- 1Department of Neurosurgery, The First Hospital of China Medical University, Shenyang, China
- 2Department of Neurosurgery, Huazhong University of Science and Technology Union Shenzhen Hospital (Nanshan Hospital), Shenzhen, China
- 3Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences, China Medical University, Shenyang, China
Purpose: Patients with intraventricular tumors are more susceptible to postoperative meningitis (POM) than other intracranial tumors. In this study, we explored the risk factors of POM in lateral ventricular trigone meningiomas (LVTMs).
Methods: Clinical features of 64 patients with LVTMs were analyzed. Age, gender, body mass index, medical history, intraoperative blood loss (IBL), intraventricular drainage placement, surgical duration, tumor grade, postoperative tumor cavity hemorrhage, and tumor size were included in univariate and multivariate analyses of POM.
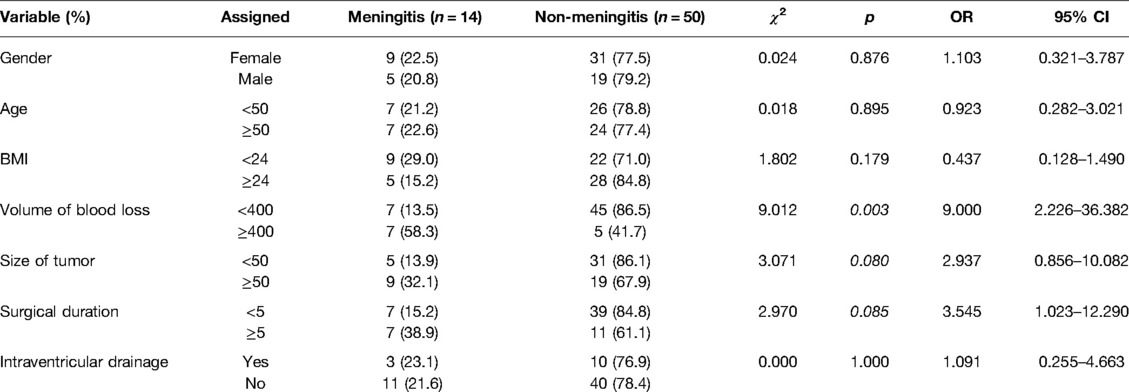
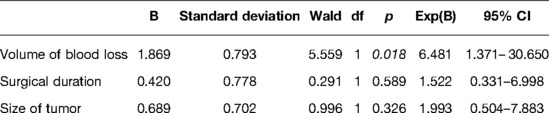
Results: Of the 64 patients, 14 patients (21.9%) received diagnosis of POM. The univariate analysis revealed IBL ≥400 mL (odds ratio [OR], 9.012; p = 0.003), tumor size ≥50 cm3 (OR, 3.071; p = 0.080), and surgical duration ≥5 h (OR, 2.970; p = 0.085) were considered possible risk factors for POM (p < 0.10). Tumor size (R = 0.514) and surgical duration (R = 0.624) were significantly correlated with IBL (p < 0.05). In the multivariate analysis, only IBL was found to be an independent risk factor for POM.
Conclusion: The IBL ≥400 mL is independently associated with the increased risk of POM in LVTM patients. Our results demonstrate the importance of controlling IBL for preventing POM, especially in large tumors and long surgeries.
Introduction
Meningioma is the second most common intracranial tumor in adults, with an incidence rate of 1.5–5.5/100,000 (1–5). Whereas the lateral ventricular trigone meningioma (LVTM), which grows in the deep area of cerebral hemisphere, accounts for only 0.39%–2.3% of all meningiomas (6–9). However, LVTMs are often detected in giant tumors with hyper-vascularization due to their unique location (10). Additionally, LVTMs are surrounded by the cortical centers and fibers associated with speaking, making surgical treatment even more challenging.
Postoperative meningitis (POM) is a challenging problem for all neurosurgeons due to severe complications. Moreover, the complications lengthen the hospital stay and increase the medical costs. Additionally, it can also cause serious neurological dysfunction and even death. Unfortunately, POM is difficult to treat. According to previous reports, the incidence of POM ranges from 0.8%–7% for all craniotomies and 19.8% for intraventricular tumor surgeries (11, 12). Intraventricular meningiomas present a much higher incidence of POM than meningiomas located in other areas (11–13). However, risk factors for POM remain unclear for LVTM surgeries. Therefore, in the current study, we explored the risk factors of POM in LVTM patients (n = 64) to bring insight for reducing the incidence of POM.
