
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg. , 26 July 2022
Sec. Genitourinary Surgery and Interventions
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.912438
This article is part of the Research Topic Optimizing Outcomes of Surgical Interventions in Urologic Oncology View all 7 articles
Introduction: Non-muscle-invasive bladder cancer (NMIBC) is a common and heterogeneous disease; many patients develop recurrent or progress to muscle-invasive disease. Intravesical drug therapy is a pillar in the current management of NMIBC; notwithstanding, Mitomycin C (MMC) and Bacillus Calmette-Guérin (BCG) have numerous limitations including international supply issues, and local and systemic toxicity. Here we review novel intravesical therapeutic options and drug delivery devices with potential for clinical use in the treatment of NMIBC.
Methods: PubMed, ClinicalTrials.gov and Cochrane Library searches were undertaken. Systematic reviews, meta-analyses, randomised controlled trials, single-arm clinical trials and national/international conference proceedings were included.
Results: Novel intravesical drugs, including chemotherapeutic agents, immune checkpoint inhibitors, monoclonal antibodies and gene therapies, have demonstrated varying efficacy in the treatment of NMIBC. Current evidence for the majority of treatments is mostly limited to single-arm trials in patients with recurrent NMIBC. Various novel methods of drug delivery have also been investigated, with encouraging preliminary results supporting the intravesical delivery of hyperthermic MMC and MMC hydrogel formulations.
Conclusions: Novel therapeutic agents and drug delivery systems will be important in the future intravesical management of NMIBC. As our understanding of the molecular diversity of NMIBC develops, molecular subtyping will become fundamental in the personalisation of intravesical treatments. Further randomised studies are urgently required to investigate the efficacy of novel intravesical treatments and novel regimens, in comparison to current standards-of-care, particularly in the context of international BCG shortages.
Bladder cancer (BCa) is the twelfth most common cancer worldwide (1), but as a consequence of an international lack of research funding, treatment has made very little progression over the last 25 years, with both pharmacological and surgical treatment options remaining largely unchanged (2). Most patients (75%–80%) present with “early-stage,” non-muscle-invasive disease (NMIBC: stages Tis/Ta/T1, Figure 1) (3); but many are sub optimally managed with recurrences occurring in up to 80%. Furthermore, 40%–50% of cases progress to muscle-invasive Bca (MIBC: stages T2+, Figure 1) which carries a 5-year survival rate of only 27%–50% (4–7), emphasising the need for early diagnosis and appropriate primary treatment.
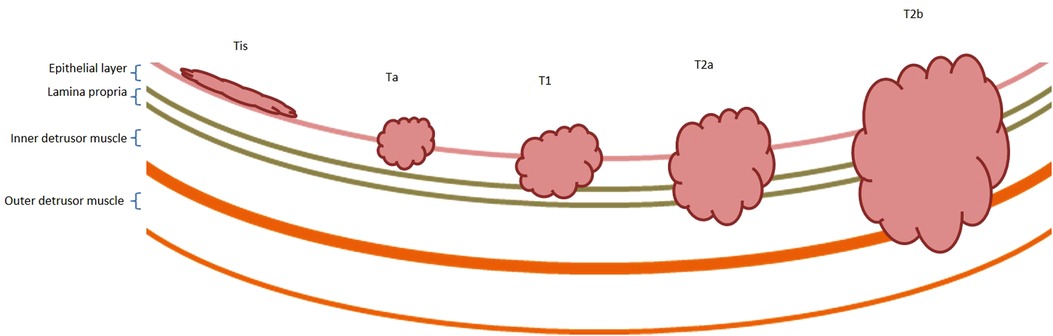
Figure 1. Non-muscle and muscle-invasive bladder cancer. Bladder cancer is described as non-muscle or muscle invasive. Non-muscle invasive bladder cancer includes Tis, Ta and T1 disease, as described by the TNM staging system (131). Tis lesions are typically located on the luminal surface. Ta tumours extend into the urothelium, and T1 tumours extend into the lamina propria. Muscle-invasive bladder cancers include T2, T3 and T4 disease. T2 tumours extend into the inner half (T2a) and outer half (T2b) of the detrusor muscle, respectively. T3 tumours extend in to the perivesical fat and lymph nodes and T4 tumours invade organ systems. T3 and T4 tumours are not shown within this diagram.
NMIBCs are highly heterogeneous both clinically and biologically (8). High-grade NMIBCs have a high mutation burden, multiple copy number changes and loss of tumour suppressors (TP53, RB1) more akin to MIBC, whereas “low-grade NMIBCs exhibit oncogene activation (FGFR3, RAS) in a relatively normal genome (9). Epigenetic changes, including histone tail modifications, microRNA expression and DNA hypermethylation, also have an association with NMIBC progression and phenotype (10, 11). Recently, “subtyping” based on gene expression has offered further insights into NMIBC biology (12), yet risk stratification and treatment selection remains entirely based upon clinico-pathological characteristics, without the inclusion of biomolecular information (3). Notwithstanding, the majority of NMIBCs are considered amenable to bladder preservation with transurethral resection of bladder tumour (TURBT) and adjuvant intravesical therapy, albeit accompanied by adverse effects (AEs) (13, 14).
The European Association of Urology (EAU) guidelines stratify NMIBC into low-, intermediate-, high- and very high-risk disease, which indicates the recommended adjuvant intravesical therapy or the need for upfront radical cystectomy (RC) (3). It is widely accepted that in low-risk tumours, single dose, post-TURBT mitomycin C (MMC) reduces the risk of recurrence and can be considered curative. Intermediate-risk disease should be managed with six instillations of MMC or one year of Bacillus Calmette-Guérin (BCG), and high-risk disease by BCG induction, followed by up to three years of maintenance BCG therapy. Patients with very high-risk disease should be offered immediate RC, or full-dose BCG for one to three years. Patients typically undergo radiological, cystoscopic, and sometimes urinary biomarker surveillance, depending upon their risk category (3). Given the intensive surveillance schedules, potentially prolonged treatment courses, and high recurrence rate (up to 85%), NMIBC is considered one of the most expensive malignancies to treat (15).
MMC and BCG both have unpleasant local side effects including chemical cystitis, and haematuria (14). Intravesical BCG can also lead to severe systemic side effects including flu-like symptoms, pneumonia and sepsis, and has ongoing global production and supply issues (14, 16–18). Despite adequate treatment, some NMIBC becomes resistant to MMC and/or BCG (19, 20). BCG-unresponsive and relapsing NMIBC is particularly challenging to treat, with few management options other than radical cystectomy (RC). Therefore, novel and effective, chemotherapeutic agents are urgently needed for the treatment of NMIBC (21).
Here we present a narrative review which explores the evidence for the use of novel intravesical drug treatments and regimens in the treatment of NMIBC, either alone or in-combination with existing standards-of-care. We will consider all new intravesical drugs and intravesical drug delivery methods which have been tested in a clinical trial setting, but which are not yet clinically approved for the treatment of NMIBC by national or international guidelines.
PubMed, Clinicaltrials.gov and the Cochrane Library were interrogated with the search terms: “non-muscle invasive bladder cancer”, “superficial bladder cancer”, “transitional cell carcinoma”, “urothelial cell carcinoma”, “intravesical therapy” and “intravesical drug delivery”. Meta-analyses, systematic reviews, randomised controlled trials (RCTs) and single-arm clinical trials were included from 2000 to 2022. Internationally and nationally-recognised conference proceedings detailing preliminary results were included for ongoing and unpublished clinical trials. Due to the small number of research in this field, studies of novel intravesical therapeutics in newly diagnosed NMIBC (stages Tis/Ta/T1), recurrent disease, and BCG “refractory”, “relapsing” and “unresponsive” disease were included. We excluded pre-clinical studies, and papers which were not published in the English language.
In this review, we use the terms “refractory”, “relapsing” and “unresponsive” NMIBC, per the definitions in the European Association of Urology (EAU) guidelines (3). BCG-refractory tumours are carcinoma in situ (Tis) or high-grade NMIBCs which remain after three months of BCG re-induction or maintenance therapy. BCG-relapsing tumours occur after completion of maintenance BCG, despite an initial treatment response. BCG-unresponsive tumours are BCG-refractory tumours and recurrences of high-grade tumours or Tis within 6 or 12 months of adequate BCG therapy, respectively. All AEs discussed are classified by the National Cancer Institute's Common Toxicity Criteria for Adverse Events (22).
