
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 20 July 2022
Sec. Reconstructive and Plastic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.918912
This article is part of the Research Topic 3D Printing for Surgical Simulation and Training: Innovative Materials and Approaches View all 9 articles
Introduction: Early exposure to practical skills in surgical training is essential in order to master technically demanding procedures such as the design and execution of local skin flaps. Changes in working patterns, increasing subspecializations, centralization of surgical services, and the publication of surgeon-specific outcomes have all made hands-on-training in a clinical environment increasingly difficult to achieve for the junior surgeon. This has been further compounded by the COVID-19 pandemic. This necessitates alternative methods of surgical skills training. To date, there are no standardized or ideal simulation models for local skin flap teaching.
Aim: This systematic review aims to summarize and evaluate local skin flap simulation and teaching models published in the literature.
Materials and Methods: A systematic review protocol was developed and undertaken in accordance with PRISMA guidelines. Key search terms encompassed both “local skin flaps” and “models” or “surgical simulation”. These were combined using Boolean logic and used to search Embase, Medline, and the Cochrane Library. Studies were collected and screened according to the inclusion criteria. The final included articles were graded for their level of evidence and recommendation based on a modified educational Oxford Center for evidence-based medicine classification system and assessed according to the CRe-DEPTH tool for articles describing training interventions in healthcare professionals.
Results: A total of 549 articles were identified, resulting in the inclusion of 16 full-text papers. Four articles used 3D simulators for local flap teaching and training, while two articles described computer simulation as an alternative method for local flap practicing. Four models were silicone based, while gelatin, Allevyn dressings, foam rubber, and ethylene-vinyl acetate-based local flap simulators were also described. Animal models such as pigs head, porcine skin, chicken leg, and rat, as well as a training model based on fresh human skin excised from body-contouring procedures, were described. Each simulation and teaching method was assessed by a group of candidates via a questionnaire or evaluation survey grading system. Most of the studies were graded as level of evidence 3 or 4.
Conclusion: Many methods of simulation for the design and execution of local skin flaps have been described. However, most of these have been assessed only in small cohort numbers, and, therefore, larger candidate sizes and a standardized method for assessment are required. Moreover, some proposed simulators, although promising, are in a very preliminary stage of development. Further development and evaluation of promising high-fidelity models is required in order to improve training in such a complex area of surgery.
Surgical training has become increasingly challenging due to restricted working hours, increasing subspecializations, centralization of surgical services, and the publication of surgeon-specific outcomes (1). All these factors have contributed to limitations in practical surgical training, which have been further confounded by the COVID-19 pandemic. This has encouraged the use of simulated and model-based surgical training and education (2). Simulation training in modern teaching and surgical education allows trainees to practice procedures effectively and safely. It can also have a positive impact on operative outcomes and can provide skills easily transferrable to the clinical setting (2–4).
Local flaps are extensively utilized in soft tissue reconstruction (2), providing wound closure when direct closure is not possible through the mobilization of adjacent skin and subcutaneous tissue (2, 5). The design and execution of flaps is a highly demanding procedure with cognitive and technical difficulties, requiring the design of appropriate flaps with respect for the local anatomy to avoid distortion (6, 7). To gain confidence and expertise in such procedures, extensive exposure and practice is required, which junior trainees lack. The expectations of reaching the level of competence required in the design and execution of a variety of flaps cannot be easily achieved due to the aforementioned causes. This necessitates a realistic simulation model that could provide surgical trainees with exposure to and familiarity with both the cognitive process of planning the flap and the procedural skills of tissue mobilization. Models have the potential benefits of affording frequent practice, skill refinement, and confidence in a safe environment so that the technically challenging execution and design of local flaps can be easily achieved (8).
A flap training model has some essential prerequisites such as cost-effectiveness, multiuse, being widely accessible and available, and last but not least, to mimic tissues closely (4, 9). Many simulator models have been introduced and suggested in the literature; however, there is no standardized or ideal model that has been widely introduced for local flap teaching. The scope of our systematic review is to highlight all the available local flap simulators and teaching models. The aim is to provide a comprehensive summary of the available flap simulation methods for surgical trainees and to provide an insight into further advancements and developments for the design of an ideal surgical flap simulator.