Materials and Methods
Patients
We reviewed a total of 2,573 consecutive patients with the diagnosis of intracranial meningioma in the First Hospital of China Medical University from December 2010 to May 2018. Intraventricular meningiomas accounted for 2.8% (71/2,573) of all meningiomas.
Exclusion Criteria: Patients with meningiomas in the third or fourth ventricle, lacking preoperative imaging data, and who had not received an operation in the First Hospital of China Medical University were excluded.
Finally, 64 (2.5%) patients with LVTMs were included for the analysis. All of the patients underwent routine preoperative physical, radiological, and laboratory examinations. The study protocol was approved by the institutional review board of the First Hospital of China Medical University. In addition, written informed consent was obtained from each patient to use clinical data for future research.
All patients received 1.0–2.0 g of intravenous ceftriaxone intraoperatively for infection prophylaxis as described previously (14–16). A second dose of ceftriaxone was administered in patients whose operation had lasted more than 3 h (16). A trans-sulcal or transcortical temporal-occipital approach was adopted, depending on the size and location of the tumor. Gross total resection was performed in all the patients (Figure 1A). In 13 (20.3%) patients, drainage was left in the ventricular tumor cavity to drain the residual debris and blood after tumor removal (17). The drainage was removed no more than five days after surgery. Pathological diagnoses were reported by the Pathology Department of China Medical University. All of the surgical procedures were performed by neurosurgeons with more than 10 years of experience.
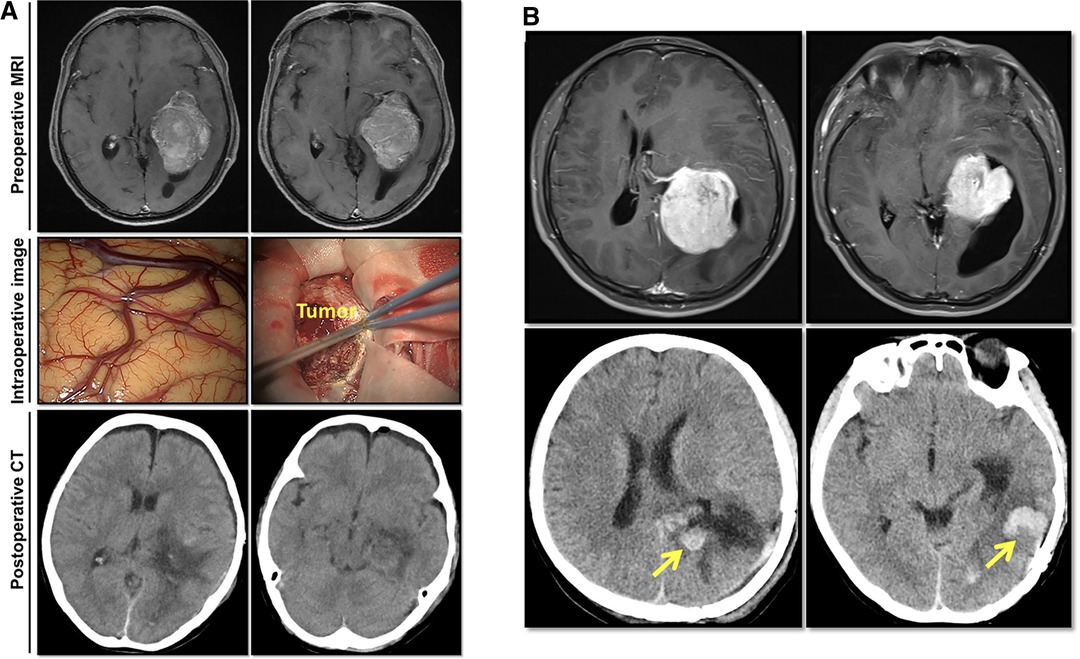
Figure 1. Imaging studies of two representative lateral ventricular trigone meningioma (LVTM) patients. (A) A 63-year-old female patient presented with dizziness and headache. Preoperative axial enhanced T1-weighted magnetic resonance imaging (MRI) showed a huge mass in the left trigone area. Intraoperative observation and postoperative computed tomography (CT) imaging showed a total tumor resection. On a 6th postoperative day, she had a fever of 38.5°C with a headache and meningeal signs. The CSF test showed elevated white cell count (394 106/L), protein level (>3,000 mg/L), and decreased glucose level (<1.1 mmol/L). Lumbar drainage was performed, and meropenem was used according to empirical therapy. Due to the negative CSF smear and culture results, meropenem was used continuously. On the 20th postoperative day, the patient had maintained a normal temperature for a week. CSF tests were repeated three times with normal results. The drainage was removed, and the meropenem was stopped. The patient was discharged from the hospital on the following day. (B) A 35-year-old male patient presented with a headache. Preoperative axial enhanced T1-weighted MRI revealed a large mass in the left trigone area (upper image). Postoperative 24-h CT showed total tumor resection with the tumor cavity hemorrhage (lower image). The patient showed mild headache without fever disturbance of consciousness. On a postoperative day 7, he had a continuous fever of 39°C. The CSF test showed elevated white cell count (754 106/L), protein level (>3,000 mg/L), and decreased glucose level (<1.1 mmol/L). Lumbar drainage was placed, and meropenem was used according to empirical therapy. As the CSF culture showed methicillin-resistant Staphylococcus aureus, meropenem was replaced by vancomycin. On the 33rd postoperative day, the patient had maintained normal temperature for a week with 3 times of normal CSF tests. The drainage was removed, and vancomycin was stopped. The patient was discharged from the hospital on the following day.
