- 1Department of Obstetrics and Gynecology, Shaoxing Maternity and Child Health Care Hospital, Shaoxing, China
- 2Department of Gynecology, The Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University, Changzhou, China
- 3Department of Gynecology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
Background and Objective: Adenomyosis focus resection has always been the main surgical method for patients with uterine preservation, but its curative effect and surgical method are still controversial. We improved this method on the basis of the “double-flap method” and combined it with the levonorgestrel intrauterine delivery system (LNG-IUS) and gonadotropin-releasing hormone agonist (GnRH-a) sequential treatment to determine the clinical effect and feasibility of this scheme in the treatment of severe adenomyosis.
Methods: This is a retrospective review. A total of 64 patients with severe adenomyosis were treated in the Department of Gynecology of Changzhou Second People's Hospital, which is affiliated to Nanjing Medical University, from December 2017 to September 2021. The transabdominal approach and laparoscopic approach were adopted for the purposes of treatment in this study. Hence, the patients were subdivided into the transabdominal approach subgroup and the laparoscopic approach subgroup. The hemoglobin, visual analog score (VAS) score, menstruation score, and other indices of each patient before and after treatment were observed, recorded, and analyzed.
Results: All 64 patients underwent the operation successfully. After the completion of sequential treatment, the CA125 decreased significantly 1 month after the operation, the average uterine volume significantly reduced, the hemoglobin value increased to a certain extent 3 months after the operation, and the menstrual score and dysmenorrhea during the first menstruation were significantly lower than they were before the operation. After the treatment, the therapeutic results of the transabdominal approach subgroup and endoscopic approach subgroup were compared on the basis of the observed indices, and no significant difference was observed (P > 0.05). Only one patient had a downward movement of the LNG-IUS, and the vaginal ultrasound showed that the upper end of the LNG-IUS was approximately 1.5 cm from the bottom of the uterine cavity. The average follow-up period was 24.02 ± 11.77 months, and no lesion progression was found in any patients.
Conclusion: For patients suffering from severe adenomyosis who have no pregnancy plans and require uterine preservation, transabdominal or laparoscopic subtotal resection of the focus of adenomyosis, combined with the LNG-IUS + GnRH-a sequential treatment, may be a safe and effective alternative when conservative treatments such as drugs fail.
Introduction
Adenomyosis (AM) is a disease caused by the invasion of the endometrium into the myometrium. It is an estrogen-dependent disease (1). The clinical symptoms are progressive secondary dysmenorrhea, increased menstruation, and enlarged uterine volume, and some patients may also suffer from infertility (2). It is reported that the prevalence rate is 5%–70% (3). The radical treatment of AM is total hysterectomy, which will cause impacts on women's physical and mental conditions and life after hysterectomy to varying degrees, so it is crucial to preserve the uterus. The most commonly used operation to preserve the uterus is local focus resection. However, the high recurrence in the long term and the improvement in clinical symptoms after focal resection are worthy of attention (4–6).
Many drugs can be used to treat AM, but they cannot cure the disease, and it is easy for the disease to relapse and progress after drug withdrawal (1). Related clinical studies have confirmed that both the levonorgestrel intrauterine delivery system (LNG-IUS) and the gonadotropin-releasing hormone agonist (GnRH-a) have certain efficacy in the treatment of AM, but they both have their own adverse reactions (7–11). Because of the high recurrence and unsatisfactory clinical effect of a single treatment approach for AM, clinicians have begun to adopt combined interventions such as high-intensity focused ultrasound (HIFU) combined with the LNG-IUS (12), local focus resection with the LNG-IUS (13), and HIFU with GnRH-a (14). However, with the increase in follow-up time, the recurrence rate also increases (14, 15–17).
We found that the “double-flap method” was more effective in the operation of preserving the uterus (18–20), but this method is suitable for patients with pregnancy intention after an operation, so a part of the myometrium will still be preserved. For patients who still have a strong desire to retain the uterus after the failure of drug treatment, this study improves the surgical method to reduce the recurrence among patients after treatment. During the operation, the focus of adenomyosis is resected as much as possible, and a part of the uterine cavity is remodeled to consolidate the curative effect and reduce the recurrence by sequential treatment with the LNG-IUS and GnRH-a after the operation. The purpose of this study was to preliminarily determine the efficacy of postoperative combined drugs in the treatment of severe adenomyosis.
Data and methods
General data
A total of 64 patients with severe adenomyosis treated in the Department of Gynecology, the Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University, from December 2017 to September 2021 were selected. The patients suffered from severe adenomyosis as determined by auxiliary examination, and the postoperative pathological results supported the diagnosis of adenomyosis. According to the guidelines of the International Federation of Obstetrics and Gynecology, the extent of the lesion was determined. If the uterus volume affected by the lesion is <25%, it is called mild, 25%–50% is called moderate, and >50% is called severe (21).
