- 1Department of obstetrics, Heji Hospital Affiliated to Changzhi Medical College, Changzhi, China
- 2Department of obstetrics, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, China
Purpose: To explore the changes of blood lipid and serum inflammatory factors in pregnant women with hypertensive disorder complicating pregnancy (HDP) and the relationship with disease development.
Methods: 107 pregnant women with HDP who had regular prenatal examination in our hospital from July 2018 to July 2021 were selected as the research objects. According to the severity of the disease, they were divided into gestational hypertension group, mild preeclampsia group and severe preeclampsia group. 30 healthy pregnant women who underwent prenatal examination in the same period were selected as the healthy group. Serum total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), lipoprotein-associated phospholipaseA2 (Lp-PLA2), C- reactive protein (CRP), interleukin -6 (IL-6), tumor necrosis factor-α (TNF-α) were measured. Receiver operating characteristic curve (ROC) was used to analyze the predictive value of blood lipid and serum inflammatory factors in pregnant women with HDP.
Results: The levels of serum TC, TG and LDL-C increased with the progression of HDP, the level of serum HDL-C decreased with the progression of HDP (P < 0.05). The levels of serum Lp-PLA2, CRP, IL-6 and TNF-α increased with the progression of HDP (P < 0.05). The AUC of serum TC, TG, HDL-C and LDL-C levels for predicting HDP were 0.759, 0.854, 0.770 and 0.785, respectively. The AUC of serum Lp-PLA2, CRP, IL-6 and TNF-α levels for predicting HDP were 0.873, 0.991, 0.966 and 0.999, respectively.
Conclusion: The levels of blood lipid and serum inflammatory factor are closely related to HDP, which has certain value in predicting the occurrence and development of HDP.
Introduction
Hypertensive disorder complicating pregnancy (HDP) is the main cause of adverse maternal and infant outcomes, with an incidence of 5%–12%, and usually occurring after the 20th week of pregnancy (1). The main typical manifestations of HDP are hypertension, edema and proteinuria, but most pregnant women with mild HDP lack obvious clinical manifestations. Severe HDP can cause coma, convulsion, organ failure and other symptoms, and even lead to complications such as placental abruption, intrauterine fetal death and cerebrovascular events (2, 3). The pathogenesis and etiology of HDP have not been completely clear, and it is generally believed to be related to maternal, placental, fetal and other pathogenic factors (4). According to the severity of the disease, HDP can be divided into gestational hypertension, preeclampsia, eclampsia, pregnancy complicated with chronic hypertension and chronic hypertension complicated with preeclampsia. In HDP, gestational hypertension and preeclampsia are more likely to occur, which may induce maternal and infant death. Therefore, finding effective indicators for early prediction of the occurrence and development of HDP has attracted more and more attention. Studies have shown that the levels of blood lipids and serum inflammatory factors in pregnant women with HDP are often abnormally expressed (5). The purpose of this study is to observe the expression of blood lipid and serum inflammatory factors in pregnant women with HDP with different conditions, in order to carry out early intervention for pregnant women with HDP.
Materials and Methods
Research Object
107 pregnant women with HDP who had regular prenatal examination in our hospital from July 2018 to July 2021 were selected as the research objects. According to the severity of the disease (6), they were divided into gestational hypertension group (42 cases: There was no history of hypertension, and blood pressure began to rise to systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg after 20 weeks of gestation), mild preeclampsia group (36 cases: The systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, and the 24 h proteinuria ≥0.3 g were measured more than twice) and severe preeclampsia group (29 cases: The systolic blood pressure ≥160 mm Hg and/or diastolic blood pressure ≥110 mmHg, and 24 h proteinuria ≥2.0 g or serum creatinine ≥1.2 mg/dL). 30 healthy pregnant women who underwent prenatal examination in the same period were selected as the healthy group. Inclusion criteria: Age ≥20 years old; Gestational week ≥20 weeks; Natural conception; Clear consciousness. Exclusion criteria: With essential hypertension; Multiple pregnancy; Non-assisted reproductive technology conception; The patients have taken drugs that affect lipid metabolism and inflammatory response; Combined diabetes; Complicated with heart disease; There is a history of alcoholism (Drinking more than 14 units of alcohol per week on average, 1 unit = 360 mL beer) and smoking (Smoking more than 1 cigarette per day).
