- Department of Breast Surgery, Henan Provincial People’s Hospital; Zhengzhou University People’s Hospital; Henan University People’s Hospital, Zhengzhou, China
Objective: This study aims to investigate the potential prognostic value of fibrinogen-to-albumin ratio (FAR) in patients with triple-negative breast cancer (TNBC).
Methods: This study used a retrospective design and enrolled 224 patients with TNBC treated between January 2009 and December 2014 at the Henan Provincial People’s Hospital. The receiver operating characteristic curve (ROC) was used to determine the optimal cut-off value for FAR. The associations between TNBC and clinicopathologic categorical variables by FAR were analyzed using the Chi-square test or Fisher’s exact test. The survival time and survival curve were determined by Kaplan-Meier survival analysis and compared using the Log-rank method. The potential prognostic factors were determined using univariate and multivariate Cox proportional hazard regression models. Prognostic nomogram was established on the basis of the multivariate analyses. The calibration curves were used to assess the predictive performance.
Results: The optimal cut-off value for FAR based on the overall survival (OS) was 0.066, as evaluated by the ROC. The 224 included patients were divided into low FAR group (<0.066) and high FAR group (≥0.066). Univariate and multivariate models shown that FAR was an potential prognostic factor for disease-free survival (DFS) and OS in patients with TNBC. The median DFS and OS of the low FAR group were longer than those of the high FAR group (χ2 = 15.080, P = 0.0001; χ2 = 13.140, P = 0.0003), including for pre-menopausal patients, and those with pathological stages I + II, and lymph vessel invasion. A nomogram based on the potential prognostic factors was efficient in predicting 3-, and 5-year DFS and OS survival probabilities.
Conclusions: The FAR, which is tested routinely and is characterized by its simplicity, objectivity, and inexpensiveness, is a potential prognostic factor of TNBC, and is potentially applicable in clinical practice.
Introduction
Breast cancer is the most common malignant tumor in females, particularly among the aging population, and its incidence rate is increasing yearly, and has become an important cause of cancer-related morbidity and mortality worldwide (1). In the 2020 global statistics, there were 19.29 million new cancer cases including 9.23 million female cases; and of the 2.26 million new breast cancer cases, about 680,000 died (2). Moreover, from the China National Cancer Center data, of the 272,400 new cancer cases, about 70,700 died (3, 4). Moreover, western lifestyle and dietary changes are the important reasons for the rapid rise in breast cancer disease burden (3, 4). Triple-negative breast cancer (TNBC) is defined by the absence of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2), and accounts for 20% of all invasive breast cancers (5). Furthermore, TNBC is characterized by high invasiveness, easy recurrence and metastasis, and poor prognosis; and it is not sensitive to traditional endocrine or targeted therapy (6).
It has been reported that tumor-associated inflammatory response (TAIR) has a critically important role in the occurrence, development of lymph node metastasis, treatment, and prognosis of tumors, and has received increasing attention from researchers (7, 8). In the occurrence and process of development of malignant tumors, coagulation dysfunction occurs with tumors, which increases the risk of thrombosis (9). Thrombosis promotes the proliferation, invasion, and metastasis of malignant tumor cells by inhibiting the functions of growth factors and natural killer cells (NK cells) (10, 11). Fibrinogen (FIB) and albumin (ALB) have attracted wide attention as noninvasive prognostic factors of various cancers. FIB is a soluble serum glycoprotein synthesized by hepatocytes, and participates in blood coagulation and platelet aggregation (12). Hypercoagulable state, acute infection, and malignant tumor can cause changes in plasma FIB levels, and the plasma FIB is driven by interleukin and cytokines (13). ALB is an important factor of nutritional status, and hypoproteinemia is a reliable indicator of malignant tumor cachexia and malnutrition (14). Moreover, malnutrition is often accompanied by autoimmune dysfunction, which can accelerate the replication and proliferation of tumor cells, and also reflects systemic inflammatory response in patients with tumor (15). Furthermore, the FAR, based on FIB and ALB, has gained credibility as a promising inflammation-based prognostic indicator in various solid tumors (16–18). Although low FAR is found to be associated with poor survival in breast cancer; however, FAR has rarely been studied in TNBC patients. Therefore, our study aimed to investigate the prognostic significance of FAR in patients with TNBC and provide a reference for the treatment of TNBC.
Materials and Methods
Ethics Approval and Informed Consent
The study design was approved by the Ethics Committee of Henan Provincial People’ s Hospital. Written informed consent was obtained from all enrolled participants. This study complied with the standards of the Declaration of Helsinki and its subsequent amendments or similar ethical standards.
Study Population
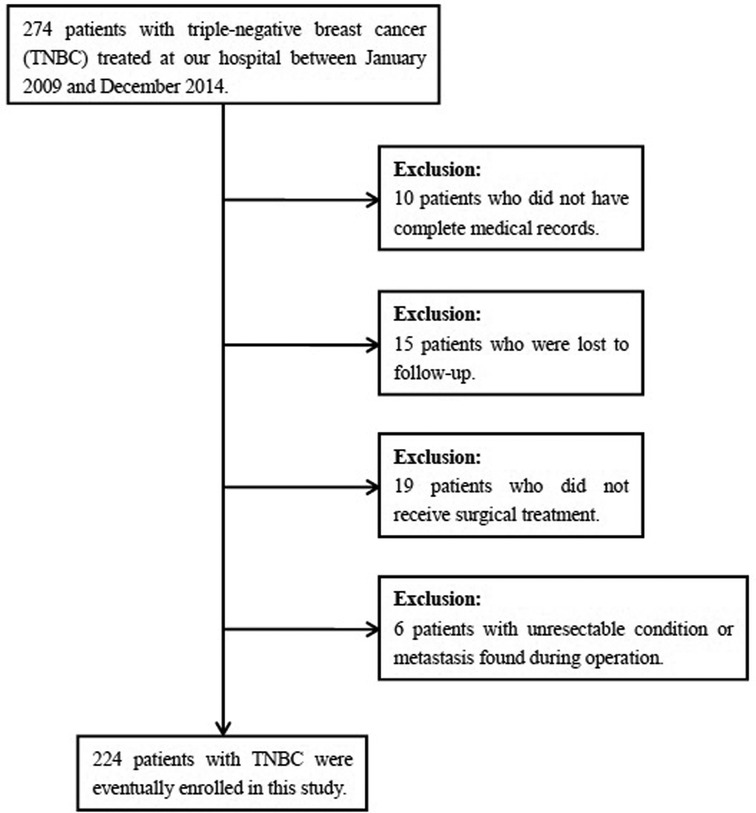
In total, 274 patients with TNBC were treated at Henan Provincial People’ s Hospital between January 2009 and December 2014. According to the inclusion and exclusion criteria, 224 patients were eventually included, while the remaining 70 patients were excluded (Figure 1). All patients were histologically-confirmed. Clinicopathologic features, detailed treatment, and follow-up data of the patients were extracted from the medical records.