(1) Case inclusion criteria: (1) The patient had a strong desire to preserve the uterus. (2) The conservative treatment with drugs failed. (3) The patient had no pregnancy plan. (4) The patient voluntarily accepted this procedure and signed the informed consent form.
(2) Case exclusion criteria: (1) The patient suffered from primary dysmenorrhea. (2) The patient had a malignant tumor of the reproductive system. (3) The patient was unwilling to accept GnRH-a treatment or was lost during follow-up.
Surgical steps
Transabdominal surgery group: Make a median longitudinal incision, pull the uterus out of the pelvic cavity, wrap it with a cotton pad soaked in normal saline, and determine the location of the lesion by touching the uterus. Inject diluted pituitrin into the normal myometrium near the focal tissue (with close attention paid to blood pressure fluctuations) (Figure 1A). If the uterine leiomyoma is to be removed at the same time (Figure 1B), make a longitudinal incision with an electrotome at the location of the focus of adenomyosis to reach the uterine cavity (Figure 1C). Resect the focal lesion from the seromuscular layer separately on the myometrium flaps (Figure 1D), and retain approximately 5 mm–10 mm of the flaps for suture (Figure 1E). The lesions around the uterine cavity should be removed as clean as possible to avoid postoperative recurrence (Figures 1F,G). Finally, the endometrium and the left and right muscle layers within 5 mm were preserved as the “uterine center” (Figure 1H). Use the LNG-IUS placed during the operation as the “ruler” to reshape the uterine cavity at a depth of approximately 7–8 cm (Figure 1I). Suture the uterine cavity (Figure 1J). Align both sides of the sarcoplasmic layers and remove the extra length of these layers (Figure 1K). Suture the seromuscular layers on both sides with the “Baseball Stitching Technique” to complete the uterine plastic surgery (Figures 1L–N).
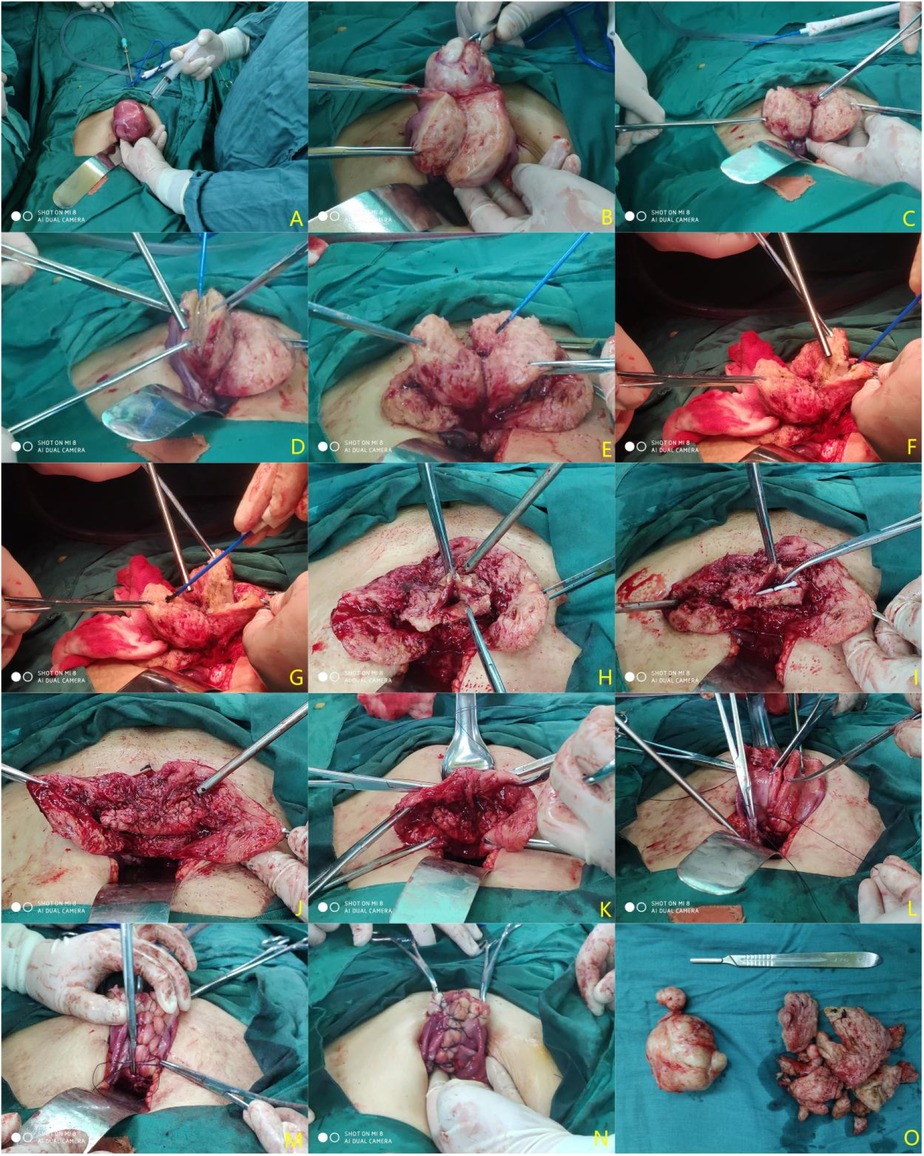
Figure 1. Procedure diagram of a transabdominal operation. (A) Inject diluted pituitrin; (B) remove uterine fibroids; (C) make a longitudinal incision of adenomyosis lesions to reach the uterine cavity; (D) Resect the lesion as much as possible (preserving approximately 0.5–1 cm of the plasmomuscular layer flaps); (E) treat the contralateral lesions with the same method; (F) gradually subtract the lesion to the uterine cavity and excise a part of the uterine cavity to reduce the uterine volume; (G) treat the contralateral lesions with the same method; (H) remodel the myometrium; (I) place the LNG-IUS (Manchester ring) and reshape the depth of the uterine cavity again based on the LNG-IUS length; (J) Mattress-suture the uterine cavity continuously; (K) align the sarcoplasmic layers and reduce the extra length; (L,M) suture the bilateral seromuscular layers with the “baseball stitching technique”; (N) repair the sutured uterus; (O) uterine fibroids and adenomyosis specimens resected during the operation.