A systematic review protocol was developed in accordance with the Prisma Guidelines (Figure 1) (10). To identify all relevant papers, a comprehensive search strategy was developed. Key search terms encompassed both “local skin flaps” and “models” or “surgical simulation”. These were combined using Boolean logic and used to search Embase, Medline, and the Cochrane Library. These papers were then screened further using specific eligibility and exclusion criteria.
Inclusion criteria were all studies and articles describing teaching or simulation models for training any kind of local flap or flaps.
In addition to having a robust and reproducible search strategy, quality control was maintained by excluding any publications published as only abstracts, letters, and those not written in the English language. Furthermore, models were not developed specifically for local flap simulation, such as those for palatoplasty and abdominal flap, the auricular model and the harvesting hand flap model. In recognition of the aim of this paper to appraise models that give both planning and execution experience, models based on z-plasties alone were not included, as z-plasties, by definition, are not used to fill defects, but they redefine a scar.
Two reviewers (EH, FB) evaluated the studies independently with a third reviewer (TG) resolving any conflicts. The article titles were initially screened to exclude duplicates. Subsequently, the abstracts’ articles were screened using the inclusion and exclusion criteria in order to retrieve the final articles for full-text review and assessment of eligibility.
Data from selected studies were extracted using Microsoft Excel 2019. The data collection included study design, type of flap procedure taught, simulation model, advantages, disadvantages, method of assessment of simulation method/training, number of candidates, origin, level of evidence, and level of recommendation.
The selected studies were graded for their level of evidence and recommendation based on a modified educational Oxford Center for evidence-based medicine classification system, where the level of recommendation of 1 is the highest and 4 is the lowest (11), and assessed further according to the CRe-DEPTH tool for articles describing training interventions in healthcare professionals (12). The CRe-DEPTH tool consists of a set of reporting criteria tools for the development and evaluation of any training interventions for healthcare professionals. It consists of 12 items on 4 main domains/categories. These are (1) development of the training, (2) characteristics of the training, (3) characteristics of the providers, and (4) assessment of the training outcomes. A detailed description of each item is out of scope of this review; however, these are summarized in Table 2. The articles were separated into four different categories according to simulation model type or teaching method as described in each article as follows: (1) 3D simulation model, (2) computer and mobile app simulation models, (3) silicone-based models, (4) animal models, and (5) other material-based models such as gelatin, human skin, allevyn dressings, foam rubber, and acetic ethylene-vinyl acetate.
The initial number of studies post-duplication removal were 349. The final articles sought for retrieval were 61, leading to a final 16 articles that fit the eligibility criteria for final review. The key characteristics of the studies were (1) Type of flap procedure, (2) Simulation model, (3) Evaluation methods, (4) Advantages and Disadvantages as described for each teaching and simulation method, and (5) Number of candidates (Table 1). The models were then categorized into Computer and Virtual Simulation, 3D Simulation Models, Animal Models, and Other Models.
Four articles used 3D simulators for local flap teaching and training, while two articles described computer simulation as an alternative method for local flap practicing. Four models were silicone based, while gelatin, Allevyn dressings, foam rubber, and ethylene-vinyl acetate-based local flap simulators were also described. Animal models such as pigs head, porcine skin, chicken leg and rat, as well as a training model based on fresh human skin excised from body-contouring procedures, were all described (Figure 2). Each simulation and teaching method was assessed by the group of candidates via a questionnaire or evaluation survey grading system. Not all studies provided the cost of production of their proposed model, making it difficult to conclude on a financial basis which was the ideal cost-effective model described so far. One cost-effective model is that of Power et al.'s, who proposed the computer-aided 3D, silicone-based model providing the cost of production estimated at 4.61–8.14$.
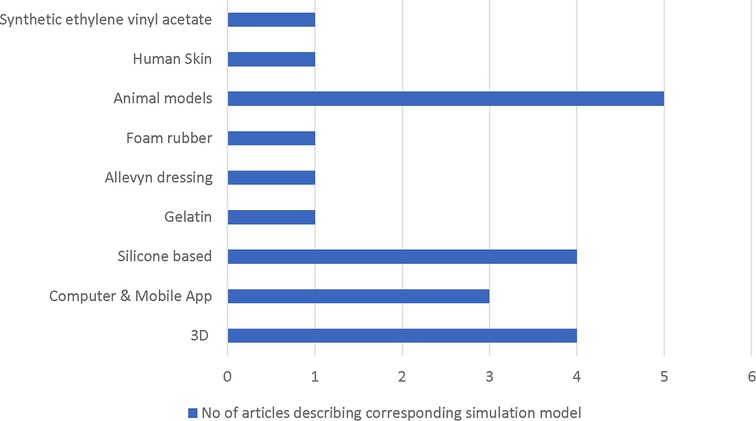
Figure 2. Flowchart indicating the number of included articles describing each category of simulation model.