Inclusion Criteria and Exclusion Criteria
The following inclusion criteria were used to select patients who: (1) were confirmed by pathology and classified as TNBC subtype; (2) had complete medical records and follow-up information; (3) Karnofsky performance status (KPS) scores ≥80 and Zubrod-Eastern Cooperative Oncology Group (ECOG)-WHO <2; (4) pathological TNM stages I–III; and (5) the blood samples were collected prior to the treatment. The following patients met the exclusion criteria: (1) those with synchronous, metachronous tumors, or distant metastases; (2) those with acute or chronic inflammatory diseases (hypertension, diabetes, and immune system diseases); (3) those receiving antitumor therapy; and (4) those who were administered anti-inflammatory medications.
Evaluation
The TNM stage classification was according to the eighth edition of the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) (19, 20). Hematoxylin and eosin (HE) staining was performed to detect lymph vessel invasion (LVI) and neural invasion in breast cancer tissues.
Peripheral Venous Blood Parameters
Peripheral venous blood was collected at definite time points before treatment. FAR’s definition was as follows: FAR = [FIB/ALB], where FIB and ALB were pretreatment peripheral FIB and ALB counts, respectively.
Follow up
All patients were followed-up regularly by telephone, or as inpatients or outpatients. The postoperative schedule included reexamination every three months for the first and second years, every six months for the third through fifth year, and then at twelve-monthly intervals thereafter. The following follow-up procedures were performed: clinical examination with laboratory tests (routine blood tests, blood biochemistry), ultrasonography of the breast, mammography, and other examinations. Disease-free survival (DFS) was defined as the time from surgery to recurrence or progression, death from any cause, or the last follow-up while overall survival (OS) was the time from surgery to death or last follow-up.
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA), GraphPad Prism software, version 8.0 (La Jolla, CA, USA) and R (version 3.6.0; Vienna, Austria. URL: http://www.R-project.org/). The receiver operating characteristic (ROC) curve was used to determine the optimal cutoff value of FAR, based on which FAR was categorized into two groups. Clinicopathologic categorical variables were analyzed using the Chi-square test or Fisher’s exact test. The Kaplan-Meier method and Log-rank test were used to determine the survival time and survival curve. Univariate and multivariate Cox proportional hazards regression models were used to evaluate the independent prognostic factors. The hazard ratio (HR) and 95% confidence intervals (CIs) for the risk of recurrence associated with FAR and breast cancer prognosis were calculated. Prognostic nomogram was established according to the multivariate analyses. The calibration curve was used to assess the predictive performance. Statistical significance was set at P < 0.05.
Results
Patient Clinicopathologic Characteristics
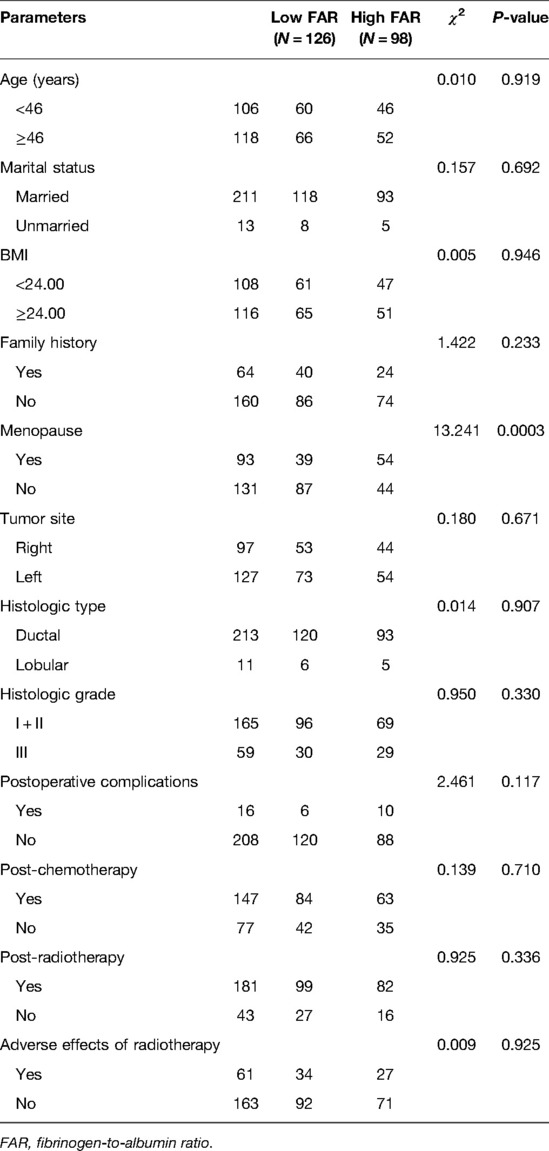
The demographic and clinicopathologic characteristics of the all female, 224 pathologically-diagnosed patients with TNBC were summarized in Table 1. The median age was 46 years, and ranged from 25 to 72 years. Of the 224 enrolled patients, 211 cases were married, 13 cases were unmarried; and 131 cases were premenopausal, 93 cases were postmenopausal, respectively. The patients were stratified into two groups: the low FAR (<0.066) group and high FAR (≥0.066) group, according to the optimal FAR cutoff value of 0.066, evaluated by ROC. There were 126 (56.25%) cases in low FAR group and 98 (43.75%) cases in high FAR group, respectively. Significant differences occurred with being menopausal (χ2 = 13.241, P = 0.0003).