Research Methods
All pregnant women were taken 5 mL of venous blood in the morning fasting state. After anticoagulation and centrifugation, the serum was separated and stored at −70°C for testing. Researchers should do a good job in quality control, require the samples to be tested under the same conditions, and avoid the influence of interference factors on the test results. ① Blood lipid: Serum total cholesterol (TC) and triglyceride (TG) were measured by phosphoglycerol oxidase method, and high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) were measured by direct method. ② Serum inflammatory factors: Serum lipoprotein-associated phospholipaseA2 (Lp-PLA2) was measured by enzymatic kinetic method, serum C-reactive protein (CRP) was measured by latex enhanced transmission turbidimetry, and serum interleukin -6(IL-6) and tumor necrosis factor-α (TNF-α) were measured by enzyme-linked immunosorbent assay.
Statistical Methods
SPSS 22.0 was used to analyze the data. Counting data was expressed by ratio, compared by χ2 test. Measurement data was expressed by mean ± standard deviation, compared by variance analysis. The predicted value was expressed by the ROC area under curve (AUC). The evaluation range of AUC: 0.5–0.7 indicates low predictive value, 0.7–0.9 indicates medium predictive value, ≥0.9 indicates high predictive value. P < 0.05 was statistically significant.
Results
Comparison of General Data in Each Group
There was no significant difference in age, gestational week, pregnancy times, pre-pregnancy body mass index and parity among the groups (P > 0.05) (Table 1).
Comparison of Blood Lipid Levels in Each Group
Compared with healthy group, the levels of serum TC, TG, LDL-C in gestational hypertension group, mild preeclampsia group and severe preeclampsia group all increased, while the level of serum HDL-C decreased. The levels of serum TC, TG and LDL-C increased with the progression of HDP, the level of serum HDL-C decreased with the progression of HDP (P < 0.05) (Figure 1).
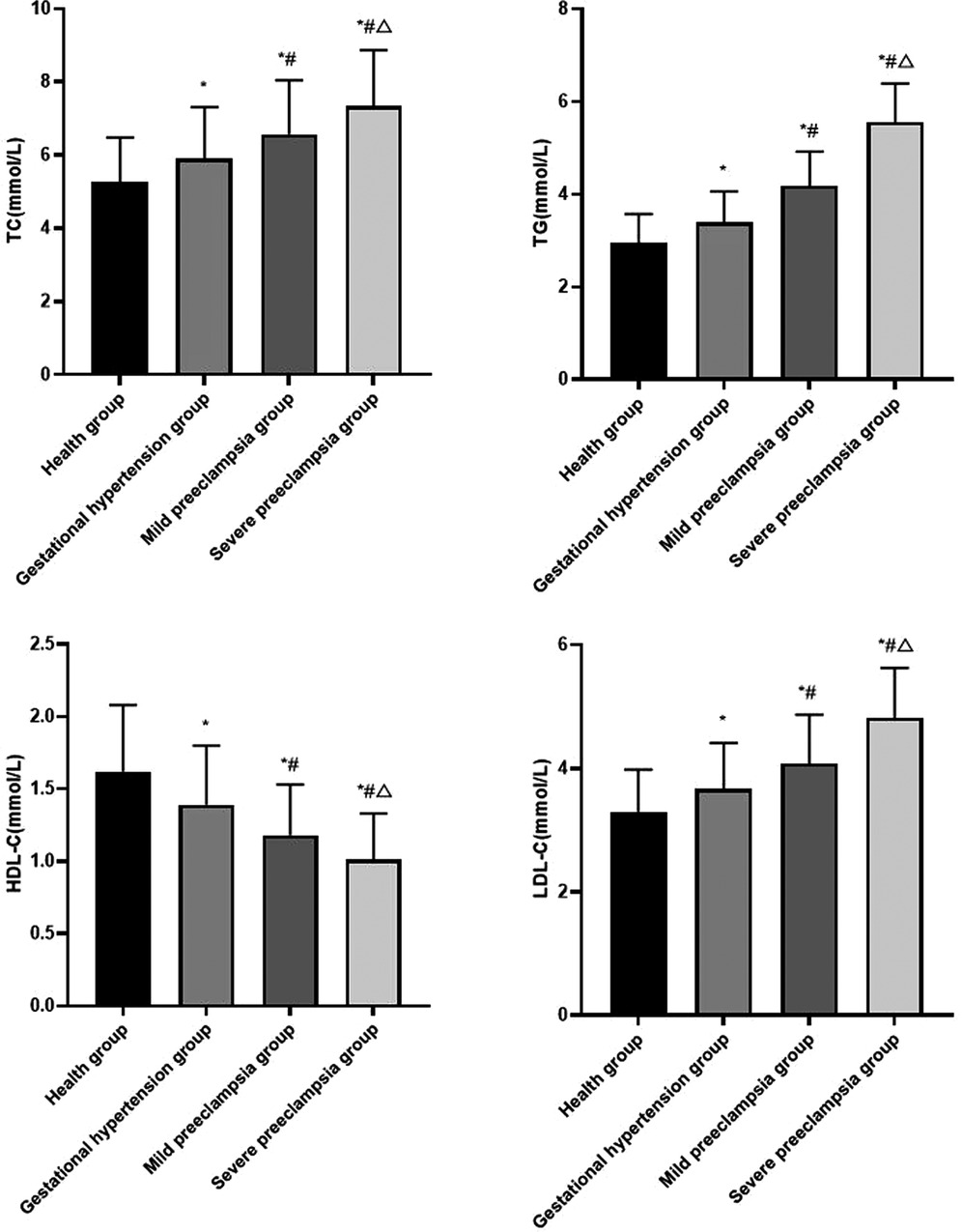