Laparoscopic surgery group: The procedure is basically the same as that of the transabdominal group. Before the operation, the location and scope of the focus were evaluated by gynecological examination, a transvaginal ultrasound, and MRI. During the operation, the focus can be resected by a rational use of energy instruments such as an ultrasonic scalpel. The specimen needs to be packed in a specimen bag and then slowly removed from the puncture hole around 1.5 cm in diameter. The “tumor-free principle” should be observed during specimen collection. Polyglactin 910: synthetic absorbable surgical suture (Model: 2-0) was used to close the uterine cavity. Polyglactin 910: synthetic absorbable surgical suture (Model: 1-0) was used to close the seromuscular layer.
After completion of either the transabdominal or laparoscopic surgery, wash the pelvic and abdominal cavity with warm saline to reduce the possibility of endometrial implantation, close the abdominal cavity layer by layer, and place drainage tubes in the pelvic cavity when necessary.
Postoperative management
No special postoperative treatment was required for the patients. After discharge, GnRH-a injection was given on the first day of menstruation. If the menstruation did not occur due to the placement of the LNG-IUS, the first injection can be administered according to the previous menstrual cycle. Administer three to six injections according to the patient's age, disease condition, and other factors, with an interval of 28 days and regular follow-up (a total of 27 patients received 6 injections of GnRH-a, and 37 patients received 3 injections of GnRH-a).
Observed indices
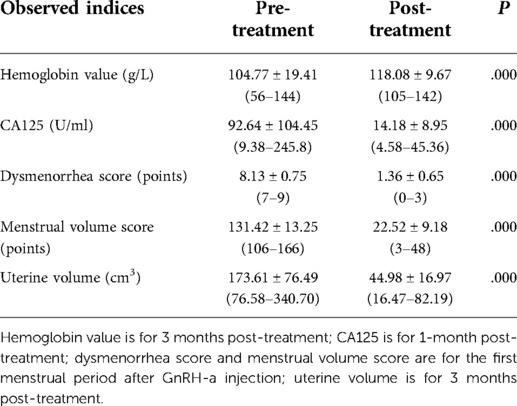
The indices before, during, and after the operation were recorded to evaluate the therapeutic effect. The indices to be observed include the following: (1) operation time and intraoperative blood loss; (2) hemoglobin value at preoperative and 3 months postoperative; (3) CA125 at preoperative and 1 month postoperative; (4) dysmenorrhea score at preoperative and the first menstrual period after GnRH-a injection; dysmenorrhea score calculated by using visual analog score (VAS) where the pain scale was subdivided into 10 grades; “no pain” was indicated on the left side of the scale, and “the maximum pain you could imagine” was designated on the right side of the scale; (5) menstrual volume score at the preoperative and the first menstrual period after GnRH-a injection, scored with the pictorial blood loss assessment chart (PBAC) (22); (6) uterine volume estimated by transvaginal ultrasound at preoperative and 3 months postoperative where the uterus size is estimated using the elliptical volume formula, which is 0.52 × length × thickness × width of the uterus measured with transvaginal ultrasound (23); (7) regular follow-up with transvaginal ultrasound to check for any progress of AM lesions and displacement of the LNG-IUS.
Statistical analysis
The data from this study were statistically analyzed using the SPSS26.0 statistical software. The results were expressed as the mean value ± standard deviation , though the measured variable values were not normally distributed. Then, the t-test was performed on the observed indices of the 64 patients before and after the operation. If P < 0.05, it means the difference was statistically significant.