Postoperative Meningitis (POM)
POM was determined as previously described (18). Briefly, patients with POM presented with the following symptoms and signs: fever >38°C, headache, meningeal signs. In addition, each patient had at least one of the following laboratory results: (1) organisms cultured from the cerebral spinal fluid (CSF); (2) elevated white cell count (0–8 106/L), protein level (120–600 mg/L), and decreased glucose level (2.2–3.9 mmol/L) in the CSF; (3) organisms observed in the CSF smear examination; and (4) positive antigen test in the CSF.
Data Collection
The clinical data were obtained from our hospital electronic information system. Patient information, including age (<50 vs. ≥50 years), gender, body mass index (BMI), past medical history (hypertension and diabetes), intraoperative blood loss (IBL), intraventricular drainage placement, surgical duration (<5 h vs. ≥5 h), tumor grade, postoperative tumor cavity hemorrhage, and tumor size (<50 cm3 vs. ≥50 cm3) were included in univariate and multivariate analyses of POM.
Statistical Analyses
Statistical analyses were performed using SPSS version 26.0 (IBM Corporation). The chi-squared test was conducted, and factors with p < 0.10 were used to input into multivariate logistic regression analysis. p < 0.05 was considered statistically significant.
The study population was divided into two groups for validation analyses depending on the patient’s admission time. The first 32 patients from December 2010 to January 2015 were included in group A, while the last 32 patients, from March 2015 to May 2018, were considered group B.
Results
Clinical Features of the LVTM Patients
As shown in Table 1, among the 64 cases, there were 24 men (37.5%) and 40 women (62.5%). The average age was 47.39 ± 13.55 years (16–72 years). According to the nearest integers of average values, the cutoff values for age, BMI, tumor size, surgical duration, and volume of IBL were determined as 50 years, 24 kg/m2, 50 cm3, 5 h, and 400 mL, respectively. There were 31 patients (48.4%) with age ≥50 years and 33 patients (51.6%) with age <50 years. The average tumor size was 52.78 ± 65.09 cm3 (1.9–301.4 cm3). There were 36 patients (56.2%) with tumors <50 cm3, while 28 patients (43.8%) with tumors ≥50 cm3. The surgery lasted for an average of 4.68 ± 1.88 h (2–11 h), whereas the average volume of IBL was 359 ± 342 mL (range, 50–1,500 mL). In 18 patients (28.1%) surgery lasted ≥5 h, while in 46 patients (71.9%) it lasted <5 h. There were 52 patients (81.2%) with IBL <400 mL and 12 patients (18.8%) with IBL ≥400 mL. There were 13 patients (20.3%) who had hypertension and no patients with diabetes. Intraventricular drainage was placed in 13 patients (20.3%). Eight patients had postoperative tumor cavity hemorrhage (12.5%; Figure 1B), and two (3.1%) of the patients required secondary surgery to remove the hematoma. Two patients (3.1%) recived a ventriculoperitoneal shunt within one year after the operation. Among the 64 patients tumors, 56 (87.5%) belonged to grade I; seven (10.9%) belonged to grade II, and one (1.6%) belonged to grade III (Table 1) (19). There was no cystic meningioma in the series of cases. Among 14 patients, one patient’s CSF smear showed Gram-positive bacteria (7.1%), and another patient’s CSF culture showed Staphylococcus aureus (7.1%).