In 2009, Sifakis et al. described a virtual surgical simulation-incision tool, which is very much in its preliminary stages of development. The idea is to provide the trainee with the virtual surgical incision and retraction tool and the ability to alternate the geometry and topology of the skin and gain a better understanding on the local flap execution and design. In this system, the plastic surgeon must consider the defect created as an organic puzzle and design the optimal pattern to close the defect aesthetically and efficiently (23). Similarly, based on this model, Mitchell et al., in 2016, described a model tested on nine resident candidates. The application was able to record the user's surgical sequences. Although this has been a big advancement with great application potential, further improvements in cleft lip surgery, breast surgery, and facial flaps, such as the use of graphics to show where secondary closure stresses in the skin are the highest, surgical action recording, the need for additional indicator graphics, and the non-use of anatomical structures are required (18). Mobile simulation Apps have also been introduced by Naveed et al. with the development of algorithms and modules that aim to teach key concepts in flap execution and design. In this study, a randomized educational trial was carried out on 18 medical students, and an assessment of the application was performed with MCQs and task analysis score. The control group obtained MCQ scores and task-based assessment scores of 56.73% and 2.58, respectively, while the intervention arm had a 62.95% MCQ score and a score of 3.53 on task assessment. The task assessment score was rated from 1 to 5 and was based on multiple domains, some of which were flap planning, coverage and suturing, excision and undermining, flap marking and planning, demarcation and margins, respect of tissue, etc. This demonstrated a statistically significant difference between the intervention group and the non-intervention group (14).
In a cohort study, Yang et al. presented a 3D-printed facial flap simulator with the aid of a CT scan, manufactured with silicone. Fifteen ENT candidates were involved in this study, with an evaluation survey on the basis of the Likert scale and a blind-folded assessment by consultants. The simulator group gave high ratings across the domains of usefulness, effectiveness, and realism of the model as a training tool. These were graded from 1 (none) to 5 (significant). The results were promising, as the overall satisfaction rate was higher. However, the sample size was small and confined to a single institute, and the mean ratings for realism, for effectiveness as a training tool, improvement in confidence levels, and expertise level were 3.22/5, 4.11/5, 3.89/5, and 3.67/5, respectively. The control group gave average to below average ratings across all survey domains. The average rating scale of 0 to 10 given by an experienced facial plastic surgeon based on the performance of both groups was 8.9 for the simulator group and 7.14 for the control group (2). Similarly, Powell et al. developed a 3-mm skin depth and a 6-mm fat depth by using CT scan. A negative casting mold was designed. Skin-colored silicone was molded on the casting mold. A ten-shore silicone was added as a second layer representing the fat layer. Seven plastic surgery and ENT trainees took a survey and evaluated the simulator on the basis of 1–4/5 Linkert scale, giving a mean domain of 3.29/4 overall on physical attributes, a mean domain of 3.19/4 on rating the realism of experience, and 4.50/5 on the performance of the flaps practiced (13). Kite et al., who studied nine plastic surgery trainees, and Ueda et al., who studied six residents, used a foam core base overlaid with fabricated multiple silicone layers to enable the layers to adhere to each other and a two-layer elastic model with the mold made by salt granules polyurethane for the surface layer and inner silicone layer, extracted by face digital imaging by using CT, MRI stereolithographic data, respectively (15, 16). In the Kite et al.’s study, 9/10 learners reported a better understanding of the local flaps theory and 8/10 candidates reported gaining more confidence in planning and execution underlying local flaps. The realistic experience of practicing undermining with the proposed flap was graded as 7/10. The flap model was scored 7/10 for simulating the design and execution of local flaps accurately (15). The candidates’ response was more generalized in Ueda et al.'s proposed model and was “an enjoyable and realistic experience” (16).