Results
Demographic details
A total of 64 patients received and completed this combined treatment, and the baseline characteristics of all these patients are given in Table 1. The average age of the 64 patients was 42.20 ± 5.39 years. The average duration of clinical symptoms such as dysmenorrhea and menorrhagia was 5.02 ± 4.72 years, and the average BMI was 24.02 ± 3.59 kg/m2. Among them, 36 patients had endometriosis and 18 patients had leiomyoma of the uterus, 9 patients had both, while 19 patients had only adenomyosis without uterine leiomyoma or endometriosis.
Pre- and post-treatment outcomes
(1) The hemoglobin value remeasured 3 months after the operation increased to a certain extent compared with that before the operation, and the difference was statistically significant (P = 0.000) (see Table 2). (2) A total of 50 patients out of the 64 patients before the operation tested positive for CA125. The remeasured CA125 1 month after the operation was lower than that before the operation, and the difference was statistically significant (P = 0.000) (see Table 2). (3) The degree of dysmenorrhea after the completion of GnRH-a sequential treatment was lower than that before the operation, and the difference was statistically significant (P = 0.000) (see Table 2). (4) The menstrual volume score after completion of GnRH-a sequential treatment was lower than that before the operation, and the difference was statistically significant (P = 0.000) (see Table 2). (5) The estimated average uterine volume re-examined 3 months after completion of the sequential treatment was smaller than that before the operation, and the difference was statistically significant (P = 0.000) (see Table 2). (6) During the postoperative follow-up, only one patient developed an intrauterine device migration, and the vaginal ultrasound showed that the upper end of the birth control ring was approximately 1.5 cm away from the bottom of the uterine cavity. The average follow-up lasted 24.02 ± 11.77 months, and no disease progression was observed.
Operation time and blood loss
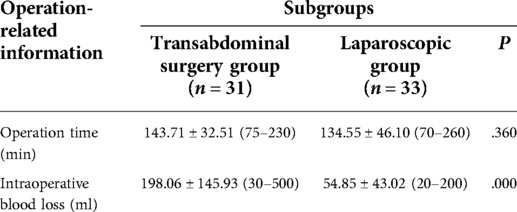
None of the 64 patients had any complications during or after the operation. There was no significant difference in the operation time between the two subgroups (P > 0.05). There was a statistically significant difference in intraoperative blood loss between the two subgroups (P = 0.000) (see Table 3).
Pre- and post-treatment outcomes of the two subgroups
After the treatment was completed, the abdominal approach treatment of this scheme was completed, and various observation indices improved after treatment (P = 0.000). The laparoscopic approach treatment of this scheme was completed, and the observation indices also improved after treatment (P = 0.000). The differences in the observed indices between the two subgroups were not statistically significant (P > 0.05) (see Table 4).
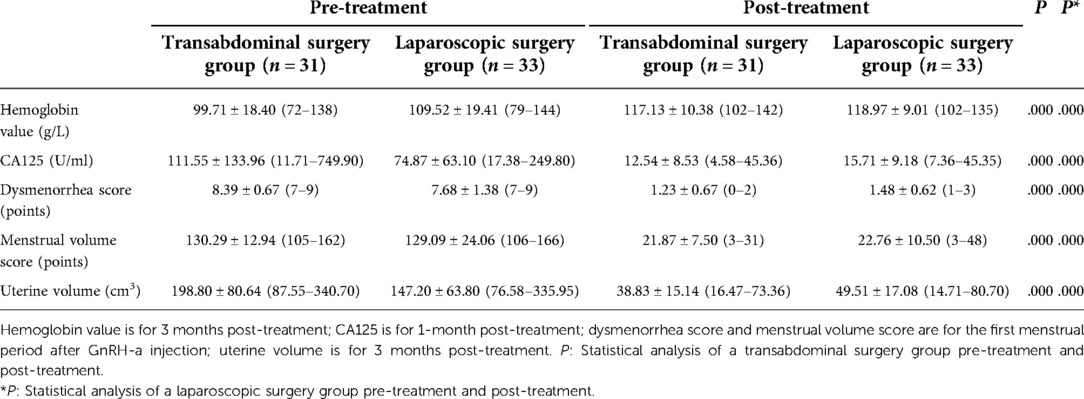
Table 4. Changes in observed indices pre-treatment and post-treatment of a transabdominal surgery group and a laparoscopic surgery group .
Discussion
According to the data in Table 1, the average age of our 64 patients is 42.20 ± 5.39 years. This is similar to the fact that most of the reported patients are over 40 years old, and the age of onset is getting younger (24–26). The average BMI value is 24.02 ± 3.59 kg/m2, which is a bit high, and it has been reported that an increase in BMI values increases the risk of uterine leiomyoma (27, 28), but whether AM is associated with patient weight gain may be an issue worth exploring. The data indicate that two patients did not have children, one of whom had two miscarriages, and the other had no history of abortion. A total of 62 (97%) patients had a history of childbearing, and 50 (78%) had a history of miscarriage, which was also related to the fact that fertility and abortion may be the causes of AM (29). In order to reduce AM incidence, we should adopt good contraceptive measures to reduce unnecessary abortions. The data indicated that a total of 18 (28%) patients were complicated with leiomyoma, which was similar to the reported 15%–57% (30), and 36 (56%) patients were complicated with endometriosis. Some studies have suggested that the incidence of adenomyosis with deep infiltrating endometriosis (DIE) is within 6.8%–25.4% (31). The reason for this may be related to our careful exploration of pelvic endometriosis, especially the occurrence of DIE.