A summary of novel intravesical drugs and drug delivery methods for the treatment of NMIBC is shown in Table 1.
Gemcitabine (2′-deoxy-2′,2′-difluorocytidine-hydrochloride, beta isomer) is a pyramidine nucleoside antimetabolite. It was originally investigated for its anti-viral properties, but was incidentally found to have potent anticancer effects. Following cellular uptake, gemcitabine is converted into the active metabolite difluorocytidine-hydrochloride-5-diphsoph-5-triphosphate, which terminates DNA replication and initiates apoptosis (23, 24) (Figure 2). As a parenteral chemotherapeutic agent, it is currently licensed for metastatic BCa (25) and is commonly used in combination with cisplatin for the neoadjuvant treatment of MIBC (5).
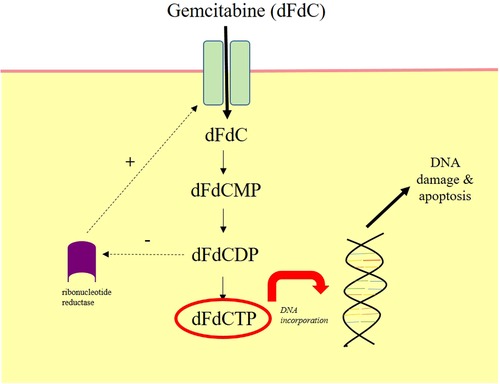
Figure 2. Mechanism of action of intravesical gemcitabine. Gemcitabine (dFdC) is prodrug. It moves intracellularly following intravesical administration through nucleoside transporters. It is phosphorylated by kinases into gemcitabine monophosphate (dFdCMP), gemcitabine diphosphate (dFdCDP) and finally gemcitabine triphosphate (dFdCTP). dFdCTP incorporates into the DNA strand and inhibits DNA synthesis, which mediates cellular apoptosis. dFdCDP also inhibits ribonucleotide reductase, which drives further dFdC uptake. Diagram redrawn from Ueno et al, 2007 (24).
Given its efficacy in metastatic BCa, there have been several studies investigating intravescial gemcitabine in the management of NMIBC. Typical dosing is 1000–2000 mg in 50–100 ml of saline, aiming to produce urinary concentrations of approximately 40 mg/ml (26). Marker lesion studies use cystoscopy to assess BCa size and morphology pre- and post-treatment, and suggest that intravesical gemcitabine is most effective when delivered as a course; bi-weekly intravesical administration (over three weeks) has demonstrated a complete response (CR, defined as complete disappearance of the lesion at nine weeks) in 40% of patients (27). Intravesical gemcitabine is generally well-tolerated, and is associated with a low frequency of grade 2 and 3 AEs such as mild dysuria, cystitis, and haematuria (28–30).
Intravesical gemcitabine may be more effective than MMC in the treatment of NMIBC (28). In a meta-analysis of 335 NMIBC patients from five RCTs, recurrence rates were significantly lower in patients receiving an eight week course of gemcitabine form to a six week course of MMC (OR 0.44, 95% CI, 0.24–0.78). Gemcitabine was also associated with a significantly lower rate of AEs than MMC.
This meta-analysis included the results of five clinical trials, but there is significant study heterogeneity, including in drug dosing and delivery schedules (28). Furthermore, many of the studies included did not carry out double blinding and the available studies may have been prone to publication bias. Further large-scale RCTs are required to compare the treatment efficacy of intravesical gemcitabine to MMC.
Intravesical gemcitabine may also be superior to MMC in the treatment of recurrent NMIBC. In 2010, a phase III trial randomised 109 patients with recurrent NMIBC to weekly, intravesical treatment with a six week course of gemcitabine (2000 mg) or a four week course of MMC (40 mg). After 36 months, recurrence free survival (RFS) was significantly greater in the gemcitabine group (72% vs. 61% vs., p = 0.002), with fewer AEs associated with intravesical gemcitabine than MMC (29, 30)
The efficacy of intravesical gemcitabine in comparison to BCG remains unclear. In one randomised controlled trial (RCT), 64 patients with high-risk NMIBC received weekly intravesical gemcitabine (2000 mg, over six weeks) or BCG (5 × 108 colony forming units) (31). After 44 months, the recurrence rate was significantly higher in the gemcitabine group (53% vs. 28% p = 0.037), suggesting that gemcitabine is less effective than BCG in high-risk tumours. Conversely, a study of 80 high-risk NMIBC patients (who had failed treatment with one course of BCG) were provided with an intravesical course of gemcitabine (2000 mg) or a further course of BCG (81 mg). Both drug regimens consisted of a six week induction period followed by maintenance doses at three, six and twelve months. After one year, 53% of patients receiving gemcitabine developed recurrent disease compared to 88% of those receiving BCG (p = 0.002) (30). Intravesical gemcitabine monotherapy therefore shows promise in the management of NMIBC, including high-risk disease.
Intravesical gemcitabine has also been investigated as a combination therapeutic in early clinical trials, including with everolimus, an mTOR inhibitor which enhances the cytotoxic effect of gemcitabine (32, 33). Bi-weekly, intravesical gemcitabine (2000 mg) was administered for six weeks, and daily oral everolimus (10 mg) for 12 months to 19 patients with BCG-refractory NMIBC (32). Only 16% of patients were disease free at one year, but 53% suffered grade 3 or 4 AEs, leading to early termination of the study. Other trials are ongoing, and are evaluating gemcitabine in combination with intravesical BCG (34), docetaxel (35), cabazitaxel, and cisplatin (36).
Docetaxel is a chemotherapeutic agent which stabilises microtubule assembly, inhibits mitosis, and initiates apoptosis (37). It is in clinical use for metastatic breast and prostate cancers, and has been shown to inhibit in vitro and in vivo growth in BCa models (38, 39). In 2013, a phase I/II trial evaluated six weeks of intravesical docetaxel followed by twelve monthly maintenance doses (5–75 mg) in 54 patients with recurrent NMIBC who had failed to respond to standard intravesical therapy with BCG, MMC or interferon. 59% had an initial response, classified as a negative biopsy and urine cytology six weeks after intravesical treatment. RFS was 40% at one year, but dropped to 25% after three years (40). Overall, docetaxel was well-tolerated and associated with very few grade 1 and 2 AEs (41).
Docetaxel has also been investigated in combination with intravesical gemcitabine (35). A case-series investigated a six week course of weekly intravesical gemcitabine (1 g) and docetaxel (37.5 mg) in 45 patients with recurrent NMIBC (following previous BCG treatment) or a contraindication to BCG treatment. Overall, this study demonstrated a RFS of 54% at 12 months (35). A recent retrospective study has also been published which compares the efficacy of intravesical gemcitabine and docetaxel therapy to intravesical BCG and interferon α-2b in 290 NMIBC patients. The 2-year high-grade RFS was 42% for the gemcitabine and docetaxel group compared to 51% for the BCG and interferon α-2b group (42).
Apaziquone is a bio-reductive alkylating agent and a derivative of MMC. It is a pro-drug which is activated by intracellular reductases to generate cytotoxic species, which initiate DNA damage and apoptosis (43). Apaziquone has demonstrated favourable efficacy in BCa models in vitro; in some cases with superiority over MMC (44).
Clinically beneficial effects have also been demonstrated using intravesical apaziquone in NMIBC (45, 46): 46 patients with Ta/T1 NMIBC underwent TURBT followed by six weekly doses of intravesical apaziquone (4 mg). A CR was seen in 67%, defined as a histological response (46). Despite these encouraging findings, subsequent trials have failed to replicate or confirm the efficacy of apaziquone.
A phase III RCT allocated 1614 NMIBC patients to treatment with TURBT plus a single intravesical dose of apaziquone (4 mg/40 ml) or TURBT plus an intravesical saline placebo. The treatment arm only saw a 7% reduction in the two-year recurrence rate of NMIBC (which was non-significant) (47). Possible reasons for lack of drug efficacy included haematuria (post TURBT) which may increase metabolism of apaziquone. Notwithstanding, some evidence from a pooled analysis found that delaying intravesical instillation of apaziquone (more than 30 minutes post TURBT) significantly improved the two-year recurrence rate compared to the placebo (35% vs 47%, p = 0.0014) (47, 48).
Most alkylating agents are associated with severe myelosupression (49); however, 40 mg of intravesical apaziquone is undetectable in the systemic circulation post administration, and is therefore safe and well tolerated (45, 50). For example, AEs are mild and similar to those seen with intravesical placebo treatments, including dysuria, bladder spasm and urgency (47). Given the encouraging early data and favourable safety and tolerability profiles, further clinical trial evaluation of intravesical apaziquone is warranted.