The Correlations Between FAR and Pathological Data in TNBC
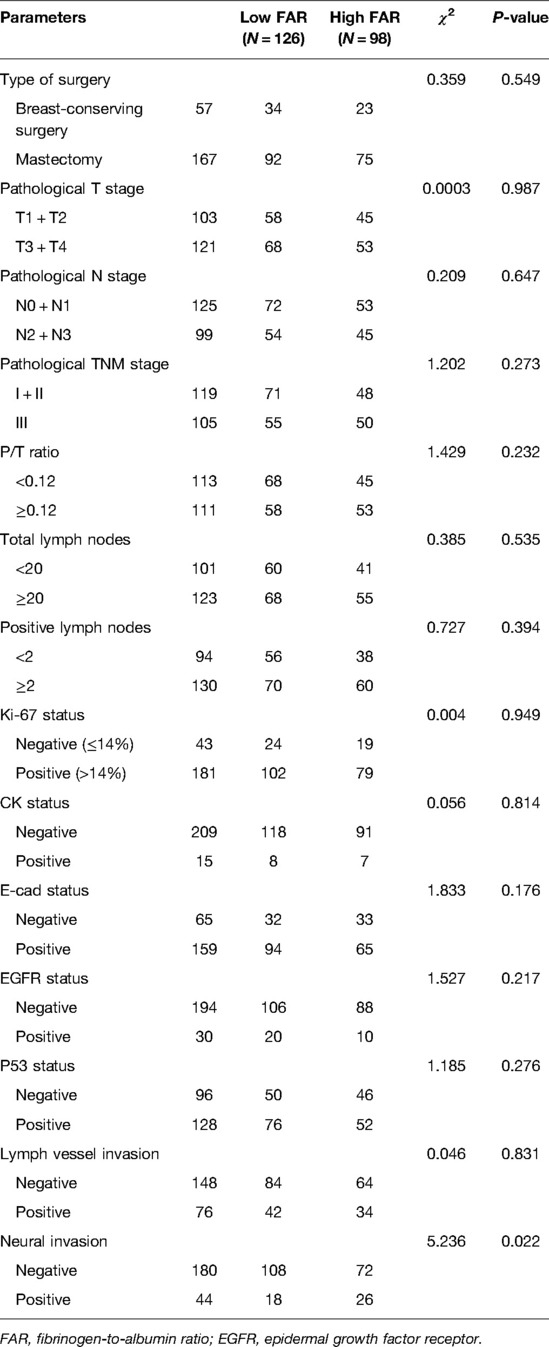
Overall, 167 and 57 patients underwent total mastectomy and breast-conserving surgery, respectively. With respect to pathological TNM stage at diagnosis, 119 (53.13%) and 105 (46.87%) patients with breast cancer had stages I + II and III disease, respectively. There were significant differences in neural invasion between the two groups (χ2 = 5.236, P = 0.022). Table 2 shown the detailed information.
Associations Between FAR and Inflammation Indexes
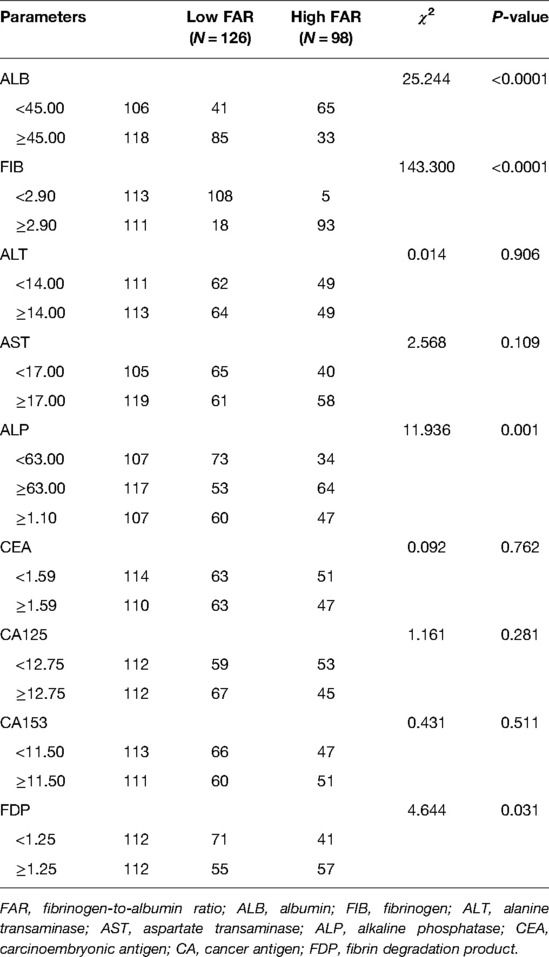
Regarding blood parameters collected before surgery, there were significant differences in ALB (χ2 = 25.244, P < 0.0001), FIB (χ2 = 143.300, P < 0.0001), alkaline phosphatase (ALP) (χ2 = 11.936, P = 0.001), and fibrin degradation products (FDPs) (χ2 = 4.644, P = 0.031). Associations between the FAR and inflammation indices were shown in Table 3.
Univariate and Multivariate Cox Regression Survival Analyses
The optimal cutoff value of 0.066 for FAR was significantly correlated with DFS and OS. Univariate and multivariate Cox regression survival analyses revealed that family history, FAR, pathological T stage, total lymph nodes, E-cadherin (E-cad) status, LVI, neural invasion, and postoperative chemotherapy were significant prognostic factors for DFS; while family history, FAR, pathological T stage, total lymph nodes, E-cad status, LVI, neural invasion, and postoperative chemotherapy were significant prognostic factors for OS. The results were presented in Table 4. And the results were displayed using forest plots, and shown in Supplementary Figure S1.