Risk Factors for POM
The potential risk factors of POM are presented in Table 2. The univariate analysis revealed IBL ≥400 mL, tumor size ≥50 cm3, and surgical duration ≥5 h were considered possible risk factors for POM (p < 0.10; Figure 2A). These were included in the multivariate logistic regression analysis. According to the Pearson correlation analysis, tumor size (R = 0.514; Figure 2B) and surgical duration (R = 0.624; Figure 2C) were significantly correlated with IBL (p < 0.05). However, gender, age, BMI, and history of hypertension did not correlate with POM. Intraventricular drainage placement was not significantly associated with POM in this study.
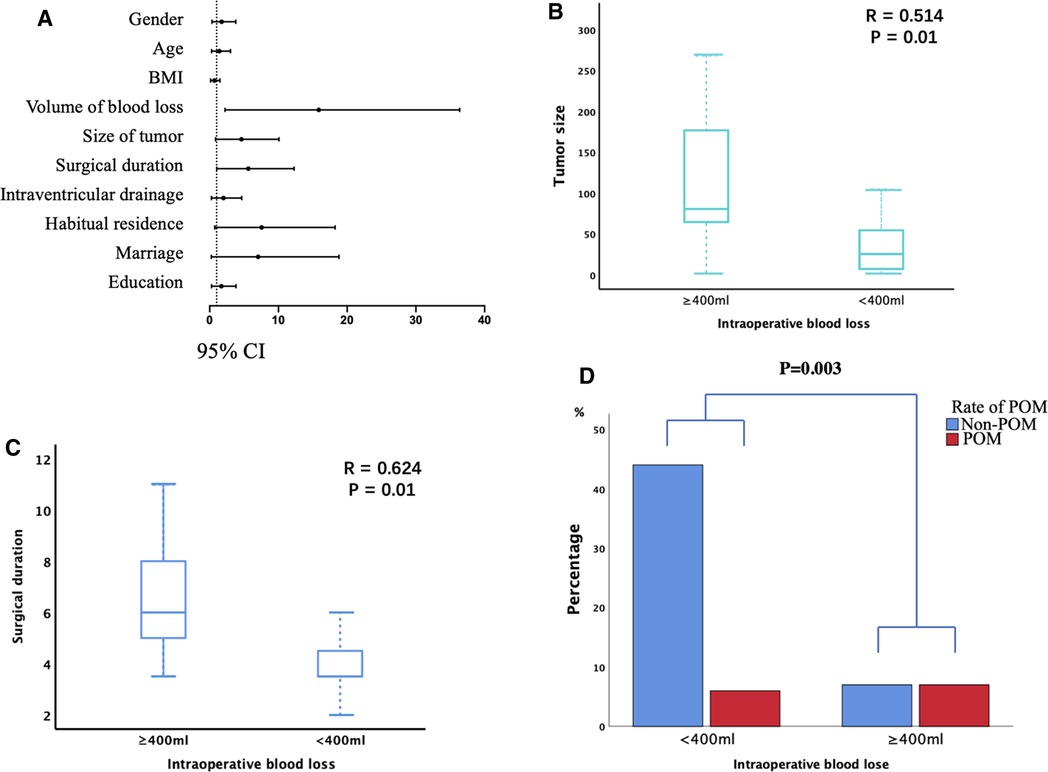
Figure 2. The risk factors for postoperative intracranial infection (POM). (A) Univariate analyses of the risk factors for POM. Odds ratios are shown with 95% CIs. (B,C) Tumor size (B) and surgical duration (C) were significantly associated with intraoperative blood loss (IBL). (D) IBL ≥400 mL was associated with a significantly higher incidence of POM.
Furthermore, IBL, tumor size, and surgical duration were analyzed through multivariate analysis. As shown in Table 3, only IBL ≥400 mL was an independent risk factor for POM (p = 0.018; Figure 2D). Our results indicated that larger tumor size and longer surgical duration might cause more IBL, further increasing the risk of POM. Finally, a similar result was obtained in the validation analysis of groups A and B: only IBL ≥400 mL was an independent risk factor for POM (P = 0.049, 0.046 for groups A and B, respectively).