Immune checkpoints are inhibitory protein complexes which downregulate immune responses and prevent autoimmune cell death. Immune checkpoints are frequently dysregulated by many cancer types; this confers protection from host immune destruction and promotes cell survival, and ultimately tumour progression and metastatic spread. Immune checkpoint complexes consist of a cell-expressed ligand and a receptor (51); monoclonal antibody blockade of this ligand-receptor interaction is a current focus of investigation in BCa therapeutics.
Of particular importance is programmed cell death protein 1 (PD-1), an immune checkpoint inhibitor which is activated by programmed cell death ligand-1 (PD-L1) that supresses T-cell responses (52). PD-L1 is highly expressed in a significant proportion of NMIBCs and can be associated with poor prognosis (53, 54). Pembrolizumab is a monoclonal antibody against PD-1, its intravenous use was approved in 2017 for locally-advanced and metastatic urothelial carcinoma (55). Early clinical studies evaluating intravesical pembrolizumab for the treatment of NMIBC are now also ongoing (56, 57).
Durvalumab is also a monoclonal antibody targeted to PD-L1; it was previously approved in the U.S for the treatment of metastatic BCa, but later withdrawn due to limited clinical efficacy (58–60). However, early-stage clinical trials are currently investigating intravesical durvalumab for NMIBC (61).
Vicinium is a recombinant fusion protein of a single-chain variable monoclonal antibody fragment to the epithelial cell adhesion molecule (EpCAM), and a truncated fragment of pseudomonas exotoxin A (62). The monoclonal antibody fragment localises the drug to EpCAM-positive BCa cells, where the pseudomonas exotoxin A blocks protein synthesis, and mediates cellular apoptosis (63–65).
Clinical trials have demonstrated that intravesical vicinium may be effective against BCG-relapsing NMIBC. 126 patients received an induction course of six weeks of bi-weekly intravesical vicinium followed by six-weeks of weekly instillations (30 mg). Maintenance therapy consisted of further fortnightly intravesical instillations (66, 67). 40% of patients with Tis and 71% of patients with Ta/T1 disease remained disease free at three months (67). In a similar trial of 22 patients treated with six-week course of weekly vicinium (up to 30 mg), 41% had CR at 3 months (68). In 2021, the U.S Food and Drug Administration denied the approval of intravesical vicinium for the treatment of NMIBC (69).
Vicinium is selective for EpCAM-positive BCa cells; with such a targeted mechanism of action, AEs should be limited (67). Also, due to its small molecular weight, almost all vicinium remains within the bladder following intravesical administration (68, 70), hence AEs tend to be localised and mild (grade 1–2); nevertheless, AEs have been reported in up to 52% of patients (67, 71).
Valrubicin, an anthracycline topoisomerase inhibitor and derivative of doxorubicin, is the only intravesical therapy approved in the U.S for the treatment of BCG-refractory NMIBC in patients unfit for cystectomy. The recommended regimen is 800 mg per week, for six weeks (72). The exact mechanism of valrubicin is incompletely understood; however, it is thought to involve interference with nucleic acid metabolism and initiation of cell cycle arrest (73).
In a pivotal trial, 90 patients with recurrent NMIBC (who had received at least one course of BCG) were treated with six weekly 800 mg instillations of intravesical valrubicin; 21% had a CR with a median follow-up time of 30 months. The median time to treatment failure was almost 18 months (74). Further clinical studies of intravesical valrubicin have found similar treatment response rates (73, 75–78).
High rates of AEs have been reported with intravesical valrubicin, with up to 86% of patients developing local reactions including urinary frequency, dysuria and skin irritation (78). AEs tended to occur immediately after drug administration, although only 5% of patients discontinue treatment due to side effects (73). Systemic AEs are uncommon, with a handful of reports of azotaemia and renal failure (76, 78)
Intravesical BCG immunotherapy is routinely given in high-risk NMIBC, but many patients develop recurrent disease. To improve efficacy, a combination therapy of BCG with intravesical ALT-803 has been proposed (79). ALT-803 is a recombinant complex of an interleukin-15 (IL-15) “superagonist”, and a human IL-15 receptor α-sushi domain/human IgG1 Fc fusion protein (IL-15 alpha Se/Fc fusion protein). IL-15 activates Natural Killer and CD8+ T cells and IL-15 alpha Se/Fc fusion protein enhances its biological activity. In a phase I clinical trial, a six-week course of weekly intravesical ALT-803 (escalating doses) and BCG (50 mg) was delivered to nine patients with BCG-naïve NMIBC. Intravesical treatment was delivered following TURBT in patients with evidence of Ta/T1 disease. All patients had a CR at 24 months, which was defined as a normal cystoscopy and negative biopsy or voided urinary cytology (79). In a case-report, a 91-year-old male with recurrent NMIBC, who was unsuitable for cystectomy, was treated with six doses of weekly intravesical ALT-803 (400 µg) and BCG (50 mg). The patient remained disease-free for more than 19 months (80). In a recent phase II/III trial, intravesical ALT-803 was delivered in combination with BCG to 80 patients with NMIBC Tis with or without additional Ta/T1 disease. Preliminary results show a 72% complete response rate (CRR) which was defined as absence of Tis at any time point. It is predicted that the CRR at 12 months will be 59% (81).
A large-scale RCT is currently ongoing and evaluating the efficacy of intravesical ALT-803 and BCG combination therapy compared to BCG monotherapy, in patients with BCG-naïve NMIBC (82). A clinical trial was planned to assess the efficacy of ALT-803 monotherapy, but was terminated in 2021 due to the coronavirus pandemic (83). AEs have unfortunately been reported in all patients receiving combination intravesical ALT-803 and BCG, and include hypertension, haematuria and urinary tract infection (84, 85). It is unclear whether BCG or ALT-803 or their combination, was the principal contributor to these side effects.
Gene therapy uses viral or plasmid vectors to introduce exogenous DNA into a host cell to produce a therapeutic response. It can be used as an oncological treatment, for example, where the introduction of genes may activate an immune antitumour response (86, 87).
Nadofaragene firadenovec (rAd-IFNα/Syn3) is a recombinant adenovirus vector which contains the human recombinant interferon α-2b gene and Syn3. IFN α-2b is an immune modulator, which has been shown to be safe as an intravesical therapy (88), but has limited therapeutic efficacy, potentially due to its short half-life (89, 90). To overcome this, rAd-IFNα/Syn3 has been developed to introduce the IFN α-2b gene into urothelial cells, which sustains intravesical IFN α-2b release (91). Syn3 is a polyamide surfactant which enhances gene transfer into BCa cells, and appears to be key for IFN α-2b gene expression (91). Intravesical rAd-IFNα/Syn3 has shown promising results in the management of BCG-unresponsive NMIBC. In a phase III clinical trial, 151 patients were treated with three, 12 weekly intravesical doses of rAd-IFNα/Syn3 (75 ml). 60% of patients had a CR at three months and 31% of patients were free from a high-grade recurrence at 12 months (90). 66% patients reported mild AEs; however, these were generally acceptable and led to only three patients stopping treatment (90). Overall, rAd-IFNα/Syn3 appears a promising intravesical therapy candidate, and further studies are currently ongoing to evaluate long-term efficacy (92).
CG0070 is an oncolytic adenovirus which selectively and preferentially replicates in cancerous cells with retinoblastoma-pathway (Rb-pathway) defects. It encodes and expresses the granulocyte-macrophage-colony stimulating factor (GM-CSF): a cytokine which stimulates granulocyte production and initiates antitumour immune responses (93). Rb-pathway defects are common in BCa and are associated with recurrence, disease progression, and high mortality rates (94).
To improve vector transduction across the BCa cell membrane, CG0070 is routinely delivered with the surfactant dodecylmaltoside (95). In a 2018 phase II clinical trial, a six week course of weekly intravesical dodecylmaltoside 0.1% (75 ml) and CG0070 (1X1012 VP) was delivered to 45 patients with treatment resistant NMIBC. All patients had failed treatment with two previous courses of intravesical therapeutics, with at least one course of BCG treatment. Six months post-treatment with CG0070, 47% of patients had a CR and only 2% of patients had progressed to muscle-invasive disease (96). In a 12 month follow-up analysis of 57 patients, 30% had a CR (97). Reported AEs included immunological symptoms such as fever and chills (97).