Figure 1. Comparison of blood lipid levels in each group Compared with healthy group, *P < 0.05; Compared with gestational hypertension group, #P < 0.05; Compared with mild preeclampsia group, △P < 0.05.
Comparison of Serum Inflammatory Factors in Each Group
Compared with healthy group, the levels of serum Lp-PLA2, CRP, IL-6 and TNF-α in gestational hypertension group, mild preeclampsia group and severe preeclampsia group all increased. The levels of serum Lp-PLA2, CRP, IL-6 and TNF-α increased with the progression of HDP (P < 0.05) (Figure 2).
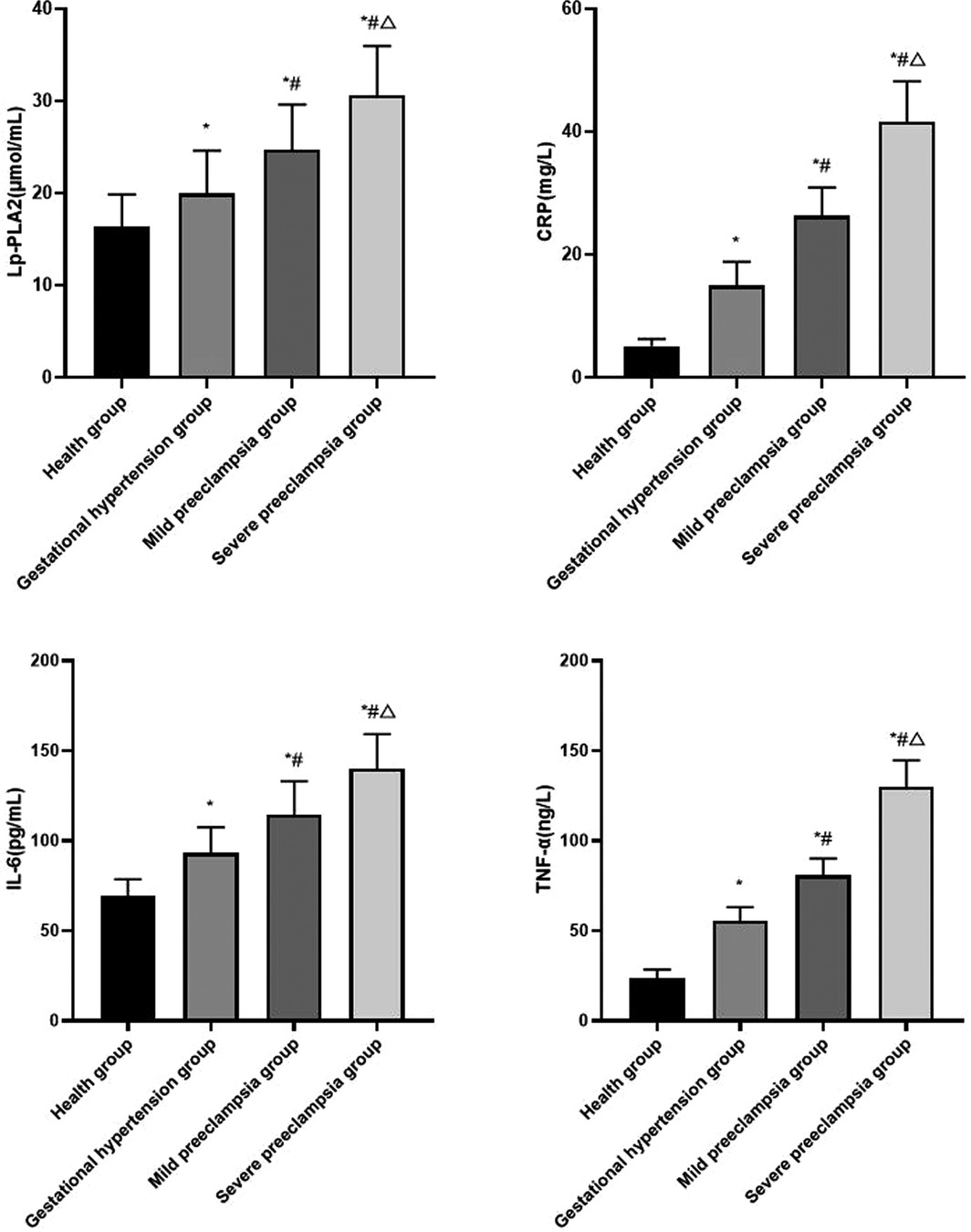
Figure 2. Comparison of serum inflammatory factors in each group Compared with healthy group, *P < 0.05; Compared with gestational hypertension group, #P < 0.05; Compared with mild preeclampsia group, △P < 0.05.
Predictive Value of Blood Lipid Level in Pregnant Women With HDP
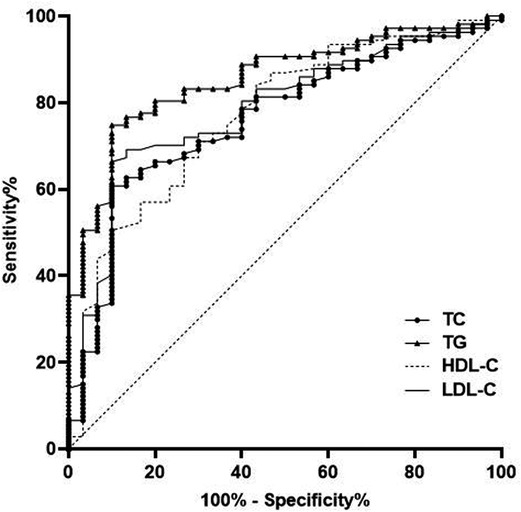
The AUC of serum TC, TG, HDL-C and LDL-C levels for predicting HDP were 0.759, 0.854, 0.770 and 0.785, respectively (Table 2 and Figure 3).
