
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 08 June 2022
Sec. Thoracic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.919227
This article is part of the Research Topic Near-Infrared Fluorescence Guided Surgery: State of the evidence from a health technology assessment perspective View all 10 articles
 Emanuele Voulaz1,2*
Emanuele Voulaz1,2* Alberto Testori2
Alberto Testori2 Felice D'Antuono3
Felice D'Antuono3 Umberto Cariboni2
Umberto Cariboni2 Marco Alloisio1,2
Marco Alloisio1,2 Giuseppe Mangiameli1,2
Giuseppe Mangiameli1,2
Localization of small-sized pulmonary nodules is challenging during video-assisted thoracoscopic surgery. Several preoperative strategies have been developed to mark these targets. We describe our localization strategy using a preoperative computed tomography-guided near-infrared dye marking.
Minimally invasive surgery, including video-assisted thoracoscopic surgery (VATS), has been ideal for the resection of small nodules because it results in minimal postoperative morbidity and mortality, less pain, and a better quality of life than with an open thoracotomy (1, 2).
Localizing small-sized pulmonary nodules is challenging during VATS. Thus, several preoperative strategies have been developed to mark these targets (3, 4). Inadequate nodule localization may lead to prolonged operative time with an increased risk of performing an unplanned thoracotomy to find the nodule (5–7).
We present a case of a no smoker 62-year-old man diagnosed with two metachronous left lung metastases (15 and 12 mm) 20 months after surgery for colorectal adenocarcinoma (Figure 1). After discussion in a multidisciplinary meeting, the patient was scheduled to undergo double video-assisted thoracoscopic wedge resection.
We describe and discuss the technical steps of our localization strategy of nodules localization by using a preoperative CT-guided near-infrared dye marking (NIDM).
Written informed consent was obtained from the patient. The first step of the surgery consists of a preoperative CT-guided injection of indocyanine green (ICG). ICG is powder at 50 mg/10 ml and diluted in 20 ml of human albumin 20% (2.5 mg/ml). A 1 ml syringe was filled with the ICG/albumin mixture and connected to a CT-guided percutaneous 21-gauge needle, and the needle lumen was filled with the marking solution prior to the marking procedure. After local anesthesia with lidocaine, a marking solution (1 ml) was injected near the nodules (Figure 2). CT scan was repeated 30 min after the injection to monitor for pneumothorax or air embolism. The entire procedure lasted 60 min and was performed by expert interventional radiologists. Surgery was performed 5 h later. During bi-portal VATS, ICG-FL was visualized using Stryker’s SPY Fluorescence technology with a 30° camera, and the thoracoscopic NIDM detection was immediate (Figure 3). Thoracoscopic instruments were used to clamp the lung to set an imaginary staple line for the intended resection (green area), and wedges resections were performed using manual staplers. After resections, the specimen was observed, and the surgical margin was inspected macroscopically; the operative time was 52 min. The postoperative course was uneventful, and the patient was discharged on day 2. Final pathology confirmed R0 resection for both metastatic nodules.
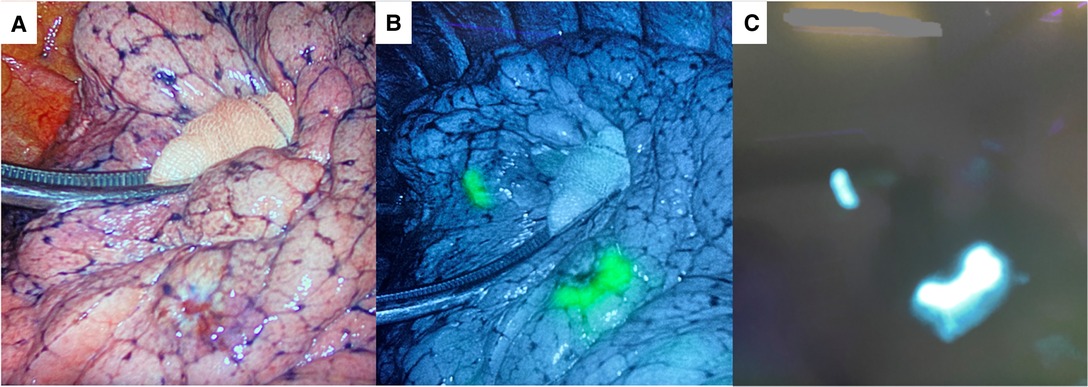
Figure 3. (A) Intraoperative field. Metastatic nodules: infrared (B) and white light (C) signal images.