A six-week combination therapy of intravesical CG0070 and IV pembrolizumab (400 mg) is now also being investigated for patients with BCG-unresponsive NMIBC (98, 99).
BC-819 is a recombinant DNA plasmid containing a regulatory H19 gene promoter that drives BCa cell expression of the diphtheria toxin A which in-turn inhibits local protein synthesis and mediates cell death (100, 101). When delivered to 39 patients with recurrent NMIBC, a six-week course of intravesical BC-819 (20 mg) led to a three month CRR of 33% (102). Unfortunately, the follow-up trial was terminated due to lack of drug efficacy (103).
Intravesical therapy requires direct drug delivery into the bladder through a urinary catheter. Following drug instillation, patients are required to maintain continence for one to two hours to ensure adequate urothelial exposure. Variations in bladder volume and rate of urine production lead to variability in drug concentration. Furthermore, early-voiding can limit drug efficacy (104). Some intravesical drugs, such as BCG, require repeat bladder instillations, which increases the risk of urinary tract infections and is highly inconvenient for both patients and clinicians (3). Various drug delivery devices and techniques have been designed to enhance and sustain the delivery of intravesical therapeutics in the treatment of NMIBC (Figure 3).
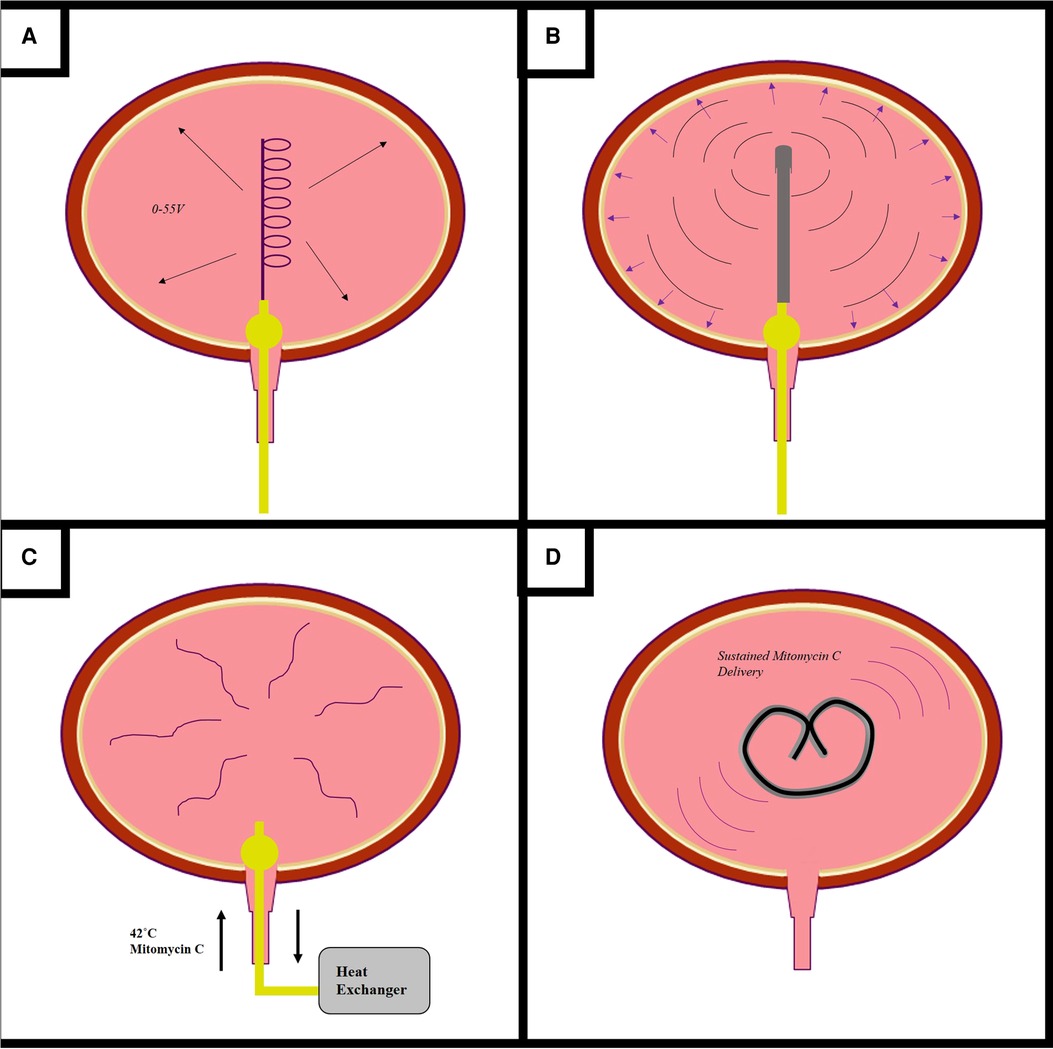
Figure 3. Novel devices for the administration of intravesical drugs. Novels drug delivery methods are being developed to enhance the efficacy of mitomycin c (MMC) and other intravesical therapeutics (A–D). Electromotive drug delivery (A) consists of inserting a cathode into into the bladder and applying an electrical current to enhance urothelial drug uptake. The Synergo system (B) uses a radiofrequency antenna to heat the bladder wall. The Combat BRS system (C) externally heats MMC, for intravesical circulation via a three-way catheter. The GemRIS device (D) is a silicone tube which provides sustained intravescial drug delivery over a two week period.
Electromotive drug administration and chemohyperthermia are well-described techniques which may enhance intravesical drug efficacy in the treatment of NMIBC. Trial evidence has been reported and reviewed elsewhere in the literature, and is outside the remit of our review (105, 106). Electromotive drug administration (EMDA) is a device-assisted therapy which uses an electrical current to enhance delivery and urothelial absorption of MMC through a combination of iontophoresis, electrophoresis, and electroporation. One electrode is inserted into the bladder via a spiral catheter, another placed on the skin of the lower abdomen, and an electrical current of 0–30 mA DC at 0–55 V is passed between them (107). EDMA is not currently recommended in the treatment of NMIBC, due to insufficient clinical trial evidence (105, 108).
Chemohyperthermia (CHT) uses heat energy to increase the urothelial uptake of MMC; it may also enhance BCa cell death through hypoxic mechanisms and by enhancing antitumour immunity (107). There are two main types of CHT. Firstly, the Synergo system uses radiofrequency microwave energy to directly heat the bladder wall. A catheter containing an integrated radiofrequency antenna is inserted into the bladder and heats the epithelium to 42°C (109). The Synergo system is well-explored within the literature, and current EAU guidelines recommend that patients with BCG-unresponsive NMIBC who are unfit for RC may be considered for chemohyperthermia using the Synergo device (3, 110).
The Combat BRS device also uses heat energy. MMC is externally heated to 43˚C and then instilled and recirculated through the bladder using through a three-way catheter. The Combat BRS device is easy and cheap to use, but its use is less explored within the literature (109).
The gemcitabine-releasing intravesical system (GemRIS) device was originally designed for the sustained delivery of lidocaine in the treatment of interstitial cystitis; however, it is under evaluation for gemcitabine delivery in the setting of NMIBC (111, 112). The device consists of a semipermeable silicone tube which is inserted into the bladder via a urinary catheter, from which gemcitabine is released over a two-week period (111). An early-stage clinical trial evaluated the safety of GemRIS in 12 patients with NMIBC (112). One group received the device for two seven-day periods and the second group received the device for two 21-day periods. Full trial data is pending publication (113).
Nanocarriers are materials between 1–200 nm, which have been developed to transport drugs. They are becoming increasingly common in clinical medicine, and various nanocarriers are currently approved in the treatment of ovarian, haematological and gastrointestinal malignancies (114).
Nanoparticle albumin bound paclitaxel (nab-paclitaxel) consists of the chemotherapeutic drug paclitaxel and the delivery component albumin. Paclitaxel (a taxane drug) targets the cytoskeletal protein tubulin and prevents microtubule disassembly and mitosis. The albumin component enhances drug transport across the cell membrane into the intracellular cytoplasm. Nab-paclitaxel is less toxic than unbound paclitaxel and can be administered in higher doses (115). In 2014, 28 patients with recurrent NMIBC received a six-week course of weekly intravesical nab-paclitaxel (500 mg). 36% of patients had an initial CR, and 67% were progression-free at an average follow up period of 21 months (116). On later analysis of this patient cohort, the one year and three year RFS rates were 32% and 18%, respectively (117).