Predictive Value of Serum Inflammatory Factors in Pregnant Women With HDP
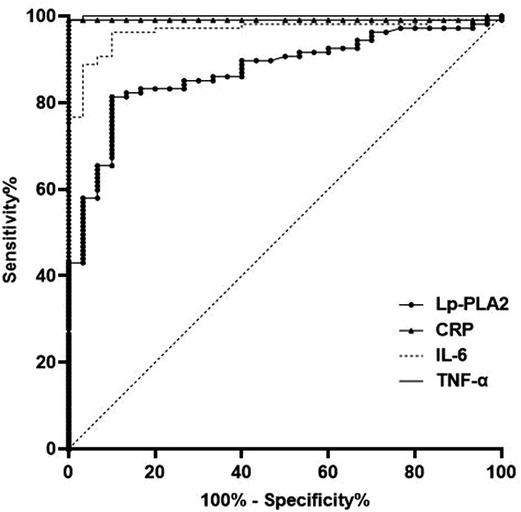
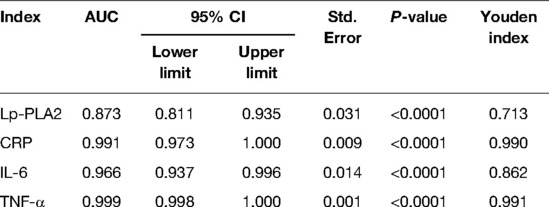
The AUC of serum Lp-PLA2, CRP, IL-6 and TNF-α levels for predicting HDP were 0.873, 0.991, 0.966 and 0.999, respectively (P < 0.05) (Table 3 and Figure 4).
Discussion
At present, clinically, it is generally believed that the occurrence of HDP is related to heredity, immunity, nutritional deficiency, inflammatory reaction, vascular endothelial damage and other factors, and its occurrence and development are affected by environmental and psychological factors (7). The progress of HDP is closely related to the life and health of mother and infant, and the disease has gradually received attention in the field of pregnancy complications. Therefore, it is clinically necessary to judge the severity of HDP by monitoring serum indicators, and then improve the maternal and infant outcomes.
In this study, the levels of serum TC, TG and LDL-C increased with the progression of HDP, while the level of serum HDL-C decreased with the progression of HDP. This indicated that dyslipidemia may increase the risk of HDP, and it is closely related to the development of HDP. TC is the precursor of bile acids, steroid hormones and other substances, which is closely related to atherosclerosis and cardiovascular diseases. The main function of TG is to supply and store energy, and it can also fix and protect internal organs. LDL-C is a risk factor for vascular endothelial injury, and it can cause atherosclerosis. HDL-C is a vascular protective factor, which can remove lipids from blood vessels. In pregnant women with HDP, with the aggravation of the disease, the levels of TC, TG, LDL-C with vascular destruction increased, while the level of HDL-C with vascular protection decreased (8). High levels of TC and TG will lead to the decrease of prostacyclin synthesis in endothelial cells, resulting in imbalance between thromboxane A2 and prostacyclin, and the spasm of blood vessels, which will lead to the occurrence of preeclampsia (9). LDL-C is easily oxidized, and participates in the formation of atherosclerotic plaque, damages smooth muscle cells and endothelial cells, increases the permeability of vascular endothelial cells, reduces arterial lumen and increases peripheral resistance, which leads to the increase of blood pressure (10). The decrease of HDL-C may weaken the protective mechanism against atherosclerosis, inhibit the anti-lipid accumulation, leading to an increase in blood lipid peroxide, which directly damages the vascular endothelial cells, which will aggravate the severity of preeclampsia (11). In addition, estrogen, as a substance that reduces the lipase activity of liver endothelial cells, plays an inhibitory role in the absorption of HDL-C by the liver. Compared with normal pregnant women, pregnant women with HDP have less estrogen secretion and lower HDL-C release, which leads to compensatory responses and increases in serum TC, TG and LDL-C levels, further affects vascular endothelial cell functions and aggravates atherosclerosis (12). Elevated blood pressure can promote the formation of acute atherosclerosis, which in turn can promote the contraction of blood vessels, resulting in thickening and hardening of blood vessel walls, slow blood flow, obstruction of blood supply and oxygen supply to placenta, resulting in further increase of blood pressure, forming a vicious circle (13). The disorder of lipid metabolism in pregnant women with HDP can aggravate atherosclerosis, enhance oxidative stress, increase the production of peroxide products and toxic substances, and then damage vascular endothelial cells (14). At the same time, abnormal blood lipid levels can reduce the synthesis of nitric oxide, resulting in vasoconstriction and relaxation disorders, showing systemic arteriolar spasm and abnormally high blood pressure, which provides conditions for the disease progression of HDP (15).