Treatment of POM
Patients with POM were treated with lumbar drainage and antibiotics (20). First, lumbar drainage was placed, and meropenem, vancomycin, or both were used as empirical therapy (21, 22). The next course of treatment depended on the results of the CSF tests. Among the 14 patients, nine patients (64.3%) received meropenem only; one (7.1%) received vancomycin only; and four patients (28.6%) received meropenem and vancomycin. The patients were cured within 8–39 days (18.57 ± 8.76 days). However, POM cases average length of postoperative hospital stay was 10 days longer than non-POM cases (p = 0.033).
Discussion
POM is a common postoperative complication of neurosurgery, causing severe consequences (23–25). Previous studies have reported that patients with intraventricular tumors were more susceptible to POM than other intracranial tumors (12, 26, 27). Therefore, identifying the risk factors for POM in LVTM patients carries significant clinical value.
In this study, 64 LVTM patients underwent craniotomy, out of which 14 patients (21.8%) had the diagnosis of POM. Previously, Wang et al. reported a 19.8% incidence of POM, 1.7% incidence of postoperative hematoma evacuation, and 3.3% incidence of postoperative ventriculoperitoneal shunt in out of 121 LVTMs (12). Though they did not analyze the risk factors for POM, their results showed a close relationship between ventricular drainage and postoperative entrapped temporal horn. However, in our study, ventricular drainage was not identified as a risk factor for POM. Besides, ventricular drainage was only used in 20.3% of the patients, which was lower than the report of Wang et al. (87.6%). Nevertheless, the incidence of postoperative complications including meningitis, severe hemorrhage in the operative cavity and hydrocephalus requiring shunting in our series of cases was not significantly different from that in Wang’s series of cases. In addition, previous studies have reported that ventricular drainage increases the risk of POM (28–31). Thus, ventricular drainage should be used with caution in LVTM surgeries to prevent complications. Intraoperative ultrasound (IoUS) provides great help in neurosurgery, which can increase the chances of total resection and reduce the risk of postoperative bleeding in the surgical cavity. Neurosurgeons can get the real-time information about the anatomical relationship between tumor and its surrounding tissue (32). Therefore, tumors can be removed more precisely with reduced intraoperative blood loss and potentially decreased risk of postoperative meningitis.
We found that IBL ≥400 mL was an independent risk factor for the POM. This observation was in-concurrence with previous studies reporting the correlation between IBL and POM (33, 34). The large amount of IBL may compromise the patient’s immune system and adversely influence the physiology of local brain tissue. The coagulation pathway might also get activated, leading to the recruitment of inflammatory cells and cytokines. As a result, tissue hypoperfusion and ischemia-reperfusion injury might occur. Further, inflammatory reaction and an ischemia-reperfusion injury increase the vascular permeability damage the integrity of the endothelium, and blood-brain barrier, enhancing the risk of POM (35–38). Massive IBL may decrease the concentration of prophylactic antibiotics in the blood and reduce its preventive effects against infection (39–44). Moreover, massive IBL is associated with allogeneic transfusion. However, the transfusion of red blood cells can contribute to POM by enhancing the inflammatory reaction and suppressing immunity (45–47).
In addition, our results also indicate that large tumor size and long surgical duration were associated with increased IBL. This association further affects the incidence of POM. Large LVTMs are usually hypervascular and fibrous and require long-lasting operations. Moreover, cystic meningioma is a special kind of meningiomas with a relatively low occurrence of about 3.5% (48). There was no cystic meningioma in our series of cases. However, according to previous study, cystic meningiomas are known to increase the length of surgery and intraoperative blood loss (48). Thus, for cystic meningiomas, surgeons should pay more attention to the risk of postoperative meningitis.
Our results emphasized the importance of controlling intraoperative bleeding. Of note, preoperative embolization, meticulous intraoperative hemostasis, and early tumor devascularization may reduce the IBL and, in turn, decrease the risk of POM (32). However, when a massive IBL occurs, an additional dose of intraoperative antibiotics might be helpful to prevent POM. Importantly, POM significantly prolongs hospital stays, increases medical costs, and affects clinical outcomes. Thus, the prevention of POM may provide significant benefits to patients.