Nanoparticle albumin bound rapamycin (nab-rapamycin) has also been explored as a novel intravesical therapeutic. Rapamycin is an inhibitor of the mTOR signalling pathway, which is thought to be implicated in the progression of NMIBC to MIBC (118). Early-stage clinical trials have found that intravesical ABI-009 (1000 mg) is safe and tolerable. Further trial data are awaiting publication (119).
Hydrogels, particularly those based upon non-synthetic materials that can dissolve, provide sustained intravesical drug release, and may improve drug efficacy. There are two types of intravesical hydrogels: mucoadhesive substances which use chemical or physical interactions to anchor onto bladder mucosa, and floating platform hydrogels which generate microbubbles and float within the bladder. In vivo BCa models have demonstrated that hydrogels can deliver and release a wide-range of chemotherapeutic agents for BCa including MMC, doxorubicin and epirubicin (120).
Phase II clinical trials have shown that intravesical MMC therapy may be curative in some patients with low-risk NMIBC. In 82 patients with a visible recurrence, 37% of patients treated with a four week course of standard intravescial MMC (without surgical intervention) had a CR at three months (121).
UGN-102 is a MMC hydrogel which may increase bladder retention time and improve MMC's efficacy as a primary chemoablative therapy. UGN-102 is administered in a liquid state and turns into a semi-solid gel at body temperature Figure 4). The gel breaks down over a six-hour period and is eliminated through urination. In a single-arm trial, 63 patients with low-grade, intermediate-risk NMIBC were treated with six, weekly instillations of UGN-102. At 12 months, 65% of patients had a CR (122). To further investigate UGN-102 a RCT is currently ongoing to compare a 6-week course of UGN-102 to TURBT only in patients with low-grade, intermediate-risk NMIBC (123).
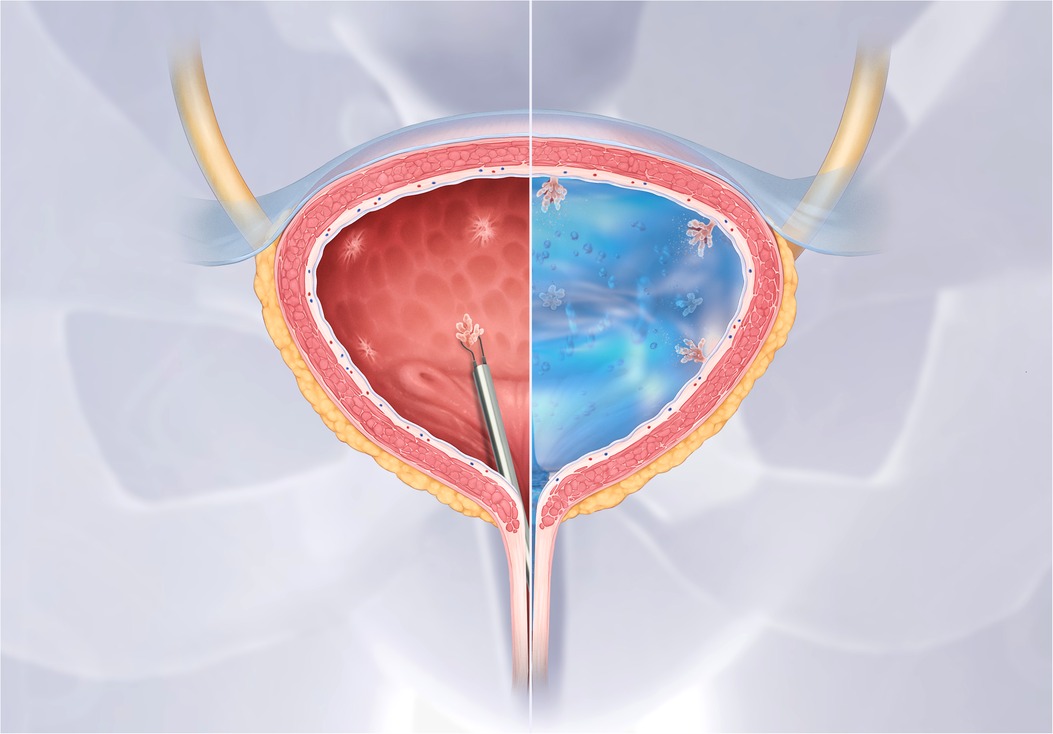
Figure 4. UGN-102 hydrogel for intravesical mitomycin C instillation. Figure supplied by UroGen. UGN-102 is a mitomycin hydrogel. When instilled into the bladder UGN-102 turns from a liquid into a gel substance, which increases drug retention time for up to six hours.
Despite advances in understanding of the natural history and molecular mechanisms of NMIBC development, intravesical MMC and BCG remain the predominant intravesical drugs (14). BCG-unresponsive and relapsing NMIBC is particularly challenging to treat, with few management options other than RC. RC is unsuitable for many patients with MMC or BCG “failure”, due to the inherent comorbidities and frailty of age typically occurring in these patients. RC is associated with reduced quality of life, potentially significant post-operative physical and psychological detriment, and is considered “over-treatment” for many patients (124). Novel and effective chemotherapeutic or immunological agents are therefore urgently needed for the treatment of NMIBC, particularly for patients whose tumours persist or progress despite MMC or BCG therapy.
Intravesical gemcitabine appears particularly promising, has demonstrated greater efficacy than MMC in several trials, and has an acceptable safety and toxicity profile (28, 29). Pending ongoing clinical trials and completed trial data analyses, it is expected that gemcitabine may become routinely available in clinical practice for the management of intermediate-risk NMIBC. Results of early-stage clinical trials are also promising for “targeted” therapies including immune checkpoint inhibitors and monoclonal antibodies. The selectivity of these drugs should minimise their toxicity and side-effects (52–54, 56, 59, 68). Chemohyperthermia and hydrogels are promising and may enhance the clinical efficacy of intravesical MMC delivery (107, 122); with international BCG supply issues, improving MMC efficacy may be particularly important for patients with intermediate- and high-risk NMIBC (125).
Many of the reviewed clinical studies are of single-arm design and some are retrospective case series; therefore, robust comparisons between novel therapeutics or the current standard-of-care with MMC or BCG, cannot currently be made. Furthermore, as outcome assessment is un-blinded, most of these studies may be prone to bias. Drug response and NMIBC recurrence is routinely assessed by cystoscopy and/or urine cytology. As Tis and small papillary tumours may be challenging to identify (due to user dependence of flexible cystoscopy and poor sensitivity of urine cytology for low-grade non-exfoliative tumours), recurrences may have been missed on follow-up. Considering variations in follow-up protocols and methods of surveillance, clinical outcome data from many of these trials described should be interpreted with caution.
Following single-arm, phase II clinical trials, both intravesical valrubicin and IV pembrolizumab were granted fast-track approval in the U.S for the treatment of BCG-refractory, and BCG-unresponsive NMIBC, respectively (74, 126). The approval of valrubicin has been criticised, as follow-up studies have demonstrated a CRR of less than 20% at three months (73, 75–78). Various novel therapeutics have demonstrated greater efficacy than valrubicin, with rAd-IFNα/Syn3 leading to a CRR of >50% at three months (90). Thus, early-approval should also be considered for other more effective intravesical therapies.
Combination therapies are common in the treatment of most cancers, and should be considered for the intravesical treatment of NMIBC. Combining two or more anticancer agents can provide a synergistic effect, prevent drug resistance and improve patient survival. This approach could also reduce the therapeutic doses of each drug required, potentially minimising drug toxicity (127). Indeed, several centres are currently trialling a combination approach to the intravesical treatment of NMIBC, some examples include gemcitabine and BCG, and pembrolizumab and BCG (34, 128).
NMIBC is a heterogeneous disease encompassing a spectrum of genomic, pathological and clinical phenotypes. Developments in technology for genomic analyses have identified molecular subtypes of NMIBC, potentially permitting the future stratification of BCa treatments and the subsequent delivery of personalised intravesical therapeutic approaches in the management of NMIBC (12). Although such precision medicine in NMIBC has not yet been realised, several drugs that target and “reset” genome-wide epigenetic modifications are being investigated in pre-clinical studies, and have shown extremely promising results in pre-clinical BCa models (129, 130).