Hysterectomy is still the main radical method for patients with severe adenomyosis. However, the uterus is a unique organ for women, especially in China where hysterectomy is not accepted by most patients, and this mindset is also observed in Japan (32). With the progress and improvement of conservative surgical methods in recent years, the double-flap method and the triple-flap method can not only preserve the uterus but also improve the short-term effects of dysmenorrhea, excessive menstruation, and infertility (19, 20). However, the boundary between the focus of AM and normal myometrium is not clear, and the focus cannot be completely removed during the operation (33). The residual small lesions may grow slowly and recur (34, 35). A 2-year follow-up study showed that the recurrence rate of patients with adenomyosis treated with GnRH-a was 28.1%, which was lower than the 49% for treatment with surgery alone (36). The LNG-IUS can release progesterone directly into the uterine cavity. In recent years, many studies have confirmed that the LNG-IUS can relieve symptoms such as dysmenorrhea and menorrhagia in patients with AM and reduce the level of CA125 to a certain extent (9, 23, 37). However, some studies have found that the slippage rate among AM patients with a larger uterus is as high as 37.5% after the implantation of the LNG-IUS (38). It can be seen that the therapeutic effect of the LNG-IUS on AM patients with a larger uterine volume is not as good as that of AM patients with a smaller uterine volume.
We improved the method of operation by cutting the uterus vertically to the uterine cavity, eradicating AM lesions as much as possible, and reducing the uterine cavity according to the depth of the LNG-IUS. The process of removing the focus is to change the adenomyosis from a larger volume to a smaller one. The postoperative combination with the GnRH-a therapy can reduce the recurrence rate of AM to a certain extent (36). The LNG-IUS has a better therapeutic effect on AM patients with a smaller uterine volume (9, 23, 37). The therapeutic effect of this regimen may be similar to that of the LNG-IUS in the treatment of small-volume adenomyosis. During the postoperative follow-up, only one patient had a downward displacement of the LNG-IUS, as observed with transvaginal ultrasound (transvaginal ultrasound showed that the upper end of the LNG-IUS was approximately 1.5 cm from the bottom of the uterine cavity). The LNG-IUS of the other patients was normal. During the follow-up, it is suggested that the incidence of LNG-IUS displacement is temporarily much lower than that suggested in other reports (39–42). We are of the opinion that the decrease in the LNG-IUS displacement may be related to the following: intraoperative remodeling of the uterine cavity to better align the LNG-IUS with the shape of the uterine cavity; and the postoperative injection of GnRH-a, which can reduce the uterine volume and uterine cavity again, reducing the probability of spondylolisthesis. However, it is necessary to increase the sample size and evaluate the change in the LNG-IUS slippage rate with time after the placement of the LNG-IUS. The effective time of the LNG-IUS is 5 years. If the patient is still menopausal after 5 years, a new LNG-IUS can be replaced until menopause to maintain the therapeutic effect.
It is difficult to remove the focus of adenomyosis completely because there is no obvious boundary between adenomyosis and normal myometrium. If there are residual small lesions during the operation, they may recur as time passes. A retrospective study showed that with the increase in follow-up time after surgery, the recurrence rate of patients with adenomyosis increases from the lowest (no recurrence) to the highest (close to 50%) (17). Some studies have also confirmed that GnRH-a or LNG-IUS treatment can reduce the recurrence rate (9, 23, 36, 37). Therefore, we designed this scheme, and it can be seen from Table 2 that the patients who completed sequential treatment after a modified subtotal resection of adenomyosis showed a significant improvement in postoperative hemoglobin, CA125, dysmenorrhea, menstrual volume, and uterine volume. The average uterine volume estimated by transvaginal ultrasound significantly reduced after treatment in both the transabdominal group and the laparoscopic group. The reduction of the volume of the uterus is the inevitable result of surgical resection of the focus of adenomyosis, but the author opines that it is very important to monitor the changes in the uterus volume after the operation. During the follow-up period, if the uterus volume increases with time, we need to be on guard against recurrence. Table 3 shows that the amount of intraoperative bleeding in the laparoscopic subgroup is lower than that in the transabdominal subgroup, which has something to do with the finer laparoscopic operation. However, there was no significant difference in operation time between the two subgroups. Table 4 shows that there is no significant difference in the therapeutic effect between the transabdominal approach subgroup and the endoscopic approach subgroup, considering that the improvement of the endoscopic technique of the operator may achieve a result similar to that of the transabdominal approach. Longer follow-up observation is still needed.