Detection of small pulmonary nodules has increased with the widespread use of chest CT screening (8, 9). Similarly, surgical resection of pulmonary metastases is nowadays widely performed in selected patients (10).
VATS is the minimally invasive procedure of choice in cases involving unsuccessful diagnosis through percutaneous transthoracic needle biopsy or the need for the resection of pulmonary nodules. However, two significant limits are strictly linked to the VATS procedure: the inability to visualize or palpate the target lesions. Furthermore, ground-glass nodule lesions do not alter the surface of the visceral pleura, and the elevation of tumors cannot be perceived in the deflated lung during VATS (11).
To overcome these problems, several localization methods have been developed to mark small-sized nodules before VATS (5–7). The most common markers are hook–thread, spiral wire needle, microcoil, fiducial marker, or dyes (methylene blue); each is injected into the lung near the target using a CT-guided percutaneous injection approach. Alternatively, barium and lipiodol or gamma-emitting radioisotopes can be injected, and the labeled nodules intraoperatively are detected using fluoroscopy or a gamma probe (12). Each of these methods is currently in clinical use but has unique drawbacks.
Kleedehn et al. reported that hook–wire needle localization has major complications like pneumothorax and pulmonary hemorrhage. The consequent dislodgement of the wire may make the surgical resection even more complex (13). The injection of methylene blue provides an effective, safe, and inexpensive method for intrathoracic localization, but diffusion away from the nodule represents the major limitation of this technique (3). Furthermore, radiation exposure is a problem for all radioisotopes. Asamura et al. first in 1994, and Lizza et al. later described a technique that involves the computed tomography-guided coil injection of a metallic coil and subsequent thoracoscopic resection under roentgenographic fluoroscopy (14, 15).
Our technique presents several specific advantages. First is the reproducibility and the absence of specific skills for an interventional radiologist who usually performs percutaneous TC lung biopsies. Second, the risk of radiation exposure is not present. Furthermore, target localization is usually not complicated by displacement or diffusion. ICG injection is well tolerated by the patient, which can freely move without the risk of any landmark dislocation. By the injection of other radiotracers like methylene blue or lipiodol is not easily identified the precise resection margin while has been described that dyes rapidly diffuse and do not remain in the target lesion. Finally, NIDM does not need immediate surgery because the marking persists from hours to days (3).
On the other side, NIDM presents some disadvantages. In cases of severe pulmonary emphysema, there is concern that liquid markers do not form a distinct spot but rather diffuse into pulmonary cysts; therefore, ICG may not be ideal in such cases. Moreover, the VATS equipment required to visualize ICG-FL is expensive and is available only in a limited number of institutions. The above-described procedure is the first European experience, having been exclusively described in oriental series (3, 4). The use of albumin as a solution for ICG is the first report in the literature.