Our systematic review showed that pig heads could be used. However, the study contained a selection bias as candidates were selected to participate (19). Isaacson et al. described the galliform model as a low-cost and reproductive simulation model. A survey of 10 participants showed that the defeathering process removes the epidermis altering the surface, resulting in a thin mobile dermis that is too easy to advance, and lacks the thick layer of dermal fat. Therefore, this will not be adequate for nasal or forehead reconstruction (20). In these two studies, there was no candidate rating or any performed statistical analysis of the teaching method and proposed simulation model. Porcine skin on mannequin heads to give a 3D was found to exhibit similarities to cadaveric head (21). However, candidates found it challenging to practice the flaps around the eyes or mouth as these areas are difficult to replicate. This study only mentions about trainee feedback without further evaluation surveys and assessments compared with previous studies that we have seen so far. Likewise, the skin of rats used in previous experimental studies properly processed has also been described (25). Interestingly, Denadai et al. compared high- (chicken leg and pig foot) and low- (rubberized line bench model synthetic ethylene-vinyl acetate) fidelity models. This comparison showed that the high- and low-fidelity groups displayed similar post-training performances, while the groups’ confidence levels in flap performance were similar compared with that of the control group (22). Participants using the low- and high-fidelity models reported more confidence in handling the rhomboid flap post training, and compared with the control group, their confidence levels were significantly high (P < 0.05).
Only a relatively small number of different techniques and methods has been described in the literature. Silicone-based models in a 3D simulator are the most described. Gelatin, allevyn, foam rubber, and synthetic ethylene-vinyl acetate are alternatives. Taylor et al. and Dinsmore et al. described gelatin- and foam rubber-based simulation models, respectively. The evaluation method in both papers relied on survey questionnaires and evaluation. However, only in the simulation model of Taylor et al., the candidates mentioned satisfaction in resemblance to fascial anatomy, with more than 80% of candidates suggesting that the gelatin model is realistic in terms of resembling the fascial anatomy and 100% opining that the model collates with the essential skills needed for fascial flap and increases the residence competency. Dinsmore et al. found that the simulator contained positive feedback relating to the basic understanding of the design, execution, biomechanics, and application of flaps (17, 26).
Most of the studies were graded as level of evidence 3 or 4 and were categorized in accordance with the Cre-Depth criteria (Tables 1 and 2). Although there are multiple variants among the proposed studies, a comparison of the level of evidence and recommendation between studies shows that the studies by Yang et al. (3D printed facial flap simulator with the aid of CT scan, manufactured with silicone), Naveed et al. (Mobile Simulation app), and Denadai et al. (high-chicken leg, pig foot-, and low-rubberized line bench model synthetic ethylene-vinyl acetate-based fidelity models) have the highest level of evidence, 2b, and the level of recommendation 3. Although measuring realism of the models is relatively objective in nature, not all studies investigated this parameter. The studies that specifically investigated the realism of the model were the silicone-based models of Yang et al., Kite et al., and Powell et al. The realistic experience was highly graded by the candidates, and, therefore, one can conclude that the 3D-based silicone models, with often some manufacturing variations, could resemble fascial anatomy. However, the data obtained in these studies are for small candidate sizes and cohort numbers, and more models are proposed models in the literature that need to be evaluated, making it difficult at this stage to flag the best simulation model.
Naveed et al.’s and Bauer et al.’s teaching method studies met most of the Cre-DePTH criteria. Naveed et al. developed novel algorithms and modules in a mobile simulation App to teach concepts required for various defect reconstruction techniques with additional resources such as videos and formal guidelines made available at relevant points in the simulation. A randomized educational trial was followed using the mobile simulation app with 18 medical students divided into intervention group learning using the new mobile simulation app, and a control group undergoing a text-based self-study. Student knowledge and skills were assessed through MCQ and task analysis. Bauer et al. perfomed two practical courses with 8 modules of 2 h for 10 students. The course modules included the surgical techniques of PRS, such as local flaps in a complex facial defect on pig heads, and were supervised by two OMFS surgeons. The identical initial and final tests examined theoretical knowledge and practical skills. Questionnaires concerning basic demographic data, future career goals, and perception of surgical disciplines before and after the completion of the course were handed out.