After the improvement of this operation and the sequential treatment with the combination of two drugs, the following advantages are observed: (1) The uterus of the patient can be preserved, the effect of cutting off the ascending branch of the uterine artery on the ovary can be avoided, the length of the vagina is not reduced, and the quality of female sex life can be preserved; (2) it retains the original normal structure around the cervix, reduces the probability of injuring the ureter, bladder, and other adjacent organs during the operation, and avoids the change of micturition and defecation habits caused by the change in the pelvic structure; (3) during the operation, the uterine cavity is repaired according to the length of the LNG-IUS, which is the difference between this operation and other uterine preservation operations. Remodeling the uterine cavity can directly and effectively reduce the amount of menstruation. Placing the LNG-IUS during the operation can also help with the treatment of AM to consolidate the surgical effect and reduce recurrence. A more appropriate size of the uterine cavity can reduce slippage of the LNG-IUS to a certain extent; (4) postoperative injection of GnRH-a can further atrophy the residual lesions, reduce the probability of abnormal uterine bleeding after the placement of the LNG-IUS, and also reduce slippage of the LNG-IUS by reducing the amount of menstruation (43).
After the improvement of this procedure, the following shortcomings are observed. (1) Endometriosis is possible during the operation, so we emphasize that the uterine cavity should be closed as soon as possible to reduce the exposure time and chance. Meanwhile, after uterine remodeling, the pelvic cavity and abdominal cavity should be fully washed to reduce the possibility of residual blood and residual endometrial cells. (2) Many tissues will be resected during the surgery, making it easy to leave dead spaces when the seromuscular layer is sutured with the myometrium retained around the uterine cavity, leading to possible hematoma and infection by errhysis. Continuous mattress suture of the uterine cavity was adopted during the operation and the bilateral seromuscular layers were sutured with the “baseball stitching technique.” This suture method can reduce needle bleeding while making the suture closer, so as to effectively avoid residual dead spaces.
In view of the fact that this study is a retrospective analysis, the number of cases is relatively small, and there may be bias in case selection, with a shorter follow-up period as well. We feel that more prospective studies with big sample sizes are also needed to evaluate and compare the efficacy and safety of this study with other treatment measures.
Conclusion
For patients suffering from severe adenomyosis who have no pregnancy plans and require uterine preservation, transabdominal or laparoscopic subtotal resection of the focus of adenomyosis, combined with the LNG-IUS + GnRH-a sequential treatment, may be a safe and effective alternative when conservative treatments such as drugs fail.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Maternal and Child Health Research Project of Jiangsu Province (F202138), the Scientific Research Support Program for Postdoctoral of Jiangsu Province (2019K064), and the Scientific Research Support Program for “333 Project” of Jiangsu Province (BRA2019161).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kho KA, Chen JS, Halvorson LM. Diagnosis, evaluation, and treatment of adenomyosis. JAMA. (2021) 326(2):177–8. doi: 10.1001/jama.2020.26436
2. Horton J, Sterrenburg M, Lane S, Maheshwari A, Li TC, Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25(5):592–632. doi: 10.1093/humupd/dmz012
3. Graziano A, Lo Monte G, Piva I, Caserta D, Karner M, Engl B, et al. Diagnostic findings in adenomyosis: a pictorial review on the major concerns. Eur Rev Med Pharmacol Sci. (2015) 19(7):1146–54.25912572
4. Mikos T, Lioupis M, Anthoulakis C, Grimbizis GF. The outcome of fertility-sparing and nonfertility-sparing surgery for the treatment of adenomyosis. A systematic review and meta-analysis. J Minim Invasive Gynecol. (2020) 27(2):309–331.e3. doi: 10.1016/j.jmig.2019.08.004
5. Tan J, Moriarty S, Taskin O, Allaire C, Williams C, Yong P, et al. Reproductive outcomes after fertility-sparing surgery for focal and diffuse adenomyosis: a systematic review. J Minim Invasive Gynecol. (2018) 25(4):608–21. doi: 10.1016/j.jmig.2017.12.020