We also found that the levels of serum Lp-PLA2, CRP, IL-6 and TNF-α were positively correlated with the severity of HDP. Lp-PLA2 can promote the release of inflammatory mediators, activate inflammatory response and damage vascular endothelial cells, which is an independent risk factor for preeclampsia. The possible mechanisms of Lp-PLA2 inducing HDP are as follows: ① Lp-PLA2 can combine apolipoprotein B with low density lipoprotein, causing lipid metabolism disorder, and then leading to atherosclerosis of uterine and placental arteries, resulting in decreased placental function, leading to the occurrence of HDP. ② Lp-PLA2 can hydrolysis and oxidation of lecithin, and produce strong pro-inflammatory mediators such as free fatty acids and lysolecithin. By activating cytokines and adhesion factors, vascular endothelial cells become dysfunctional, thereby increasing blood pressure. ③ Lp-PLA2 promotes the release of inflammatory mediators, collagen deposition and fibroblast proliferation in inflammatory reaction sites, and platelet aggregation, which plays an anti-inflammatory and anti-atherosclerosis role and promotes the progress of HDP (16–18). As a marker of inflammatory reaction in the body, CRP can activate platelet activity, inhibit platelet activating factor, promote platelet release of arachidonic acid and platelet aggregation, and inhibit the binding of platelet activating factor and neutrophils, which plays a role in regulating inflammatory reaction (19). CRP can not only aggravates the inflammatory reaction, but also reduces the sensitivity of tissue cells to insulin, and play a certain role in lipid metabolism disorder (20). There is inflammation in artery wall of pregnant women with HDP, and there are many trophoblast cells in blood from placenta, which can combine with antibodies in maternal blood to form immune complexes. CRP can combine with immune complex, activate the complement system of the body, produce a large amount of terminal reaction protein deposition, resulting in vascular endothelial cell damage, increase the production of vasoconstrictor substances, and then cause contraction and spasm of small blood vessels in the whole body, and increase blood pressure (21). The body of pregnant women with HDP is in a state of oxidative stress, which can promote neutrophil infiltration, release of a variety of proteases, inhibit vascular endothelial function, and lead to the increase of IL-6 and TNF-α levels (22). IL-6 can maintain chronic inflammation and promote the cascade amplification of inflammation. At the same time, IL-6 can interact with a variety of cytokines and induce with TNF-α, IL-1 and other inflammatory factors in the body (23). High levels of IL-6 can damage vascular endothelial cells, increase vascular permeability, interfere with vasoconstriction and relaxation, and lead to an increase in blood pressure, eventually leads to a series of clinical manifestations of preeclampsia (24). The increase of TNF-α can directly damage the vascular endothelium by releasing oxygen free radicals, causing systemic arteriole spasm, resulting in lumen stenosis and increased peripheral resistance, thereby exacerbating the progression of HDP (25). In pregnant women with HDP, TNF-α can promote the formation of neovascularization and thrombosis, inhibit the activities of lipolytic acid and lipoprotein lipic acids, which makes it difficult for lipids to dissolve, and then deposits on the wall of blood vessels, resulting in an increase in blood pressure (26).
In addition, ROC was used for further analysis in this study. The results showed that the AUC of serum TC, TG, HDL-C and LDL-C levels for predicting HDP were 0.759, 0.854, 0.770 and 0.785, respectively. The AUC of serum Lp-PLA2, CRP, IL-6 and TNF-α levels for predicting HDP were 0.873, 0.991, 0.966 and 0.999, respectively. This results further confirmed that the levels of blood lipid and serum inflammatory factor are closely related to HDP, which has certain value in predicting the occurrence and development of HDP. This is helpful to judge the occurrence of HDP and evaluate the status of HDP.