In our experience, ICG diluted human albumin effectively overcame the diffusion and limitations of most common markers, facilitating accurate localization and resection of pulmonary nodules during VATS. Further investigation, including larger series, could confirm our initial report.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
EV, GM, and FD contributed to the conception and design of the study. AT, UC, and MA revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early-stage lung cancer: a randomised controlled trial. Lancet Oncol. (2016) 17(6):836–44. doi: 10.1016/S1470-2045(16)00173-X
2. Vannucci F, Gonzalez-Rivas D. Is VATS lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer. (2016) 100:114–9. doi: 10.1016/j.lungcan.2016.08.004
3. Anayama T, Hirohashi K, Miyazaki R, Okada H, Kawamoto N, Yamamoto M, et al. Near-infrared dye marking for thoracoscopic resection of small-sized pulmonary nodules: comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg. (2018) 13(1):5. doi: 10.1186/s13019-018-0697-6
4. Yang YL, Li1 ZZ, Huang WC, Zhuang J, Lin DY, Zhong WZ, et al. Electromagnetic navigation bronchoscopic localization versus percutaneous CT-guided localization for thoracoscopic resection of small pulmonary nodules. Thorac Cancer. (2021) 12(4):468–74. doi: 10.1111/1759-7714.13775
5. Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. (1999) 115(2):563–8. doi: 10.1378/chest.115.2.563
6. Saito H, Minamiya Y, Matsuzaki I, Tozawa K, Taguchi K, Nakagawa T, et al. Indication for preoperative localization of small peripheral pulmonary nodules in thoracoscopic surgery. J Thorac Cardiovasc Surg. (2002) 124(6):1198–202. doi: 10.1067/mtc.2002.127331
7. Powell TI, Jangra D, Clifton JC, Lara-Guerra H, Church N, English J, et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum micro coils. Ann Surg. (2004) 240(3):481–8. doi: 10.1097/01.sla.0000137132.01881.57
8. Crucitti P, Gallo IF, Santoro G, Mangiameli G. Lung cancer screening with low dose CT: experience at Campus Bio-Medico of Rome on 1500 patients. Minerva Chir. (2015) 70(6):393–9.25700151
9. Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. (2015) 191(10):1166–75. doi: 10.1164/rccm.201408-1475OC
10. Gonzalez M, Zellweger M, Nardini M, Migliore M. Precision surgery in lung metastasectomy. Future Oncol. (2020) 16(16s):7–13. doi: 10.2217/fon-2018-0713
11. Mangiameli G, Crucitti P, Rocco G. Microsized lung adenocarcinoma vs. small-sized lung adenocarcinoma: clinical characteristics, advantages and surgical implications. J Thorac Dis. (2016) 8(9):E1003–5. doi: 10.21037/jtd.2016.08.04
12. Rho J, Lee JW, Quan YH, Choi BH, Shin BK, Han KN, et al. Fluorescent and iodized emulsion for preoperative localization of pulmonary nodules. Ann Surg. (2021) 273(5):989–96. doi: 10.1097/SLA.0000000000003300
13. Kleedehn M, Kim DH, Lee FT, Lubner MG, Robbins JB, Ziemlewicz TJ, et al. Preoperative pulmonary nodule localization: a comparison of methylene blue and Hookwire techniques. AJR Am J Roentgenol. (2016) 207(6):1334–9. doi: 10.2214/AJR.16.16272
14. Asamura H, Kondo H, Naruke T, Tsuchiya R, Wakao F, Kaneko M, et al. Computed tomography-guided coil injection and thoracoscopic pulmonary resection under roentgenographic fluoroscopy. Ann Thorac Surg. (1994) 58(5):1542–4. doi: 10.1016/0003-4975(94)91957-7
Keywords: preoperative marking for pulmonary nodule, indocyanin green (ICG), lung resection, lung metastases, CT guide
Citation: Voulaz E, Testori A, D'Antuono F, Cariboni U, Alloisio M and Mangiameli G (2022) Preoperative CT-Guided Near-Infrared Dye Marking for Thoracoscopic Resection of Pulmonary Nodules: A Case Report. Front. Surg. 9:919227. doi: 10.3389/fsurg.2022.919227
Received: 13 April 2022; Accepted: 17 May 2022;
Published: 8 June 2022.
Edited by:
Luca Bertolaccini, European Institute of Oncology (IEO), ItalyReviewed by:
Dania Nachira, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2022 Voulaz, testori, D'Antuono, cariboni, Alloisio and mangiameli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emanuele Voulaz ZW1hbnVlbGUudm91bGF6QGh1bWFuaXRhcy5pdA==
Specialty section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.