6. Huang YF, Deng J, Wei XL, Sun X, Xue M, Zhu XG, et al. A comparison of reproductive outcomes of patients with adenomyosis and infertility treated with high-intensity focused ultrasound and laparoscopic excision. Int J Hyperthermia. (2020) 37(1):301–7. doi: 10.1080/02656736.2020.1742390
7. Song SY, Lee SY, Kim HY, Park DB, Kim DE, Lee KH, et al. Long-term efficacy and feasibility of levonorgestrel-releasing intrauterine device use in patients with adenomyosis. Medicine (Baltimore). (2020) 99(22):e20421. doi: 10.1097/MD.0000000000020421
8. Abbas AM, Samy A, Atwa K, Ghoneim HM, Lotfy M, Saber Mohammed H, et al. The role of levonorgestrel intra-uterine system in the management of adenomyosis: a systematic review and meta-analysis of prospective studies. Acta Obstet Gynecol Scand. (2020) 99(5):571–81. doi: 10.1111/aogs.13798
9. Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. (2009) 79(3):189–93. doi: 10.1016/j.contraception.2008.11.004
10. Magon N. Gonadotropin releasing hormone agonists: expanding vistas. Indian J Endocrinol Metab. (2011) 15(4):261–7. doi: 10.4103/2230-8210.85575
11. Kang JL, Wang XX, Nie ML, Huang XH. Efficacy of gonadotropin-releasing hormone agonist and an extended-interval dosing regimen in the treatment of patients with adenomyosis and endometriosis. Gynecol Obstet Invest. (2010) 69(2):73–7. doi: 10.1159/000258683
12. Guo Q, Xu F, Ding Z, Li P, Wang X, Gao B. High intensity focused ultrasound treatment of adenomyosis: a comparative study. Int J Hyperthermia. (2018) 35(1):505–9. doi: 10.1080/02656736.2018.1509238
13. Wu Q, Lian Y, Chen L, Yu Y, Lin T. Alleviation of symptoms and improvement of endometrial receptivity following laparoscopic adenomyoma excision and secondary therapy with the levonorgestrel-releasing intrauterine system. Reprod Sci. (2020) 27(6):1259–65. doi: 10.1007/s43032-019-00130-4
14. Pang LL, Mei J, Fan LX, Zhao TT, Li RN, Wen Y. Efficacy of high-intensity focused ultrasound combined with GnRH-a for adenomyosis: a systematic review and meta-analysis. Front Public Health. (2021) 9:688264. doi: 10.3389/fpubh.2021.688264
15. Haiyan S, Lin W, Shuhua H, Wang W. High-intensity focused ultrasound (HIFU) combined with gonadotropin-releasing hormone analogs (GnRHa) and levonorgestrel-releasing intrauterine system (LNG-IUS) for adenomyosis: a case series with long-term follow up. Int J Hyperthermia. (2019) 36(1):1179–85. doi: 10.1080/02656736.2019.1679892
16. Wang W, Ma X, Zhang W, Li Z, Wang Y, Yu Z, et al. Relapse after conservative surgery combined with triptorelin acetate versus conservative surgery only in women with focal adenomyosis: study protocol for a multicenter, prospective, randomized controlled trial. Trials. (2020) 21(1):364. doi: 10.1186/s13063-020-04294-2
17. Younes G, Tulandi T. Conservative surgery for adenomyosis and results: a systematic review. J Minim Invasive Gynecol. (2018) 25(2):265–76. doi: 10.1016/j.jmig.2017.07.014
18. Kim JK, Shin CS, Ko YB, Nam SY, Yim HS, Lee KH. Laparoscopic assisted adenomyomectomy using double flap method. Obstet Gynecol Sci. (2014) 57(2):128–35. doi: 10.5468/ogs.2014.57.2.128
19. Huang X, Huang Q, Chen S, Zhang J, Lin K, Zhang X. Efficacy of laparoscopic adenomyomectomy using double-flap method for diffuse uterine adenomyosis. BMC Womens Health. (2015) 15:24. doi: 10.1186/s12905-015-0182-5
20. Zhu L, Chen S, Che X, Xu P, Huang X, Zhang X. Comparisons of the efficacy and recurrence of adenomyomectomy for severe uterine diffuse adenomyosis via laparotomy versus laparoscopy: a long-term result in a single institution. J Pain Res. (2019) 12:1917–24. doi: 10.2147/JPR.S205561
21. Munro MG, Critchley HOD, Fraser IS, FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. (2018) 143(3):393–408. doi: 10.1002/ijgo.12666. Erratum in: Int J Gynaecol Obstet. (2019 Feb) 144(2):237. doi: 10.1002/ijgo.12666
22. Herman MC, Mak N, Geomini PM, Winkens B, Mol BW, Bongers MY, et al. Is the Pictorial Blood Loss Assessment Chart (PBAC) score associated with treatment outcome after endometrial ablation for heavy menstrual bleeding? A cohort study. BJOG. (2017) 124(2):277–82. doi: 10.1111/1471-0528.14434
23. Cho S, Nam A, Kim H, Chay D, Park K, Cho DJ, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. (2008) 198(4):373.e1–7. doi: 10.1016/j.ajog.2007.10.798
24. Naftalin J, Hoo W, Pateman K, Mavrelos D, Holland T, Jurkovic D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. (2012) 27(12):3432–9. doi: 10.1093/humrep/des332
25. Chapron C, Tosti C, Marcellin L, Bourdon M, Lafay-Pillet MC, Millischer AE, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. (2017) 32(7):1393–401. doi: 10.1093/humrep/dex088