The current study had some limitations. One major limitation was the lack of distinction between bacterial and aseptic meningitis. As a result, they show similar symptoms (new headache, fever, seizures, etc.), CSF test (abnormalities of CSF cell count, glucose, and protein), except CSF culture and smear. However, CSF culture and smear show a low positive rate, which can be reduced by prophylactic antibiotics (49). In this study, all 14 patients with POM showed some improvement and finally recovered after antibiotic therapy, suggesting the potentially beneficial effect of antibiotic therapy.
Conclusion
The IBL ≥400 mL is independently associated with the increased risk of POM in LVTM patients. Our results demonstrate the importance of controlling IBL for preventing POM, especially in large tumors and long surgeries.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection was performed by TH, SH, LZ and JY, analysis was performed by XH, RW and LL. The first draft of the manuscript was written by XH and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants from the LiaoNing Revitalization Talents Program (no. XLYC1807253) and ShenYang Science and Technology Innovation Project (no. RC200610).
Acknowledgments
We thank Junqi Wu, Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, for assistance in the statistical analyses.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Staneczek W, Jänisch W. Epidemiologic data on meningiomas in East Germany 1961–1986: incidence, localization, age and sex distribution. Clin Neuropathol. (1992) 11(3):135–41. PMID: 1611725
2. Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. (2005) 57(6):1088–95; discussion -95. doi: 10.1227/01.neu.0000188281.91351.b9
3. Criscuolo GR, Symon L. Intraventricular meningioma. A review of 10 cases of the national hospital, Queen Square (1974–1985) with reference to the literature. Acta Neurochir (Wien). (1986) 83(3–4):83–91. doi: 10.1007/bf01402383
4. McDermott MW. Intraventricular meningiomas. Neurosurg Clin N Am. (2003) 14(4):559–69. doi: 10.1016/s1042-3680(03)00055-x
5. Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Neuner M, et al. Intraventricular meningiomas: a report of 16 cases. Neurosurg Rev. (2006) 29(1):30–5. doi: 10.1007/s10143-005-0414-5
6. Grujicic D, Cavallo LM, Somma T, Illic R, Milicevic M, Raicevic S, et al. Intraventricular meningiomas: a series of 42 patients at a single institution and literature review. World Neurosurg. (2017) 97:178–88. doi: 10.1016/j.wneu.2016.09.068
7. Wang X, Cai BW, You C, He M. Microsurgical management of lateral ventricular meningiomas: a report of 51 cases. Minim Invasive Neurosurg. (2007) 50(6):346–9. doi: 10.1055/s-2007-993205
8. Zhang WH, Xie M, Liu H, Wang X, Lin MH. Surgical challenges for lateral ventricle meningiomas: a consecutive series of 21 patients. J Huazhong Univ Sci Technolog Med Sci. (2015) 35(5):742–6. doi: 10.1007/s11596-015-1500-8
9. Moliterno J, Cope WP, Vartanian ED, Reiner AS, Kellen R, Ogilvie SQ, et al. Survival in patients treated for anaplastic meningioma. J Neurosurg. (2015) 123(1):23–30. doi: 10.3171/2014.10.Jns14502
10. Nayar VV, DeMonte F, Yoshor D, Blacklock JB, Sawaya R. Surgical approaches to meningiomas of the lateral ventricles. Clin Neurol Neurosurg. (2010) 112(5):400–5. doi: 10.1016/j.clineuro.2010.02.005
11. McClelland S 3rdHall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. (2007) 45(1):55–9. doi: 10.1086/518580
12. Wang Y, Lin Z, Li Z, Zhao M, Hu M, Zhang H, et al. The incidence and risk factors of postoperative entrapped temporal horn in trigone meningiomas. World Neurosurg. (2016) 90:511–7. doi: 10.1016/j.wneu.2016.03.040
13. Tenney JH, Vlahov D, Salcman M, Ducker TB. Wide variation in risk of wound infection following clean neurosurgery. Implications for perioperative antibiotic prophylaxis. J Neurosurg. (1985) 62(2):243–7. doi: 10.3171/jns.1985.62.2.0243