Novel intravesical therapeutics are urgently needed for the treatment of NMIBC, particularly during the current BCG crisis. Gemcitabine and chemohyperthermia-assisted MMC have both demonstrated superiority over standard MMC therapy in some types of NMIBC. Early-stage clinical trials have also shown very promising results for immune checkpoint inhibitors, monoclonal antibody therapies, and gene therapies. Unfortunately, hitherto, novel intravesical therapeutics have most often been assessed within single-arm study settings, and therefore high quality RCTs are required to drive changes in clinical practice. In the near future, it is hoped that tumour (NMIBC) genomic profiling will allow more accurate risk stratification and targeted intravesical treatments.
KW was responsible for researching and drafting this manuscript. MK and SJM were involved in proofreading and feedback. FK and RTB supervised this project and were key in idea generation and proofreading.
We would like to thank the Metchley Park Medical Society for funding KW's Research Fellowship at the Bladder Cancer Research Centre, University of Birmingham, UK.
RT Bryan contributes to advisory boards for Nonacus Limited and undertakes research funded by Janssen; he has previously conducted research funded by UroGen Pharma and QED Therapeutics. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Boormans JL, Zwarthoff EC. Limited funds for bladder cancer research and what can we do about it. Bladder Cancer. (2016) 2(1):49–51. doi: 10.3233/BLC-150042
3. Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, Dominguez Escrig JL, et al. European Association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. (2022) 81(1):75–94. doi: 10.1016/j.eururo.2021.08.010
4. Sylvester RJ, Rodriguez O, Hernandez V, Turturica D, Bauerova L, Bruins HM, et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur Urol. (2021) 79(4):480–88. doi: 10.1016/j.eururo.2020.12.033
5. Witjes JA, Bruins HM, Cathomas R, Comperat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. (2021) 79(1):82–104. doi: 10.1016/j.eururo.2020.03.055
6. Wallace DM, Bryan RT, Dunn JA, Begum G, Bathers S. Delay and survival in bladder cancer. BJU Int. (2002) 89(9):868–78. doi: 10.1046/j.1464-410X.2002.02776.x
7. Bryan RT, Arnold R, Khanim FL, Shepherd DE, Patel P, Ward DG. Establishing the bladder cancer research centre at the university of birmingham. Nat Rev Urol. (2021) 18(6):318–20. doi: 10.1038/s41585-021-00448-2
8. Ward DG, Arnold R, Bryan RT. Molecular subtypes of T1 bladder cancer: biomolecular characteristics versus clinical utility. Eur Urol. (2020) 78(4):538–9. doi: 10.1016/j.eururo.2020.07.021
9. Hurst CD, Platt FM, Taylor CF, Knowles MA. Novel tumor subgroups of urothelial carcinoma of the bladder defined by integrated genomic analysis. Clin Cancer Res. (2012) 18(21):5865–77. doi: 10.1158/1078-0432.CCR-12-1807
10. Li H-T, Duymich CE, Weisenberger DJ, Liang G. Genetic and epigenetic alterations in bladder cancer. Int Neurourol J. (2016) 20(Suppl 2):S84. doi: 10.5213/inj.1632752.376
11. Kitchen MO, Bryan RT, Emes RD, Glossop JR, Luscombe C, Cheng K, et al. Quantitative genome-wide methylation analysis of high-grade non-muscle invasive bladder cancer. Epigenetics. (2016) 11(3):237–46. doi: 10.1080/15592294.2016.1154246
12. Lindskrog SV, Prip F, Lamy P, Taber A, Groeneveld CS, Birkenkamp-Demtröder K, et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun. (2021) 12(1):1–18. doi: 10.1038/s41467-021-22465-w
13. Nissenkorn I, Herrod H, Soloway MS. Side effects associated with intravesical mitomycin. J Urol. (1981) 126(5):596–7. doi: 10.1016/S0022-5347(17)54642-X
14. Shariat SF, Chade DC, Karakiewicz PI, Scherr DS, Dalbagni G. Update on intravesical agents for non-muscle-invasive bladder cancer. Immunotherapy. (2010) 2(3):381–92. doi: 10.2217/imt.10.1
15. Cox E, Saramago P, Kelly J, Porta N, Hall E, Tan WS, et al. Effects of bladder cancer on UK healthcare costs and patient health-related quality of life: evidence from the BOXIT trial. Clin Genitourin Cancer. (2020) 18(4):e418–42. doi: 10.1016/j.clgc.2019.12.004
16. Waked R, Choucair J, Chehata N, Haddad E, Saliba G. Intravesical Bacillus Calmette-Guérin (BCG) treatment's severe complications: a single institution review of incidence, presentation and treatment outcome. J Clin Tuberc Other Mycobact Dis. (2020) 19:100149. doi: 10.1016/j.jctube.2020.100149
17. Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol. (2006) 175(6):2004–10. doi: 10.1016/S0022-5347(06)00264-3
18. Messing EM. The BCG shortage. Bladder Cancer (Amsterdam, Netherlands). (2017) 3(3):227. doi: 10.3233/BLC-179018
19. Zargar H, Aning J, Ischia J, So A, Black P. Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. . (2014) 11(4):220–30. doi: 10.1038/nrurol.2014.52
20. Alhunaidi O, Zlotta AR. The use of intravesical BCG in urothelial carcinoma of the bladder. Ecancermedicalscience. (2019) 26(13):905. doi: 10.3332/ecancer.2019.905
21. Bryan RT, Arnold R, Khanim FL, Shepherd DE, Patel P, Ward DG. Establishing the bladder cancer research centre at the university of birmingham. Nat Rev Urol. (2021) 18(6):318–20. doi: 10.1038/s41585-021-00448-2
22. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Bethesda, Maryland: U.S. Department of Health and Human Services (2022).
23. Noble S, Goa KL. Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs. (1997) 54(3):447–72. doi: 10.2165/00003495-199754030-00009
24. Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer. (2007) 97(2):145–51. doi: 10.1038/sj.bjc.6603860
25. Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Future Oncol. (2005) 1(1):7–17. doi: 10.1517/14796694.1.1.7
26. Shelley MD, Jones G, Cleves A, Wilt TJ, Mason MD, Kynaston HG. Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): a systematic review. BJU Int. (2012) 109(4):496–505. doi: 10.1111/j.1464-410X.2011.10880.x
27. Gardmark T, Carringer M, Beckman E, Malmstrom PU, Members of the Intravesical Gemcitabine Study G. Randomized phase II marker lesion study evaluating effect of scheduling on response to intravesical gemcitabine in recurrent Stage Ta urothelial cell carcinoma of the bladder. Urology. (2005) 66(3):527–30. doi: 10.1016/j.urology.2005.03.084
28. Li R, Li Y, Song J, Gao K, Chen K, Yang X, et al. Intravesical gemcitabine versus mitomycin for non-muscle invasive bladder cancer: a systematic review and meta-analysis of randomized controlled trial. BMC Urol. (2020) 20(1):1–8. doi: 10.1186/s12894-019-0555-4
29. Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol. (2010) 28(4):543–8. doi: 10.1200/JCO.2008.20.8199
30. Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. (2010) 116(8):1893–900. doi: 10.1002/cncr.24914
31. Porena M, Del Zingaro M, Lazzeri M, Mearini L, Giannantoni A, Bini V, et al. Bacillus Calmette-Guérin versus gemcitabine for intravesical therapy in high-risk superficial bladder cancer: a randomised prospective study. Urol Int. (2010) 84(1):23–7. doi: 10.1159/000273461