26. Pinzauti S, Lazzeri L, Tosti C, Centini G, Orlandini C, Luisi S, et al. Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol. (2015) 46(6):730–6. doi: 10.1002/uog.14834
27. Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. (2016) 59(1):2–24. doi: 10.1097/GRF.0000000000000164
28. Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. (2018) 46:3–11. doi: 10.1016/j.bpobgyn.2017.09.004
29. Genc M, Genc B, Cengiz H. Adenomyosis and accompanying gynecological pathologies. Arch Gynecol Obstet. (2015) 291(4):877–81. doi: 10.1007/s00404-014-3498-8
30. Weiss G, Maseelall P, Schott LL, Brockwell SE, Schocken M, Johnston JM. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women’s Health Across the Nation (SWAN). Fertil Steril. (2009) 91(1):201–6. doi: 10.1016/j.fertnstert.2007.11.025
31. Khan KN, Fujishita A, Koshiba A, Kuroboshi H, Mori T, Ogi H, et al. Biological differences between intrinsic and extrinsic adenomyosis with coexisting deep infiltrating endometriosis. Reprod Biomed Online. (2019) 39(2):343–53. doi: 10.1016/j.rbmo.2019.03.210
32. Osada H, Silber S, Kakinuma T, Nagaishi M, Kato K, Kato O. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online. (2011) 22(1):94–9. doi: 10.1016/j.rbmo.2010.09.014
33. Di Spiezio Sardo A, Calagna G, Santangelo F, Zizolfi B, Tanos V, Perino A, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int. (2017) 2017:2518396. doi: 10.1155/2017/2518396
34. Louis LS, Saso S, Chatterjee J, Barsoum E, Al-Samarrai M. Adenomyosis and infertility. Reprod Biomed Online. (2012) 24(5):586. doi: 10.1016/j.rbmo.2012.02.013. author reply 587.22459492
35. Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. (2012) 18(4):374–92. doi: 10.1093/humupd/dms006
36. Wang PH, Liu WM, Fuh JL, Cheng MH, Chao HT. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. (2009) 92(3):876–85. doi: 10.1016/j.fertnstert.2008.07.1744
37. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. (2007) 76(3):195–9. doi: 10.1016/j.contraception.2007.05.091
38. Park DS, Kim ML, Song T, Yun BS, Kim MK, Jun HS, et al. Clinical experiences of the levonorgestrel-releasing intrauterine system in patients with large symptomatic adenomyosis. Taiwan J Obstet Gynecol. (2015) 54(4):412–5. doi: 10.1016/j.tjog.2014.05.009
39. Kriplani A, Singh BM, Lal S, Agarwal N. Efficacy, acceptability and side effects of the levonorgestrel intrauterine system for menorrhagia. Int J Gynaecol Obstet. (2007) 97(3):190–4. doi: 10.1016/j.ijgo.2007.01.009
40. Maldonado LY, Sergison JE, Gao X, Hubacher D. Menstrual bleeding and spotting with the levonorgestrel intrauterine system (52 mg) during the first-year after insertion: a systematic review and meta-analysis. Am J Obstet Gynecol. (2020) 222(5):451–468.e9. doi: 10.1016/j.ajog.2019.09.044
41. Saloranta TH, Gyllenberg FK, But A, Gissler M, Laine MK, Heikinheimo O. Free-of-charge long-acting reversible contraception: two-year discontinuation, its risk factors, and reasons. Am J Obstet Gynecol. (2020) 223(6):886.e1–886.e17. doi: 10.1016/j.ajog.2020.06.023
42. Bahamondes MV, de Lima Y, Teich V, Bahamondes L, Monteiro I. Resources and procedures in the treatment of heavy menstrual bleeding with the levonorgestrel-releasing intrauterine system (LNG-IUS) or hysterectomy in Brazil. Contraception. (2012) 86(3):244–50. doi: 10.1016/j.contraception.2011.12.005
Keywords: severe adenomyosis, subtotal resection of adenomyosis, levonorgestrel intrauterine delivery system, gonadotropin-releasing hormone agonist, dysmenorrhea
Citation: Qin Z, Dong Z, Tang H, Zhang S, Wang H, Bao M, Wei W, Shi R, Chen J and Xia B (2022) Application of modified subtotal resection of adenomyosis combined with LNG-IUS and GnRH-a sequential therapy in severe adenomyosis: A case series. Front. Surg. 9:914725. doi: 10.3389/fsurg.2022.914725
Received: 7 April 2022; Accepted: 11 July 2022;
Published: 18 August 2022.
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Diana Buzinskiene, Vilnius University, LithuaniaMuhammad Azrai Abu, UKM Medical Centre, Malaysia
© 2022 Qin, Dong, Tang, Zhang, Wang, Bao, Wei, Shi, Chen and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiming Chen Y2ptaW5nQDEyNi5jb20= Bairong Xia eGlhYmFpcm9uZ0B1c3RjLmVkdS5jbg==
†These authors share first authorship.
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
 Zhenyue Qin
Zhenyue Qin Zhiyong Dong
Zhiyong Dong Huimin Tang2,†
Huimin Tang2,† Huihui Wang
Huihui Wang Weiwei Wei
Weiwei Wei Jiming Chen
Jiming Chen Bairong Xia
Bairong Xia