14. Gaillard T, Gilsbach JM. Intra-operative antibiotic prophylaxis in neurosurgery. A prospective, randomized, controlled study on cefotiam. Acta Neurochir (Wien). (1991) 113(3–4):103–9. doi: 10.1007/bf01403193
15. Rocca B, Mallet MN, Scemama F, Malca S, Chevalier A, Gouin F. Perioperative remote infections in neurosurgery. Role of antibiotic prophylaxis. Presse Med. (1992) 21(42):2037–40. PMID: 1294976
16. Zhao JZ, Wang S, Li JS, Yang J, Zang JT, He Q, et al. The perioperative use of ceftriaxone as infection prophylaxis in neurosurgery. Clin Neurol Neurosurg. (1995) 97(4):285–9. doi: 10.1016/0303-8467(95)00054-n
17. Fernandez-Miranda JC, Xu Y, Hendricks BK, Cohen-Gadol A. Contralateral interhemispheric transfalcine transprecuneus approach: advancing operative angles to deep-seated lesions. World Neurosurg. (2020) 144:341–50. doi: 10.1016/j.wneu.2020.08.150
18. Kourbeti IS, Jacobs AV, Koslow M, Karabetsos D, Holzman RS. Risk factors associated with postcraniotomy meningitis. Neurosurgery. (2007) 60(2):317–25; discussion 25–6. doi: 10.1227/01.Neu.0000249266.26322.25
19. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
20. Abulhasan YB, Al-Jehani H, Valiquette MA, McManus A, Dolan-Cake M, Ayoub O, et al. Lumbar drainage for the treatment of severe bacterial meningitis. Neurocrit Care. (2013) 19(2):199–205. doi: 10.1007/s12028-013-9853-y
21. Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, et al. 2017 Infectious diseases society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. (2017) 64(6):e34–e65. doi: 10.1093/cid/ciw861
22. van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. (2010) 362(2):146–54. doi: 10.1056/NEJMra0804573
23. Rebuck JA, Murry KR, Rhoney DH, Michael DB, Coplin WM. Infection related to intracranial pressure monitors in adults: analysis of risk factors and antibiotic prophylaxis. J Neurol Neurosurg Psychiatry. (2000) 69(3):381–4. doi: 10.1136/jnnp.69.3.381
24. Dashti SR, Baharvahdat H, Spetzler RF, Sauvageau E, Chang SW, Stiefel MF, et al. Operative intracranial infection following craniotomy. Neurosurg Focus. (2008) 24(6):E10. doi: 10.3171/FOC/2008/24/6/E10
25. Korinek AM, Golmard JL, Elcheick A, Bismuth R, van Effenterre R, Coriat P, et al. Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg. (2005) 19(2):155–62. doi: 10.1080/02688690500145639
26. Shi ZH, Xu M, Wang YZ, Luo XY, Chen GQ, Wang X, et al. Post-craniotomy intracranial infection in patients with brain tumors: a retrospective analysis of 5723 consecutive patients. Br J Neurosurg. (2017) 31(1):5–9. doi: 10.1080/02688697.2016.1253827
27. Gungor A, Danyeli AE, Akbas A, Eksi MS, Guduk M, Ozduman K, et al. Ventricular meningiomas: surgical strategies and a new finding that suggest an origin from the choroid plexus epithelium. World Neurosurg. (2019) 129:e177–90. doi: 10.1016/j.wneu.2019.05.092
28. Reichert MC, Medeiros EA, Ferraz FA. Hospital-acquired meningitis in patients undergoing craniotomy: incidence, evolution, and risk factors. Am J Infect Control. (2002) 30(3):158–64. doi: 10.1067/mic.2002.119925
29. Mahé V, Kermarrec N, Ecoffey C. Infections related to external ventricular drainage. Ann Fr Anesth Reanim. (1995) 14(1):8–12. doi: 10.1016/s0750-7658(05)80144-3
30. Korinek AM. [Infectious risk from ventriculostomy]. Ann Fr Anesth Reanim. (1999) 18(5):554–7. doi: 10.1016/s0750-7658(99)80132-4
31. Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections. a prospective epidemiologic study. N Engl J Med. (1984) 310(9):553–9. doi: 10.1056/nejm198403013100903
32. Ganau M, Ligarotti GK, Apostolopoulos V. Real-time intraoperative ultrasound in brain surgery: neuronavigation and use of contrast-enhanced image fusion. Quant Imaging Med Surg. (2019) 9(3):350–8. doi: 10.21037/qims.2019.03.06