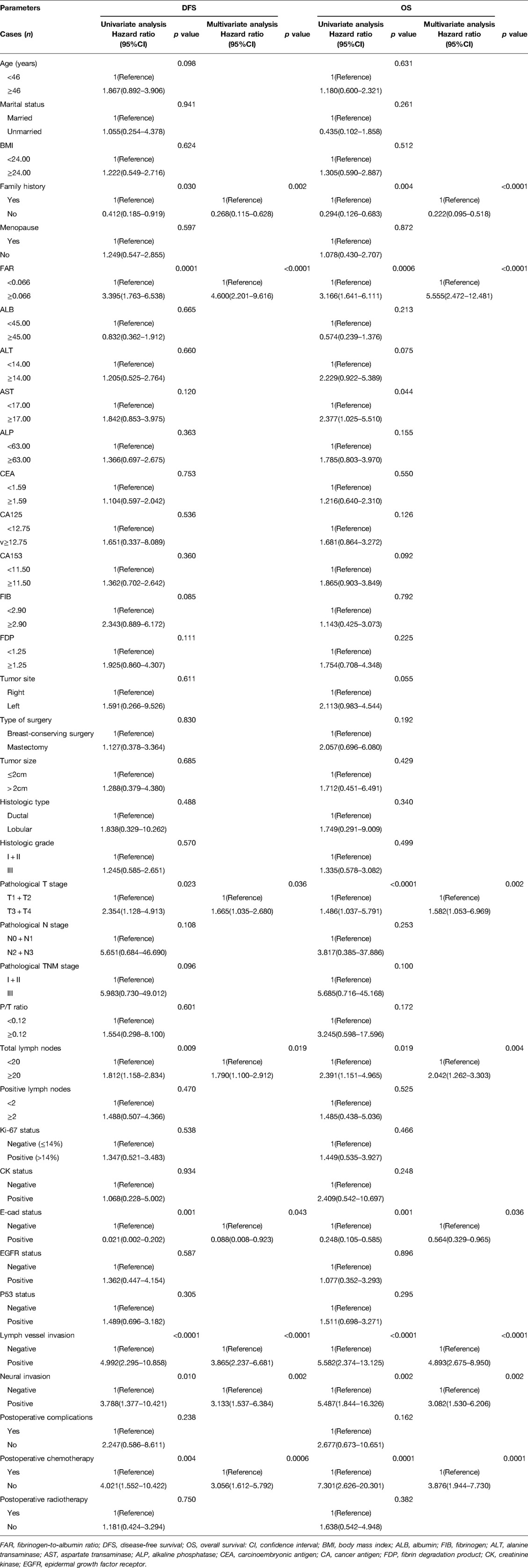
Table 4. Univariate and multivariate Cox regression survival analyses of the FAR for the prediction of DFS and OS in triple-negative breast cancer.
Survival and Prognosis
The median DFS and OS was 39.90 and 60.37 months, respectively. FAR shown significant prognostic association with DFS (P = 0.0001, HR: 3.395, 95% CI, 1.763–6.538; P < 0.0001, HR: 4.600, 95% CI, 2.201–9.616) and OS (P = 0.0006, HR: 3.166, 95% CI, 1.641–6.111; P < 0.0001, HR: 5.555, 95% CI, 2.472–12.481). The median DFS and OS were 42.58 vs. 63.64 months, and 38.02 vs. 57.72 months, for the low and high FAR groups, respectively. Compared with the high FAR group, the median DFS and OS in the low FAR group were significantly longer (χ2 = 15.080, P = 0.0001; χ2 = 13.140, P = 0.0003). The results were shown in Figure 2.

Figure 2. Disease free survival (DFS) and overall survival (OS) in triple-negative breast cancer (TNBC). (A) All enrolled patients for DFS, (B) All enrolled patients for OS, (C) Correlation of FAR with DFS, (D) Correlation of FAR with OS. Statistical analyses were performed using log-rank tests.
Establishment of the Nomogram
Though the results of the multivariate Cox proportional hazards model, we constructed an novel nomogram for DFS and OS. In this nomogram, each variable was imputed a weighted point, and the sum of the points can predict 1-, 3-, and 5-year survival probabilities for DFS, and 3-, 5-, and 10-year survival probabilities for OS. A higher patient grade was associated with a lower survival probability. The nomogram for DFS and OS had unique features, and integrated family history, FAR, pathological T stage, total lymph nodes, E-cad status, LVI, neural invasion, and postoperative chemotherapy (Figure 3). Moreover, we also used the calibration curve to evaluate the nomogram for predicted and the actual probability of DFS and OS. The prediction line matched the reference line well for postoperative 3- and 5-year DFS and OS, showing good performance of the nomogram (Figure 4).
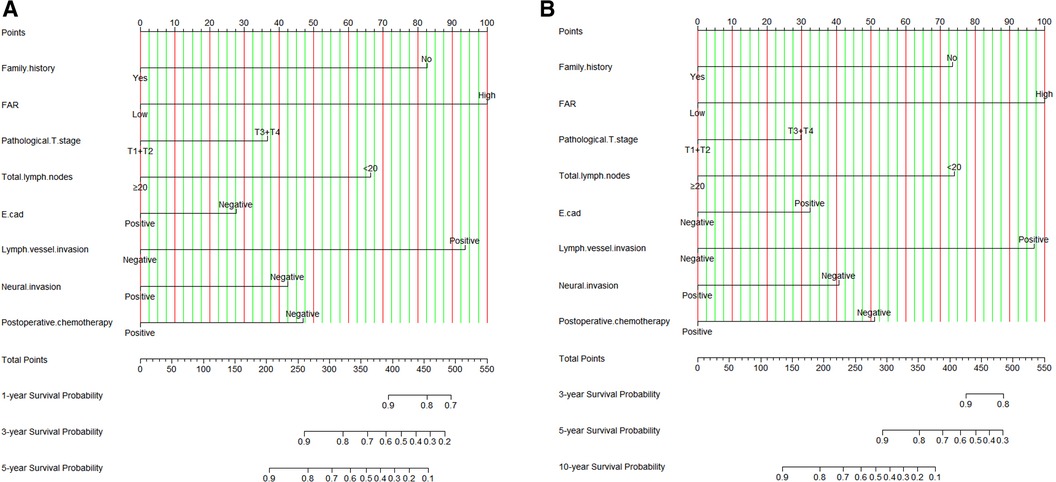
Figure 3. FAR based nomogram for predicting disease free survival (DFS) and overall survival (OS). (A) FAR based nomogram for predicting DFS; (B) FAR based nomogram for predicting OS.