The complexity of the processes involved in the planning and execution of flap-based reconstructions is reflected by the variability in simulation models yielded by this review. The inconsistency in outcomes reported between the studies, the lack of a standardized reporting and assessment tool, as well as variation among the study designs themselves, make the drawing of any firm conclusions or assertions unfeasible. To perform a local flap-based reconstruction requires a consideration of a multitude of factors, including the availability of local donor tissue, the effects of redistributing tension on adjacent structures, and ensuring the viability of the transposed tissue. The physical steps of performing this surgery represent an extra dimension in the cognitive process required of an operating surgeon. The emphasis on which of these processes requires honing will depend on the experience of the trainee and their familiarity with dealing with the defect or flap in question. Some studies included in this review focused on the technical execution of flaps as judged by expert faculty; others reported the perceived outcomes and confidence of trainees using the models. This reflects the spectrum of skills that can be honed and addressed with simulation-based training.
Furthermore, the availability of resources will also influence the suitability of a particular flap for a specified application; where reported, the financial costs of all the models are purported to be reasonable. Clearly, these will vary and should be considered in the context of other constraints such as the availability of human or animal tissue as reported in some studies. Clearly, the financial costs associated with digital models are less straightforward to analyze, depending on whether the initial design and programming costs should be considered or whether the costs of simply accessing an established software should be considered, and will be influenced by the direct cost per user.
Several of the models reported in this review are in early stages of development. These have been included as they are doubtless of interest and significance in signposting the potential future directions of simulation training in local flap surgery.
This review is mainly limited by the quality of the included studies, and as such it is difficult to draw firm conclusions as to which model is the best.
In our systematic analysis, most of the described models have been assessed only in small cohort numbers, and therefore larger candidate sizes and standardized methods for assessment are required. Moreover, some proposed simulators, although promising, are still in a very early stage of development. Further development and evaluation of promising high-fidelity models is required to improve training in a complex area of surgery such as this.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
EH extracted data, reviewed articles, and evaluated studies. He wrote a majority of the articles and assembled all papers. FB was the second reviewer, who reviewed the articles and evaluated the studies. He wrote a part of the Introduction and extracted data. TG was the third reviewer, who solved conflicts and differences that occurred during article evaluations. TD drafted the work and carried out the necessary corrections for finalizing the manuscript and for the final analysis. All authors contributed to the article and approved the submitted version. IW oversaw the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Khatib M, Hald N, Brenton H, Barakat MF, Sarker SK, Standfield N, et al. Validation of open inguinal hernia repair simulation model: a randomized controlled educational trial. Am J Surg. (2014) 208(2):295–301. doi: 10.1016/j.amjsurg.2013.12.007
2. Yang SF, Powell A, Srinivasan S, Kim JC, Baker SR, Green GE, et al. Addressing the pandemic training deficiency: filling the void with simulation in facial reconstruction. Laryngoscope. (2021) 131(8):E2444-8. doi: 10.1002/lary.29490
3. Sarker S, Patel B. Simulation and surgical training. Int J Clin Practise. (2007) 61(12):2120–5. doi: 10.1111/j.1742-1241.2007.01435.x
4. Tan SSY, Sarker SK. Simulation in surgery: a review. Scott Med J. (2011) 56(2):104–9. doi: 10.1258/smj.2011.011098
5. Simman R. Wound closure and the reconstructive ladder in plastic surgery. J Am Coll Certif Wound Spec. (2009) 1(1):6–11. doi: 10.1016/j.jcws.2008.10.003
7. Yang S, Truesdale C, Moyer J. Local flaps for facial reconstruction. Open Access Atlas Otolaryngol Head Neck Oper Surg. (2019) 1:5.