Conclusion
To sum up, the levels of blood lipid and serum inflammatory factor are closely related to HDP, which has certain value in predicting the occurrence and development of HDP. We believe that dietary control and lifestyle changes can control blood lipids and reduce inflammation, and drug treatment can be used when necessary, which is conducive to improving the pathological changes of HDP. The sample size of this study is small, and a large number of clinical samples are still needed to provide further theoretical basis in the future, and it is also necessary to further explore the specific pathogenesis of HDP.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by This study was approved by the ethics committee of our hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WC is the mainly responsible for the writing of the article. YG is mainly responsible for research design. XY is mainly responsible for data analysis. DZ is responsible for the guidance of the entire research. The corresponding author is WC and she is responsible for ensuring that the descriptions are accurate and agreed by all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. (2020) 75:2323–34. doi: 10.1016/j.jacc.2020.03.028
2. Sinkey RG, Battarbee AN, Bello NA, Ives CW, Oparil S, Tita ATN. Prevention, diagnosis, and management of hypertensive disorders of pregnancy: a comparison of international guidelines. Curr Hypertens Rep. (2020) 22:66. doi: 10.1007/s11906-020-01082-w
3. Vahedi FA, Gholizadeh L, Heydari M. Hypertensive disorders of pregnancy and risk of future cardiovascular disease in women. Nurs Womens Health. (2020) 24:91–100. doi: 10.1016/j.nwh.2020.02.001
4. Magro-Malosso ER, Saccone G, Di Tommaso M, Roman A, Berghella V. Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2017) 96:921–31. doi: 10.1111/aogs.13151
5. Zhang Y, Lan X, Cai C, Li R, Gao Y, Yang L, et al. Associations between maternal lipid profiles and pregnancy complications: a prospective population-based study. Am J Perinatol. (2021) 38:834–40. doi: 10.1055/s-0039-3402724
6. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803
7. Tan S, Tan X, Chi Z, Zhang D, Li W. In vitro assessment of the toxicity of lead (Pb2+) to phycocyanin. Chemosphere. (2018) 192:171–7. doi: 10.1016/j.chemosphere.2017.10.159
8. Stoenescu M, Şerbănescu MS, Dijmarescu AL, Manolea MM, Sandulescu S, Vrabie S, et al. Maternal lipid profile as predictor for mother and fetus outcome-an artificial neural network approach. Curr Health Sci J. (2021) 47:215–20. doi: 10.12865/CHSJ.47.02.11
9. Zhang ZQ, Chen AP, Yu T, Yang SJ, Yu DS, Yang R, et al. Exploring the pharmacological mechanism of danhe granulesagainst hyperlipidemia by means of network pharmacology and verified by preliminary experiments. World J Tradit Chin Med. (2021) 7:436–44. doi: 10.4103/wjtcm.wjtcm_59_21
10. Mou AD, Barman Z, Hasan M, Miah R, Hafsa JM, Das Trisha A, et al. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci Rep. (2021) 11:21339. doi: 10.1038/s41598-021-00839-w
11. Niyaty S, Moghaddam-Banaem L, Sourinejad H, Mokhlesi S. Are maternal metabolic syndrome and lipid profile associated with preterm delivery and preterm premature rupture of membranes? Arch Gynecol Obstet. (2021) 303:113–9. doi: 10.1007/s00404-020-05738-5
12. Hessami K, Kasraeian M, Asadi N, Vafaei H, Foroughinia L. Association of maternal and umbilical cord blood lipid parameters with uterine and fetal-placental blood flow in hypertensive and normotensive pregnancies. Int J Womens Health. (2020) 12:115–25. doi: 10.2147/IJWH.S233029
13. Cabunac P, Karadžov Orlić N, Ardalić D, Banjac G, Ivanišević J, Janać J, et al. Unraveling the role of oxidative stress and lipid status parameters in the onset of preeclampsia. Hypertens Pregnancy. (2021) 40:162–70. doi: 10.1080/10641955.2021.1921790