33. Wang LY, Cao XH, Shi LK, Ma ZZ, Wang Y, Liu Y. Risk factors for intracranial infection after craniotomy: a case-control study. Brain Behav. (2020) 10(7):e01658. doi: 10.1002/brb3.1658
34. Huang B, Ren Y, Wang C, Lan Z, Hui X, Liu W, et al. Risk factors for postoperative meningitis after microsurgery for vestibular schwannoma. PLoS One. (2019) 14(7):e0217253. doi: 10.1371/journal.pone.0217253
35. Liu MH, Tian J, Su YP, Wang T, Xiang Q, Wen L. Cervical sympathetic block regulates early systemic inflammatory response in severe trauma patients. Med Sci Monit. (2013) 19:194–201. doi: 10.12659/msm.883833
36. Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci. (1995) 18(3):130–6. doi: 10.1016/0166-2236(95)93890-a
37. Hall S, Kumaria A, Belli A. The role of vagus nerve overactivity in the increased incidence of pneumonia following traumatic brain injury. Br J Neurosurg. (2014) 28(2):181–6. doi: 10.3109/02688697.2013.835373
38. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. (2014) 384(9952):1455–65. doi: 10.1016/s0140-6736(14)60687-5
39. Nix DE, DiPiro JT, Bowden TA Jr, Vallner JJ. Cephalosporins for surgical prophylaxis: computer projections of intraoperative availability. South Med J. (1985) 78(8):962–6. doi: 10.1097/00007611-198508000-00018
40. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. (1961) 50:161–8. PMID: 16722001
41. Markantonis SL, Kostopanagiotou G, Panidis D, Smirniotis V, Voros D. Effects of blood loss and fluid volume replacement on serum and tissue gentamicin concentrations during colorectal surgery. Clin Ther. (2004) 26(2):271–81. doi: 10.1016/s0149-2918(04)90025-2
42. Polk HC J, Simpson CJ, Simmons BP, Alexander JW. Guidelines for prevention of surgical wound infection. Arch Surg. (1983) 118(10):1213–7. doi: 10.1001/archsurg.1983.01390100075019
43. Vogelman B, Craig WA. Kinetics of antimicrobial activity. J Pediatr. (1986) 108(5 Pt 2):835–40. doi: 10.1016/s0022-3476(86)80754-5
44. Burke JF. Preventing bacterial infection by coordinating antibiotic and host activity: a time-dependent relationship. South Med J. (1977) 70(Suppl 1):24–6. doi: 10.1097/00007611-197710001-00006
45. Patil H, Garg N, Navakar D, Banabokade L. Clinical experience of autologous blood transfusion in neurosurgery: prospective study in central india. World Neurosurg. (2018) 115:e539–43. doi: 10.1016/j.wneu.2018.04.091
46. Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery Patients. J Am Coll Surg. (2009) 208(5):931–7, 7.e1-2; discussion 8–9. doi: 10.1016/j.jamcollsurg.2008.11.019
47. Vamvakas EC. Platelet transfusion and postoperative infection in cardiac surgery. Transfusion. (2007) 47(2):352–4; author reply 4–6. doi: 10.1111/j.1537-2995.2007.01115.x
48. Boukobza M, Cebula H, Pop R, Kouakou F, Sadoun A, Coca HA, et al. Cystic meningioma: radiological, histological, and surgical particularities in 43 patients. Acta Neurochir (Wien). (2016) 158(10):1955–64. doi: 10.1007/s00701-016-2898-x
Keywords: lateral ventricular trigone meningioma, postoperative meningitis, blood loss, risk factor, cerebral spinal fluid
Citation: Han X, Hu T, Wang R, Li L, Yu J, Zhang L and Han S (2022) Risk Factors of Postoperative Meningitis in Lateral Ventricular Trigone Meningiomas: A Clinical Analysis of 64 Patients. Front. Surg. 9:916053. doi: 10.3389/fsurg.2022.916053
Received: 8 April 2022; Accepted: 9 May 2022;
Published: 25 May 2022.
Edited by:
Mario Ganau Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Νικοlaos CH. Syrmos, Aristotle University of Thessaloniki, GreeceLiangzhao Chu, The First Affiliated Hospital of GuiZhou Medical University, China
Copyright © 2022 Han, Hu, Wang, Li, Yu, Zhang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Han aGFuc2hlbmdAY211LmVkdS5jbg== Li Zhang Y211emhhbmdsaUAxMjYuY29t
Specialty section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Xiaodi Han1
Xiaodi Han1 Tianhao Hu
Tianhao Hu Run Wang
Run Wang Sheng Han
Sheng Han