199711000-00003
23. Min BW, Song KS, Cho CH, Bae KC, Lee KJ. Femoral osteolysis around the unrevised stem during isolated acetabular revision. Clin Orthop Relat Res. (2008) 467(6):1501. doi: 10.1007/s11999-008-0499-6
24. Gruen TA, Mcneice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. (1979) 141:17–27. doi: 10.1007/978-1-4471-5451-8_8
25. Li H, Qu X, Mao Y, Dai K, Zhu Z. Custom acetabular cages offer stable fixation and improved hip scores for revision THA with severe bone defects. Clin Orthop Relat Res. (2016) 474(3):731–40. doi: 10.1007/s11999-015-4587-0
26. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. (2006) 444:176–83. doi: 10.1097/01.blo.0000201149.14078.50
27. Gie GA, Linder L, Ling RS, Simon JP, Slooff TJ, Timperley AJ. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg Br. (1993) 75(1):14–21. doi: 10.1302/0301-620X.75B1.8421012
28. Shetty AA, Sharma P, Singh S, Tindall A, Kumar SV, Rand C. Bulk femoral-head autografting in uncemented total hip arthroplasty for acetabular dysplasia. J Arthroplasty. (2004) 19(6):706–13. doi: 10.1016/j.arth.2004.02.032
29. Malahias M-A, Mancino F, Gu A, Adriani M, De Martino I, Boettner F, et al. Acetabular impaction grafting with mesh for acetabular bone defects: a systematic review. Hip Int. (2020) 32(2):185–96. doi: 10.1177/1120700020971851
30. Verspeek J, Nijenhuis TA, Kuijpers MFL, Rijnen WHC, Schreurs BW. What are the long-term results of cemented revision THA with use of both acetabular and femoral impaction bone grafting in patients younger than 55 years?. Clin Orthop Relat Res. (2021) 479(1):84–91. doi: 10.1097/corr.0000000000001462
31. Beswick A, Blom AW. Bone graft substitutes in hip revision surgery: a comprehensive overview. Injury. (2011) 42(Supp-S2):S40–6. doi: 10.1016/j.injury.2011.06.009
32. Lee CH, Chung YS, Lee SH, Yang HJ, Son YJ. Analysis of the factors influencing bone graft infection after cranioplasty. J Trauma Acute Care Surg. (2012) 73(1):255–60. doi: 10.1097/TA.0b013e318256a150
33. Grimaud E, Soubigou L, Couillaud S, Coipeau P, Moreau A, Passuti N, et al. Receptor activator of nuclear factor kappa B ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol. (2003) 163(5):2021–31. doi: 10.1016/s0002-9440(10)63560-2
34. Mochida Y, Bauer TW, Koshino T, Hirakawa K, Saito T. Histologic and quantitative wear particle analyses of tissue around cementless ceramic total knee prostheses. J Arthroplasty. (2002) 17(1):121–8. doi: 10.1054/arth.2002.29330
35. Kobayashi A. Number of polyethylene particles and osteolysis in total joint replacements. A quantitative study using a tissue-digestion method. J Bone Joint Surg Br. (1997) 79(5):844–8. doi: 10.1302/0301-620X.79B5.0790844
36. Villatte G, Erivan R, Salles G, Pereira B, Galvin M, Descamps S, et al. Acetabular bone defects in THA revision: reconstruction using morsellised virus-inactivated bone allograft and reinforcement ring. Seven-year outcomes in 95 patients. Orthop Traumatol Surg Res. (2017) 103(4):543–8. doi: 10.1016/j.otsr.2017.03.008
Keywords: bone grafting, osteolytic lesions, revision total hip arthroplasty (rTHA), osteolysis progression, debridement
Citation: Kong K, Li F, Qiao H, Chang Y, Hu Y, Li H and Zhang J (2023) Debridement without bone grafting prevents osteolytic lesions progression in revision THAs with prosthesis revised. Front. Surg. 9:925940. doi: 10.3389/fsurg.2022.925940
Received: 22 April 2022; Accepted: 7 November 2022;
Published: 6 January 2023.
Edited by:
Zeyu Luo, Sichuan University, ChinaReviewed by:
Xing Du, Chongqing Medical University, ChinaJincheng Wang, Second Affiliated Hospital of Jilin University, China
© 2023 Kong, Li, Qiao, Chang, Hu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiwu Li aHVpd3UxMjIzQDE2My5jb20= Jingwei Zhang emp3X3lzQDE2My5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Keyu Kong
Keyu Kong Fupeng Li
Fupeng Li Hua Qiao
Hua Qiao Yongyun Chang
Yongyun Chang Yi Hu
Yi Hu Huiwu Li
Huiwu Li Jingwei Zhang
Jingwei Zhang