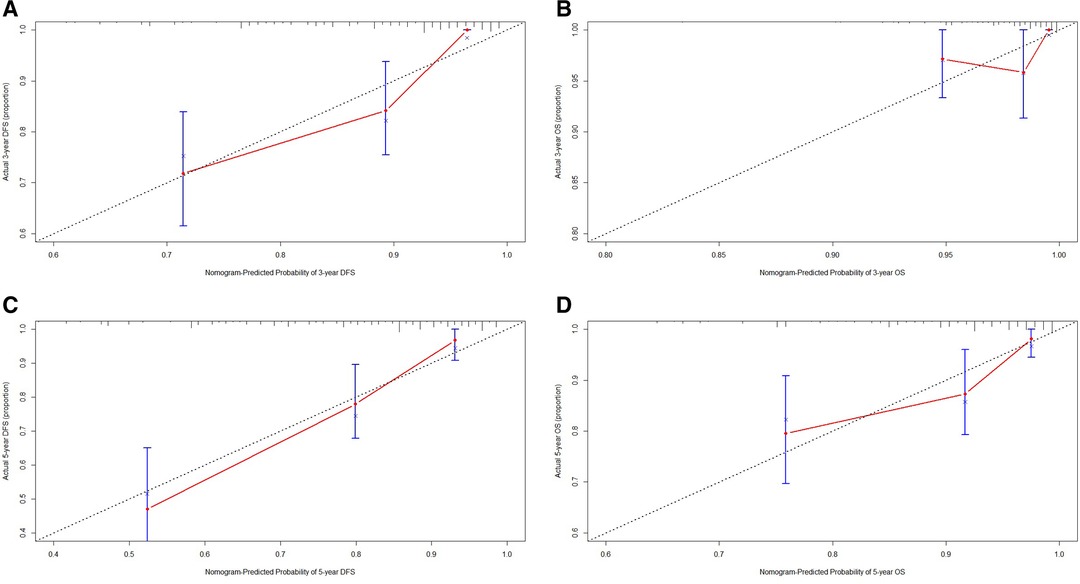
Figure 4. The calibration curves for predicting the 3-, 5-year DFS and OS rates. (A) The calibration curves for predicting the 3-year DFS rate in patients with triple-negative breast cancer (TNBC); (B) The calibration curves for predicting the 3-year OS rate in patients with TNBC; (C) The calibration curves for predicting the 5-year DFS rate in patients with TNBC; (D) The calibration curves for predicting the 5-year OS rate in patients with TNBC.
Correlation Between FAR and Menopause
Overall, 93 and 131 patients were post-menopausal and pre-menopausal, respectively. To investigate the prognostic efficiency of the FAR, a reanalysis according to menopause status was conducted. Pre-menopausal patients survived longer than post-menopausal patients (χ2 = 7.372, P = 0.007; χ2 = 5.115, P = 0.024). The low FAR post-menopausal patients survived significantly longer than the high FAR group (χ2 = 7.123, P = 0.008; χ2 = 6.512, P = 0.011). The low FAR pre-menopausal patients survived significantly longer than their high FAR counterparts (χ2 = 5.023, P = 0.025; χ2 = 4.879, P = 0.027). The results were shown in Supplementary Figure S2.
The Prognostic Significance of FAR in TNBC Patients by Pathological TNM Stage
Further, we determined the correlation of FAR with pathological TNM stage, including 119 (53.13%) and 105 (46.87%) patients diagnosed with pathological stages I + II and III, respectively. Not surprisingly, patients with pathological stages I + II survived longer compared to those with pathological stage III (χ2 = 4.840, P = 0.028; χ2 = 7.558, P = 0.006). Among patients with pathological stages I + II, the low FAR group survived significantly longer than the high FAR group (χ2 = 10.280, P = 0.001; χ2 = 8.075, P = 0.005). Among patients with pathological stage III, the low FAR group survived significantly longer than the high FAR group (χ2 = 3.619, P = 0.057; χ2 = 3.438, P = 0.064). The results were shown in Supplementary Figure S3.
Correlation Between FAR and Lymph Vessel Invasion
From the univariate and multivariate analyses, LVI was a prognostic factor of DFS (P < 0.0001, HR: 4.992, 95% CI, 2.295–10.858; P < 0.0001, HR: 3.865, 95% CI, 2.237–6.681) and OS (P < 0.0001, HR: 5.582, 95% CI, 2.374–13.125; P < 0.0001, HR: 4.893, 95% CI, 2.675–8.950). To investigate the prognostic efficiency of FAR, reanalysis according to LVI was performed. Overall, 76 (33.93%) and 148 (66.07%) patients were diagnosed with and without LVI, respectively. Patients without LVI survived longer than those with LVI (χ2 = 8.851, P = 0.003; χ2 = 14.620, P = 0.0001, respectively). Among patients without LVI, the low FAR group survived significantly longer than their high FAR counterparts (χ2 = 18.600, P < 0.0001; χ2 = 15.990, P < 0.0001). Among patients with LVI, the low FAR group survived significantly longer than their high FAR counterparts (χ2 = 0.011, P = 0.918; χ2 = 0.222, P = 0.638). The results were shown in Supplementary Figure S4.