32. Dalbagni G, Benfante N, Sjoberg DD, Bochner BH, Machele Donat S, Herr HW, et al. Single arm phase I/II study of everolimus and intravesical gemcitabine in patients with primary or secondary carcinoma in situ of the bladder who failed bacillus Calmette Guerin (NCT01259063). Bl Cancer. (2017) 3(2):113–9. doi: 10.3233/BLC-170095
33. Pinto-Leite R, Arantes-Rodrigues R, Palmeira C, Gaivao I, Cardoso ML, Colaço A, et al. Everolimus enhances gemcitabine-induced cytotoxicity in bladder-cancer cell lines. J Toxicol Environ Health, Part A. (2012) 75(13-15):788–99. doi: 10.1080/15287394.2012.690325
34. U.S National Library of Medicine. Bacillus Calmette-Guérin (BCG) and Gemcitabine in People With High-Grade Non-Muscle Invasive Bladder Cancer That Came Back After BCG Treatment (2019). Available from: https://clinicaltrials.gov/ct2/show/NCT04179162?term = intravesical + gemcitabine&draw=2&rank=8
35. Steinberg RL, Thomas LJ, O’Donnell MA, Nepple KG. Sequential intravesical gemcitabine and docetaxel for the salvage treatment of non-muscle invasive bladder cancer. Bl Cancer. (2015) 1(1):65–72. doi: 10.3233/BLC-150008
36. U.S National Library of Medicine. Intravesical Cabazitaxel, Gemcitabine, and Cisplatin (CGC) in the Treatment Urothelial Carcinoma of the Bladder (CGC) (2018). Available from:https://clinicaltrials.gov/ct2/show/NCT02202772?term = intravesical + gemcitabine&draw=2&rank=17
37. Imran M, Saleem S, Chaudhuri A, Ali J, Baboota S. Docetaxel: an update on its molecular mechanisms, therapeutic trajectory and nanotechnology in the treatment of breast, lung and prostate cancer. J Drug Deliv Sci Technol. (2020) 60:101959. doi: 10.1016/j.jddst.2020.101959
38. Rangel C, Niell H, Miller A, Cox C. Taxol and taxotere in bladder cancer: in vitro activity and urine stability. Cancer Chemother Pharmacol. (1994) 33(6):460–4. doi: 10.1007/BF00686501
39. Song D, Wientjes MG, Au J-S. Bladder tissue pharmacokinetics of intravesical taxol. Cancer Chemother Pharmacol. (1997) 40(4):285–92. doi: 10.1007/s002800050660
40. Barlow LJ, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guerin therapy. J Urol. (2013) 189(3):834–9. doi: 10.1016/j.juro.2012.10.068
41. McKiernan JM, Masson P, Murphy AM, Goetzl M, Olsson CA, Petrylak DP, et al. Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol. (2006) 24(19):3075–80. doi: 10.1200/JCO.2005.03.1161
42. Steinberg RL, Packiam VT, Thomas LJ, Brooks N, Vitale A, Mott SL, et al. editors. Intravesical sequential gemcitabine and docetaxel versus bacillus calmette-guerin (BCG) plus interferon in patients with recurrent non-muscle invasive bladder cancer following a single induction course of BCG. Urol Oncol: Semin Orig Invest. (2022) 40(1):e1–9. doi: 10.1016/j.urolonc.2021.03.024
43. Phillips RM, Hendriks HR, Sweeney JB, Reddy G, Peters GJ. Efficacy, pharmacokinetic and pharmacodynamic evaluation of apaziquone in the treatment of non-muscle invasive bladder cancer. Expert Opin Drug Metab Toxicol. (2017) 13(7):783–91. doi: 10.1080/17425255.2017.1341490
44. van der Heijden AG, Verhaegh G, Jansen CF, Schalken JA, Witjes JA. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol. (2005) 173(4):1375–80. doi: 10.1097/01.ju.0000146274.85012.e1
45. Puri R, Palit V, Loadman PM, Flannigan M, Shah T, Choudry GA, et al. Phase I/II pilot study of intravesical apaziquone (EO9) for superficial bladder cancer. J Urol. (2006) 176(4):1344–8. doi: 10.1016/j.juro.2006.06.047
46. van der Heijden AG, Moonen PM, Cornel EB, Vergunst H, de Reijke TM, van Boven E, et al. Phase II marker lesion study with intravesical instillation of apaziquone for superficial bladder cancer: toxicity and marker response. J Urol. (2006) 176(4):1349–53. doi: 10.1016/j.juro.2006.06.007
47. Karsh L, Shore N, Soloway M, Bhat G, Reddy G, Leu S-Y, et al. Double-blind, randomized, placebo-controlled studies evaluating apaziquone (E09, Qapzola™) intravesical instillation post transurethral resection of bladder tumors for the treatment of low-risk non-muscle invasive bladder cancer. Bl Cancer. (2018) 4(3):293–301. doi: 10.3233/BLC-180166
48. Phillips RM, Loadman PM, Reddy G. Inactivation of apaziquone by haematuria: implications for the design of phase III clinical trials against non-muscle invasive bladder cancer. Cancer Chemother Pharmacol. (2019) 83(6):1183–9. doi: 10.1007/s00280-019-03812-7
49. Moudi M, Go R, Yien CYS, Nazre M. Vinca alkaloids. Int J Prev Med. (2013) 4(11):1231. PMID: 24404355
50. Schellens JH, Planting AST, van Acker BA, Loos WJ, Boer-Dennert M, van der Burg ME, et al. Phase I and pharmacologic study of the novel indoloquinone bioreductive alkylating cytotoxic drug E09. J Natl Cancer Inst. (1994) 86(12):906–12. doi: 10.1093/jnci/86.12.906
51. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. (2020) 11(1):1–3. doi: 10.1038/s41467-019-13993-7
52. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. (2020) 10(3):727. PMID: 32266087
53. Hashizume A, Umemoto S, Yokose T, Nakamura Y, Yoshihara M, Shoji K, et al. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget. (2018) 9(75):34066. doi: 10.18632/oncotarget.26122
54. Fukumoto K, Kikuchi E, Mikami S, Hayakawa N, Matsumoto K, Niwa N, et al. Clinical role of programmed cell death-1 expression in patients with non-muscle-invasive bladder cancer recurring after initial bacillus Calmette–Guérin therapy. Ann Surg Oncol. (2018) 25(8):2484–91. doi: 10.1245/s10434-018-6498-2
55. National Institute for Health and Care Excellence. Pembrolizumab for treating locally advanced or metastatic urothelial carcinoma after platinum-containing chemotherapy (2021). Available from: https://www.nice.org.uk/guidance/ta692
56. Woodcock VK, Purshouse K, Butcher C, Haddon C, Verrall G, Elhussein L, et al. A phase I study to assess the safety and tolerability of intravesical pembrolizumab in recurrent non-muscle invasive bladder cancer (NMIBC). Am J Clin Oncol. (2019) 37(7):406. doi: 10.1200/JCO.2019.37.7_suppl.406
57. U.S National Library of Medicine. Pembrolizumab and BCG Solution in Treating Patients With Recurrent Non-Muscle-Invasive Bladder Cancer. (2017).
58. U.S Food and Drug Administration. Durvalumab (Imfinzi) (2017). Available from:https://www.fda.gov/drugs/resources-information-approved-drugs/durvalumab-imfinzi
59. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. (2020) 21(12):1574–88. doi: 10.1016/S1470-2045(20)30541-6
60. Cancer Network. AstraZeneca Withdraws Durvalumab Indication for Previously Treated Locally Advanced or Metastatic Bladder Cancer 2021 [cited 2022 7th Janurary 2022]. Available from: https://www.cancernetwork.com/view/astrazeneca-withdraws-durvalumab-indication-for-previously-treated-locally-advanced-or-metastatic-bladder-cancer
61. U.S National Library of Medicine. Efficacy of Durvalumab in Non-muscle-invasive Bladder Cancer (2018). Available from: https://clinicaltrials.gov/ct2/show/NCT03759496
62. Marlin C, Brown J, Rasamoelisoloa M, Cizeau J, Bosc D, Entwistle J, et al. Pre-clinical safety assessment of VB4-845, an EpCAM binding immunoconjugate. AACR. (2008) 68(9_Supplement):2136.