8. Ederer IA, Reutzsch FL, Schäfer RC, Wahler T, Daigeler A, Rieger UM, et al. A training model for local flaps using fresh human skin excised during body contouring procedures. J Surg Res. (2021):262:190–6. doi: 10.1016/j.jss.2021.01.011
9. Reznick RK, MacRae H. Teaching surgical skills — changes in the wind. N Engl J Med. (2006) 355(25):2664–9. doi: 10.1056/NEJMra054785
10. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:N71. doi: 10.1136/bmj.n71
11. Carter FJ, Schijven MP, Aggarwal R, Grantcharov T, Francis NK, Hanna GB, et al. Consensus guidelines for validation of virtual reality surgical simulators. Surg Endosc Other Interv Tech. (2005) 19:1523–32. doi: 10.1007/s00464-005-0384-2
12. Ann VH, Veerle D, Peter P, Dimitri B, Sofie V. Criteria for describing and evaluating training interventions in healthcare professions – CRe-DEPTH. Nurse Educ Today. (2020) 84:104254. doi: 10.1016/j.nedt.2019.104254
13. Powell AR, Srinivasan S, Green G, Kim J, Zopf DA. Computer-aided design, 3-D-printed manufacturing, and expert validation of a high-fidelity facial flap surgical simulator. JAMA Facial Plast Surg. (2019) 21(4):327–31. doi: 10.1001/jamafacial.2019.0050
14. Naveed H, Hudson R, Khatib M, Bello F. Basic skin surgery interactive simulation: system description and randomised educational trial. Adv Simul. (2018) 3(1):1–9. doi: 10.1186/s41077-018-0074-5
15. Kite AC, Yacoe M, Rhodes JL. The use of a novel local flap trainer in plastic surgery education. Plast Reconstr Surg. (2018) 6(6):e1786. doi: 10.1097/GOX.0000000000001786
16. Ueda K, Shigemura Y, Otsuki Y, Fuse A, Mitsuno D. Three-dimensional computer-assisted two-layer elastic models of the face. Plast Reconstr Surg. (2017) 140(5):983–6. doi: 10.1097/PRS.0000000000003764
17. Taylor SR, David Chang CW. Gelatin facial skin simulator for cutaneous reconstruction. Otolaryngol Head Neck Surg (US). (2016) 154(2):279–81. doi: 10.1177/0194599815618389
18. Mitchell NM, Cutting CB, King TW, Oliker A, Sifakis ED. A real-time local flaps surgical simulator based on advances in computational algorithms for finite element models. Plast Reconstr Surg. (2016) 137(2):445e–52e. doi: 10.1097/01.prs.0000475793.38984.7e
19. Bauer F, Koerdt S, Rommel N, Wolff KD, Kesting MR, Weitz J. Reconstruction of facial defects with local flaps – a training model for medical students? Head Face Med. (2015) 11(1):1–6. doi: 10.1186/s13005-015-0087-4
20. Isaacson DS, Edmonds PR, Isaacson G. The galliform (Turkey thigh) model for resident training in facial plastic surgery. Laryngoscope. (2014) 124(4):866–8. doi: 10.1002/lary.24350
21. Hassan Z, Hogg F, Graham K. A 3-dimensional model for teaching local flaps using porcine skin. Ann Plast Surg. (2014) 73(4):362–3. doi: 10.1097/SAP.0b013e3182996efd
22. Denadai R, Saad-Hossne R, Raposo-Amaral CE. Simulation-based rhomboid flap skills training during medical education: comparing low- and high-fidelity bench models. J Craniofac Surg. (2014) 25(6):2134–8. doi: 10.1097/SCS.0000000000001094
23. Sifakis E, Hellrung J, Teran J, Oliker A, Cutting C. Local flaps: a real-time finite element based solution to the plastic surgery defect puzzle. Stud Health Technol Inform. (2009) 142:313–8. doi: 10.33233/978-1-58603-964-6-313
24. Bjellerup M. Novel method for training skin flap surgery: polyurethane foam dressing used as a skin equivalent. Dermatol Surg. (2005) 31(9 PART 1):1107–11. doi: 10.1097/00042728-200509000-00004
25. Altinyazar HC, Hosnuter M, Ünalacak M, Koca R, Babucçu O. A training model for cutaneous surgery. Dermatol Surg. (2003) 29(11):1122–4. doi: 10.1046/j.1524-4725.2003.29350.x
Keywords: plastic surgery training, teaching, local flaps, simulation models, local flap design, training
Citation: Hadjikyriacou E, Goldsmith T, Bowerman FI, Dobbs TD and Whitaker IS (2022) Simulation models for learning local skin flap design and execution: A systematic review of the literature. Front. Surg. 9:918912. doi: 10.3389/fsurg.2022.918912
Received: 12 April 2022; Accepted: 29 June 2022;
Published: 20 July 2022.
Edited by:
Stefania Marconi, University of Pavia, ItalyReviewed by:
Eleonora M.C. Trecca, IRCCS Casa Sollievo della Sofferenza Ospedale di San Pio da Pietrelcina, Italy© 2022 Hadjikyriacou, Goldsmith, Bowerman, Dobbs and Whitaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleni Hadjikyriacou ZWxlbmkuaGFkamlreXJpYWNvdUBuaHMubmV0
Specialty Section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.