Chemotherapy and Radiotherapy after Operation
From the univariate and multivariate analyses, chemotherapy was a prognostic factor of DFS (P = 0.004, HR: 4.021, 95% CI, 1.552–10.422; P = 0.001, HR: 3.056, 95% CI, 1.612–5.792) and OS (P = 0.0001, HR: 7.301, 95% CI, 2.626–20.301; P = 0.0001, HR: 3.876, 95% CI, 1.944–7.730). Overall, 147 (65.63%) and 77 (34.37%) patients received or did not receive chemotherapy, respectively. Patients who received chemotherapy after surgery survived longer than those that did not receive chemotherapy (χ2 = 5.027, P = 0.025; χ2 = 4.510, P = 0.034). Among patients who did not receive chemotherapy, the low FAR group survived longer than the high FAR counterparts (χ2 = 5.858, P = 0.016; χ2 = 3.762, P = 0.052). In patients who received chemotherapy, survival was significantly longer in the low FAR group than in their high FAR counterparts (χ2 = 9.367, P = 0.002; χ2 = 9.203, P = 0.002).
Moreover, we also analyzed the correlation between FAR and radiotherapy; and 181 (80.80%) and 43 (19.20%) patients received or did not receive radiotherapy after operation, respectively. Patients who received radiotherapy after surgery survived longer than those who did not (χ2 = 1.727, P = 0.189; χ2 = 4.542, P = 0.033, respectively). Among patients who did not receive radiotherapy, those in the low FAR group survived longer than their high FAR group counterparts (χ2 = 4.465, P = 0.035; χ2 = 2.613, P = 0.106). Among patients who received radiotherapy, those in the low FAR group survived longer than their high FAR group counterparts (χ2 = 9.935, P = 0.002; χ2 = 8.837, P = 0.003).
Discussion
TNBC is characterized by high histological grade and invasiveness; however, there is still a lack of effective treatment (21). In breast cancer development, inflammation plays an important role by promoting tumor occurrence and progression, and can also affect proliferation, invasion, apoptosis, and angiogenesis of tumor cells, and inhibit anti-tumor immune response (22, 23). Some studies have indicated FIB as a multifunctional protein, usually accompanied by tissue abnormalities, infection, and inflammation (24). Moreover, coagulation system activation and coagulation factor release play important roles in the process of tumor occurrence and progression (25). Hyperfibrinogenemia increases tumor progression and inhibits tumor cell immunity by natural killer cell-mediated cytotoxicity (26). In addition to reflecting the nutritional status of the host, serum ALB can also be affected by inflammatory factors, which in turn reflects the level of inflammation in the body. ALB, which is used for tissue repair and as a carrier protein mainly, is also used assessing metabolism and immunity. Furthermore, in patients with hypoproteinemia, the occurrence of tumor cachexia would be aggravated, and the nutritional status will further deteriorate (27). The inflammatory immune parameters, such as FIB, systemic inflammation response index (SIRI), systemic immune-inflammation index (SII), and C-reactive protein (CRP), were included to assess breast cancer prognosis, and parameters with high values were independent prognostic risk factors (28–31). Recently, FAR, proven to be related to prognosis in various malignant tumors, has become a key prognostic factor (32, 33). Therefore, it is important to further assess the clinical prognosis in TNBC.
In this study, 224 TNBC patients were enrolled and retrospectively analyzed. The results indicated FAR as a prognostic factor, and the median DFS and OS in the low FAR group were longer than those in the high FAR group. Zheng et al. shown that combined preoperative fibrinogen-albumin ratio and platelet-lymphocyte ratio score (FAR-PLR score) was a potential new biomarker for predicting survival and prognosis of breast cancer, and may facilitate better clinical decision making for breast cancer treatment by the physicians (34). Cao et al. indicated that patients with preoperative high FAR were prone to short progression-free survival and low OS rate, and that preoperative FAR was a potential prognostic factor of breast cancer (35). Another study shown that preoperative plasma FIB was independently associated with poor prognosis and might serve as a valuable parameter for risk assessment in patients with breast cancer, especially in stage II-III, Luminal subtypes and TNBC patients (36). Moreover, we constructed a nomogram based on the potential prognostic factors by the multivariate analysis. The nomogram predicts the 1-, 3-, and 5-year survival probabilities of DFS, and the 3-, 5-, and 10-year survival probabilities of OS. Moreover, the prediction line matches the reference line well for postoperative survival DFS and OS survival by calibration curves.
Early menarche and late menopause increase the risk of breast cancer in women. Our study indicated that patients with pre-menopausal status survived longer than those with post-menopausal status, and patients with low FAR survived longer than those with high FAR. The underlying mechanism could be related to the use of hormone therapy in postmenopausal women with a greater increased risk of ER-positive than ER-negative tumors; moreover, young age at menarche and older age at menopause increase breast cancer risk (37). Further, we determined the correlation of FAR with pathological TNM stage, and shown that patients with pathological stages I + II survived longer than the pathological stage III patients, while patients with low FAR survived longer than the high FAR group. Commonly, the lymphatic vessel density and lymphovascular invasion are evaluated to identify the clinicopathological outcomes in breast cancer (38). We also analyzed the correlation between FAR and LVI and found LVI to be a prognostic factor, and patients without LVI survived longer than those with LVI. Kurozumi et al. found that LVI was associated with metastasis development in invasive breast cancer as well as with a specific transcriptomic profile of potential prognostic value (39). Another study reported a strong association of LVI with both breast cancer-specific survival (BCSS) and distant metastasis-free survival (DMFS). LVI, a strong predictor of patients’ outcome in invasive breast cancer, should be included in the breast cancer staging systems (40).