14. Adank MC, Benschop L, Peterbroers KR, Smak Gregoor AM, Kors AW, Mulder MT, et al. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am J Obstet Gynecol. (2019) 221:150.e1–e13. doi: 10.1016/j.ajog.2019.03.025
15. Serrano-Berrones MÁ, Barragán-Padilla SB. Study on the association of hypertriglyceridemia with hypertensive states of pregnancy. Gac Med Mex. (2019) 155:S17–S21. doi: 10.24875/GMM.M19000284
16. Qiao J, Zhou K, Huang C, Fu S, Xing Y, Zhang B. Comparison of serum Lp-PLA2 levels in ischemic stroke patients with H-type hypertension or non-H-type hypertension. J Clin Lab Anal. (2020) 34:e23068. doi: 10.1002/jcla.23068
17. De Mauri A, Vidali M, Chiarinotti D, Bellomo G, Rolla R. Lipoprotein-associated phospholipase A2 predicts cardiovascular events in dialyzed patients. J Nephrol. (2019) 32:283–8. doi: 10.1007/s40620-018-0521-3
18. Fras Z, Tršan J, Banach M. On the present and future role of Lp-PLA2 in atherosclerosis-related cardiovascular risk prediction and management. Arch Med Sci. (2020) 17:954–64. doi: 10.5114/aoms.2020.98195
19. Hamadeh R, Mohsen A, Kobeissy F, Karouni A, Akoum H. C-reactive protein for prediction or early detection of pre-eclampsia: a systematic review. Gynecol Obstet Invest. (2021) 86:13–26. doi: 10.1159/000515530
20. Chen H, Zhang J, Qin F, Chen X, Jiang X. Evaluation of the predictive value of high sensitivity C-reactive protein in pregnancy-induced hypertension syndrome. Exp Ther Med. (2018) 16:619–22. doi: 10.3892/etm.2018.6246
21. Vecchié A, Bonaventura A, Carbone F, Maggi D, Ferraiolo A, Carloni B, et al. C-reactive protein levels at the midpregnancy can predict gestational complications. Biomed Res Int. (2018) 2018:1070151. doi: 10.1155/2018/1070151
22. Kong D, Wang H, Liu Y, Li H, Wang H, Zhu P. Correlation between the expression of inflammatory cytokines IL-6, TNF-α and hs-CRP and unfavorable fetal outcomes in patients with pregnancy-induced hypertension. Exp Ther Med. (2018) 16:1982–6. doi: 10.3892/etm.2018.6393
23. Gencheva D, Nikolov F, Uchikova E, Mihaylov R, Pencheva B, Vasileva M. Interleukin-6 and its correlations with maternal characteristics and echocardiographic parameters in pre-eclampsia, gestational hypertension and normotensive pregnancy. Cardiovasc J Afr. (2021) 32:1–9. doi: 10.5830/CVJA-2021-040
24. Wang Y, Gu Y, Alexander JS, Lewis DF. Preeclampsia status controls interleukin-6 and soluble IL-6 receptor release from neutrophils and endothelial cells: relevance to increased inflammatory responses. Pathophysiology. (2021) 28:202–11. doi: 10.3390/pathophysiology28020013
25. Jayaram A, Deer E, Amaral LM, Campbell N, Vaka VR, Cunningham M, et al. The role of tumor necrosis factor in triggering activation of natural killer cell, multi-organ mitochondrial dysfunction and hypertension during pregnancy. Pregnancy Hypertens. (2021) 24:65–72. doi: 10.1016/j.preghy.2021.02.006
Keywords: hypertensive disorder complicating pregnancy, blood lipid, inflammatory factors, preeclampsia, predictive value
Citation: Chen W, Guo Y, Yao X and Zhao D (2022) Correlation of Blood Lipid and Serum Inflammatory Factor Levels With Hypertensive Disorder Complicating Pregnancy. Front. Surg. 9:917458. doi: 10.3389/fsurg.2022.917458
Received: 11 April 2022; Accepted: 31 May 2022;
Published: 17 June 2022.
Edited by:
Songwen Tan, Central South University, ChinaReviewed by:
Lihong Chen, The First Affiliated Hospital of Fujian Medical University, ChinaFang Wei, Second Xiangya Hospital, Central South University, China
Copyright © 2022 Chen, Guo, Yao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangxiang Chen Y3d4Y2dsMDUwMTAxQDE2My5jb20=
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Wangxiang Chen
Wangxiang Chen Yan Guo2
Yan Guo2