Some of the potential mechanisms to explain the clinical significance of FAR in breast cancer include the following. FIB is upregulated by the inflammatory cytokine, IL-6, and has been demonstrated to mediate cell proliferation and form an extracellular matrix that binds to tumor cell surfaces, further increasing cancer cell adhesion, invasion, and metastasis (41–43). Serum ALB, a common indicator of nutritional status, is related to the immune status and prognosis of breast cancer. Moreover, tumor necrosis factor (TNF)-α selectively inhibits ALB gene expression and reduces ALB level ultimately (44, 45). FAR is a more comprehensive serum marker, reflecting the inflammation and nutritional status of patients with tumors, and acting as a new promising prognostic indicator (46). The FAR and fibrinogen-prealbumin ratio (FPR) for patients with cancer were assessed in a meta-analysis. They reported correlation of a low FAR and high FPR with increased risk of cancer mortality and recurrence and might be promising prognostic markers for malignant tumors (25).
There were several limitations to this study. First, this was a retrospective study, and a small number of patients were included; therefore, more patients should be enrolled for further study. Second, peripheral blood parameters were not compared with inflammatory parameters for the primary tumor tissue. Third, power calculation for an estimation of the sample size used for the study was not done. Finally, some heterogeneities were observed in the patients’ treatment approaches after surgery, which might have led to a different clinical prognosis. Thus, large-scale, multicenter, prospective studies should be conducted to further assess the prognostic role of FAR and determine the high-risk population of patients with breast cancer.
Conclusion
In conclusion, FAR is significantly associated with the survival and prognosis of TNBC. It is a strong and independent factor and is of great significance for identifying high-risk patients and providing accurate treatment. Furthermore, FAR is a simple, objective, and inexpensive biomarker for preoperative clinical evaluation, and is potentially applicable in clinical practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The study design was approved by the Ethics Committee of Henan Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Writing-original draft and Writing-review & editing, YQ; Formal analysis, LF; Data curation, LD; Investigation, YY; Methodology and Supervision, YQ. All authors contributed to the article and approved the submitted version.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.916298/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66(2):115–32. doi: 10.3322/caac.21338
4. Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. (2020) 21(7):e342–9. doi: 10.1016/S1470-2045(20)30073-5
5. Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. (2020) 22(1):61. doi: 10.1186/s13058-020-01296-5
6. Medina MA, Oza G, Sharma A, Arriaga LG, Hernández Hernández JM, Rotello VM, et al. Triple-negative breast cancer: A review of conventional and advanced therapeutic strategies. Int J Environ Res Public Health. (2020) 17(6):2078. doi: 10.3390/ijerph17062078
7. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3
8. Shinko D, Diakos CI, Clarke SJ, Charles KA. Cancer-related systemic inflammation: The challenges and therapeutic opportunities for personalized medicine. Clin Pharmacol Ther. (2017) 102(4):599–610. doi: 10.1002/cpt.789
9. Repetto O, De Re V. Coagulation and fibrinolysis in gastric cancer. Ann N Y Acad Sci. (2017) 1404(1):27–48. doi: 10.1111/nyas.13454
10. Chan AK, Paredes N. The coagulation system in humans. Methods Mol Biol. (2013) 992:3–12. doi: 10.1007/978-1-62703-339-8_1
11. Kim N, Lee HH, Lee HJ, Choi WS, Lee J, Kim HS. Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch Pharm Res. (2019) 42(7):591–606. doi: 10.1007/s12272-019-01143-y
12. Zhang Y, Liu N, Liu C, Cao B, Zhou P, Yang B. High fibrinogen and platelets correlate with poor survival in gastric cancer patients. Ann Clin Lab Sci. (2020) 50(4):457–62.32826241
13. Kwaan HC, Lindholm PF. Fibrin and fibrinolysis in cancer. Semin Thromb Hemost. (2019) 45(4):413–22. doi: 10.1055/s-0039-1688495
14. Li SQ, Jiang YH, Lin J, Zhang J, Sun F, Gao QF, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med. (2018) 7(4):1221–31. doi: 10.1002/cam4.1428
15. Chen C, Liu Y, Han P, Cui B. Research progress of preoperative FPR, FAR or AFR in patients with colorectal cancer. Cancer Manag Res. (2021) 13:1791–801. doi: 10.2147/CMAR.S292605
16. Zhang J, Ding Y, Wang W, Lu Y, Wang H, Wang H, et al. Combining the fibrinogen/albumin ratio and systemic inflammation response index predicts survival in resectable gastric cancer. Gastroenterol Res Pract. (2020) 2020:3207345. doi: 10.1155/2020/3207345
17. Liang Y, Wang W, Que Y, Guan Y, Xiao W, Fang C, et al. Prognostic value of the fibrinogen/albumin ratio (FAR) in patients with operable soft tissue sarcoma. BMC Cancer. (2018) 18(1):942. doi: 10.1186/s12885-018-4856-x
18. Liu J, Gan Y, Song H, Zhu K, Zhang Q. The predictive value of the preoperative fibrinogen-albumin ratio on the postoperative prognosis of renal cell carcinoma. Transl Androl Urol. (2020) 9(3):1053–61. doi: 10.21037/tau-19-873
19. Abdel-Rahman O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat. (2018) 168(1):269–75. doi: 10.1007/s10549-017-4577-x
20. Fouad TM, Barrera AMG, Reuben JM, Lucci A, Woodward WA, Stauder MC, et al. Inflammatory breast cancer: A proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol. (2017) 18(4):e228–32. doi: 10.1016/S1470-2045(17)30192-4
21. Kim C, Gao R, Sei E, Brandt R, Hartman J, Hatschek T, et al. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell. (2018) 173(4):879–93.e13. doi: 10.1016/j.cell.2018.03.041
22. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. (2013) 13(11):759–71. doi: 10.1038/nrc3611
23. Singh R, Mishra MK, Aggarwal H. Inflammation, immunity, and cancer. Mediators Inflamm. (2017) 2017:6027305. doi: 10.1155/2017/6027305
24. Everett CJ, Wells BJ, Frithsen IL, Koopman RJ. Smoking, fibrinogen and cancer mortality. J Natl Med Assoc. (2007) 99(4):328–33.17444421
25. Sun DW, An L, Lv GY. Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: An updated meta-analysis. World J Surg Oncol. (2020) 18(1):9. doi: 10.1186/s12957-020-1786-2
26. Yamashita H, Kitayama J, Nagawa H. Hyperfibrinogenemia is a useful predictor for lymphatic metastasis in human gastric cancer. Jpn J Clin Oncol. (2005) 35(10):595–600. doi: 10.1093/jjco/hyi150
27. Critselis E, Panagiotakos DB, Machairas A, Zampelas A, Critselis AN, Polychronopoulos E. Postoperative hypoproteinemia in cancer patients following extensive abdominal surgery despite parenteral nutritional support. Nutr Cancer. (2011) 63(7):1021–8. doi: 10.1080/01635581.2011.606392
28. Tang MJ, Ding SB, Hu WY. Fibrinogen and albumin score changes during preoperative treatment can predict prognosis in patients with locally advanced rectal cancer. Gastroenterol Res Pract. (2019) 2019:3514586. doi: 10.1155/2019/3514586
29. Wang L, Zhou Y, Xia S, Lu L, Dai T, Li A, et al. Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. (2020) 28(4):537–47. doi: 10.3233/CBM-201682
30. Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, et al. Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. (2020) 24(3):610–8. doi: 10.1007/s11605-019-04187-z
31. Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. (2011) 48(4):155–70. doi: 10.3109/10408363.2011.599831
32. Xu WY, Zhang HH, Xiong JP, Yang XB, Bai Y, Lin JZ, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. (2018) 24(29):3281–92. doi: 10.3748/wjg.v24.i29.3281
33. An Q, Liu W, Yang Y, Yang B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer. (2020) 20(1):691. doi: 10.1186/s12885-020-07191-8
34. Zheng Y, Wu C, Yan H, Chen S. Prognostic value of combined preoperative fibrinogen-albumin ratio and platelet-lymphocyte ratio score in patients with breast cancer: A prognostic nomogram study. Clin Chim Acta. (2020) 506:110–21. doi: 10.1016/j.cca.2020.03.011
35. Xi C, Yidong Z, Feng M, Yan L, Xin H, Qiang S. Effect of fibrinogen to albumin ratio on prognosis of operable breast cancer patients. Chin J Breast Dis (Electronic Edition). (2020) 14(4):213–20.
36. Wen J, Yang Y, Ye F, Huang X, Li S, Wang Q, et al. The preoperative plasma fibrinogen level is an independent prognostic factor for overall survival of breast cancer patients who underwent surgical treatment. Breast. (2015) 24(6): 745–50. doi: 10.1016/j.breast.2015.09.007
37. Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob Health. (2020) 8(8):e1027–37. doi: 10.1016/S2214-109X(20)30215-1
38. Zhang S, Zhang D, Gong M, Wen L, Liao C, Zou L. High lymphatic vessel density and presence of lymphovascular invasion both predict poor prognosis in breast cancer. BMC Cancer. (2017) 17(1):335. doi: 10.1186/s12885-017-3338-x
39. Kurozumi S, Joseph C, Sonbul S, Alsaeed S, Kariri Y, Aljohani A, et al. A key genomic subtype associated with lymphovascular invasion in invasive breast cancer. Br J Cancer. (2019) 120(12):1129–36. doi: 10.1038/s41416-019-0486-6
40. Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. (2012) 118(15):3670–80. doi: 10.1002/cncr.26711
41. Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. (2001) 936:406–25.11460495
42. Rybarczyk BJ, Simpson-Haidaris PJ. Fibrinogen assembly, secretion, and deposition into extracellular matrix by MCF-7 human breast carcinoma cells. Cancer Res. (2000) 60(7):2033–9.10766195
43. Redman CM, Xia H. Fibrinogen biosynthesis. Assembly, intracellular degradation, and association with lipid synthesis and secretion. Ann N Y Acad Sci. (2001) 936:480–95.11460506
44. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr Cancer. (2017) 69(8):1151–76. doi: 10.1080/01635581.2017.1367947
45. Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. (2003) 27(1):10–5. doi: 10.1177/014860710302700110
Keywords: fibrinogen-to-albumin ratio, triple-negative breast cancer, fibrinogen, albumin, chemotherapy
Citation: Yang Q, Liang D, Yu Y and Lv F (2022) The Prognostic Significance of the Fibrinogen-to-Albumin Ratio in Patients With Triple-Negative Breast Cancer: A Retrospective Study. Front. Surg. 9:916298. doi: 10.3389/fsurg.2022.916298
Received: 9 April 2022; Accepted: 24 May 2022;
Published: 14 June 2022.
Edited by:
Alba Di Leone, Children’s Health and Public Health, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Li Chen, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaHongjiang Song, Harbin Medical University, China
Copyright © 2022 Yang, Liang, Yu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinheng Yang Qinhengyang002@163.com
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Abbreviations: AFR, albumin-fibrinogen ratio; AJCC, American Joint Committee on Cancer; ALB, albumin; ALP, alkaline phosphatase; BCSS, breast cancer-specific survival; CI, confidence interval; CRP, C-reactive protein; DFS, disease-free survival; DMFS, distant metastasis-free survival; E-cad, E-cadherin; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; FAR, fibrinogen-to-albumin ratio; FDP, fibrin degradation product; FIB, fibrinogen; FPR, fibrinogen-prealbumin ratio; HE, hematoxylin and eosin; HER2, human epidermal growth factor receptor-2; HR, hazard ratio; NK cells, natural killer cells; OS, overall survival; PLR, platelet-lymphocyte ratio; PR, progesterone receptor; ROC, receiver operating characteristic; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; TAIR, tumor-associated inflammatory response; TNBC, triple-negative breast cancer; UICC, Union for International Cancer Control.
 Qinheng Yang
Qinheng Yang