63. Brunner A, Prelog M, Verdorfer I, Tzankov A, Mikuz G, Ensinger C. EpCAM is predominantly expressed in high grade and advanced stage urothelial carcinoma of the bladder. J Clin Pathol. (2008) 61(3):307–10. doi: 10.1136/jcp.2007.049460
64. Di Paolo C, Willuda J, Kubetzko S, Lauffer I, Tschudi D, Waibel R, et al. A recombinant immunotoxin derived from a humanized epithelial cell adhesion molecule-specific single-chain antibody fragment has potent and selective antitumor activity. Clin Cancer Res. (2003) 9(7):2837–48. PMID: 12855664
65. Kowalski M, Entwistle J, Cizeau J, Niforos D, Loewen S, Chapman W, et al. A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCGrefractory and BCG-intolerant patients. Drug Des Devel Ther. (2010) 15(4):313–20. doi: 10.2147/DDDT.S14071
66. U.S National Library of Medicine. Vicinium Treatment for Subjects With Non-muscle Invasive Bladder Cancer Previously Treated With BCG 2015 [Available at: https://clinicaltrials.gov/ct2/show/NCT02449239
67. Shore N, O’Donnell M, Keane T, Jewett MA, Kulkarni GS, Dickstein R, et al. PD03-02 Phase 3 results of vicinium in BCG-unresponsive non-muscle invasive bladder cancer. J Urol. (2020) 203(Suppl. 4):e72. doi: 10.1097/JU.0000000000000823.02
68. Kowalski M, Jones N, Jewett M, Cuthbert W. POD-07.03: treatment with intravesical vicinium™ results in durable responses in patients with carcinoma in situ (CIS) previously treated with bacille calmette-guérin (BCG). Urology. (2009) 74(4):S21. doi: 10.1016/j.urology.2009.07.1142
69. Li G, Suzuki H, Asano T, Tanaka T, Suzuki H, Kaneko MK, et al. Development of A novel anti-epcam monoclonal antibody for various applications. Antibodies (Basel). (2022) 11(2):41. doi: 10.3390/antib11020041
70. Fitsialos D, Seitz S, Wiecek E, Rasamoelisolo M, Entwistle J, Jewett M, et al. Phase I/II study of vicinium given by intravesical administration in patients with superficial transitional cell carcinoma of the bladder: Phase I results. J Clin Oncol. (2006) 24(18_suppl):4580. doi: 10.1200/jco.2006.24.18_suppl.4580
71. Dickstein R, Wu N, Cowan B, Dunshee C, Franks M, Wolk F, et al. LBA27 Phase 3 study of Vicinium in BCG-unresponsive non-muscle invasive bladder cancer: initial results. J Urol. (2018) 199(4S):e1167. doi: 10.1016/j.juro.2018.03.099
72. U.S. Food and Drug Administration. Valstar (Valrubicin) Sterile Solution 2001 [cited 2022 11th January 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20892.cfm
73. Onrust SV, Lamb HM. Valrubicin. Drugs Aging. (1999) 15(1):69–75. doi: 10.2165/00002512-199915010-00006
74. Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M, et al. Efficacy and safety of valrubicin for the treatment of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. J Urol. (2000) 163(3):761–7. doi: 10.1016/S0022-5347(05)67799-3
75. Greenberg RE, Bahnson RR, Wood D, Childs SJ, Bellingham C, Edson M, et al. Initial report on intraves1cal administration of N-trifluoroacetyladriamycin-14-valerate (AD 32) to patients with refractory superficial transitional cell carcinoma of the urinary bladder. Urology. (1997) 49(3):471–5. doi: 10.1016/S0090-4295(96)00621-8
76. Dinney CP, Greenberg RE, Steinberg GD, editors. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol: Semin Orig Invest. (2013) 31(8):1635–42. doi: 10.1016/j.urolonc.2012.04.010
77. Ignatoff JM, Chen Y-H, Greenberg RE, Pow-Sang JM, Messing EM, Wilding G, editors. Phase II study of intravesical therapy with AD32 in patients with papillary urothelial carcinoma or carcinoma in situ (CIS) refractory to prior therapy with bacillus Calmette-Guerin (E3897): a trial of the Eastern Cooperative Oncology Group. Urol Oncol: Semin Orig Invest. (2009) 27(5):496–501. doi: 10.1016/j.urolonc.2008.05.004
78. Cookson MS, Chang SS, Lihou C, Li T, Harper SQ, Lang Z, et al. Use of intravesical valrubicin in clinical practice for treatment of nonmuscle-invasive bladder cancer, including carcinoma in situ of the bladder. Ther Adv Urol. (2014) 6(5):181–91. doi: 10.1177/1756287214541798
79. Rosser CJ, Nix J, Ferguson L, Hernandez L, Wong HC. Phase Ib trial of ALT-803, an IL-15 superagonist, plus BCG for the treatment of BCG-naïve patients with non-muscle-invasive bladder cancer. Am J Clin Oncol. (2018) 36(6_Supplement):510. doi: 10.1200/JCO.2018.36.6_suppl.510
80. Huang J, Schisler J, Wong HC, Rosser CJ, Sterbis J. Intravesical ALT-803 for BCG-unresponsive bladder cancer–A case report. Urol Case Rep. (2017) 14:15–7. doi: 10.1016/j.eucr.2017.04.015
81. Chamie K, Chang S, Gonzalgo ML, Kramolowsky EV, Sexton WJ, Reddy SK, et al. Phase II/III clinical results of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ (CIS) patients. Am J Clin Oncol. (2021) 206(3_Supplement):e120–1. doi: 10.1097/JU.0000000000001977.05
82. U.S National Library of Medicine. A Study of Intravesical BCG in Combination With ALT-803 in Patients With Non-Muscle Invasive Bladder Cancer 2014 [cited 2022 10th January 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT02138734
83. U.S National Library of Medicine. PK Sub-Study of QUILT-3.032 (CA ALT-803-01-16) and of QUILT-2.005 (CA ALT-803-01-14) 2019 [cited 2022 10th January 2022]. Available from:https://clinicaltrials.gov/ct2/show/NCT04142359
84. Chamie K, Lee JH, Rock A, Rhode PR, Soon-Shiong P. Preliminary phase 2 clinical results of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) patients. Am J Clin Oncol. (2019) 37(15_Supplement):4561. doi: 10.1200/JCO.2019.37.15_suppl.4561
85. Rosser CJ, Tikhonenkov S, Nix JW, Chan OT, Ianculescu I, Reddy S, et al. Safety, tolerability, and long-term clinical outcomes of an IL-15 analogue (N-803) admixed with bacillus calmette-guérin (BCG) for the treatment of bladder cancer. Oncoimmunology. (2021) 10(1):1912885. doi: 10.1080/2162402X.2021.1912885
86. Narayan VM, Dinney CPN. Intravesical gene therapy. Urol Clin North Am. (2020) 47(1):93–101. doi: 10.1016/j.ucl.2019.09.011
87. Kuball J, Wen SF, Leissner J, Atkins D, Meinhardt P, Quijano E, et al. Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. J Clin Oncol. (2002) 20(4):957–65. doi: 10.1200/JCO.2002.20.4.957
88. O'Donnell MA, Lilli K, Leopold C, National Bacillus Calmette-Guerin/Interferon Phase 2 Investigator Group. Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer [published correction appears in J Urol. 2004 Dec; 172(6 Pt 1):2485]. J Urol. 2004;172(3):888–93. doi: 10.1097/01.ju.0000136446.37840.0a
89. O’Donnell MA, Lilli K, Leopold C, Group NBC-GIPI. Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. (2004) 172(3):888–93. doi: 10.1097/01.ju.0000136446.37840.0a
90. Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. (2021) 22(1):107–17. doi: 10.1016/S1470-2045(20)30540-4
91. Benedict WF, Tao Z, Kim C-S, Zhang X, Zhou J-H, Adam L, et al. Intravesical Ad-IFNα causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-α protein. Mol Ther. (2004) 10(3):525–32. doi: 10.1016/j.ymthe.2004.05.027
92. U.S. National Library of Medicine. INSTILADRIN® in Patients With Bacillus Calmette-Guerin (BCG) Unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC) 2016 [cited 2022 12th Janurary 2022]. Available from: INSTILADRIN® in Patients With Bacillus Calmette-Guerin (BCG) Unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC)
93. Ramesh N, Ge Y, Ennist DL, Zhu M, Mina M, Ganesh S, et al. CG0070, A conditionally replicating granulocyte macrophage colony-stimulating factor–armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. (2006) 12(1):305–13. doi: 10.1158/1078-0432.CCR-05-1059
94. Mitra AP, Birkhahn M, Cote RJ. P53 and retinoblastoma pathways in bladder cancer. World J Urol. (2007) 25(6):563–71. doi: 10.1007/s00345-007-0197-0
95. U.S. National Library of Medicine. Study of CG0070 Given in Patients With Non-Muscle Invasive Bladder Cancer, Unresponsive to Bacillus-Calmette-Guerin (BOND-003) 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT04452591?cond = CG0070&draw=2&rank=2
96. Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL 3rd, et al., An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: interim results. Urol Oncol. (2018) 36(10):440–7. doi: 10.1016/j.urolonc.2017.07.005
97. Packiam VT, Barocas DA, Chamie K, Davis RL III, Kader AK, Lamm DL, et al. LBA24 CG0070, an oncolytic adenovirus, for bcg-unresponsive non-muscle-invasive bladder cancer (NMIBC): 12 month interim results from a multicenter phase II trial. J Urol. (2018) 199(4S):e1166. doi: 10.1016/j.juro.2018.03.096
98. U.S National Library of Medicine. Study of CG0070 Given in Combination With Pembrolizumab, in Non-Muscle Invasive Bladder Cancer, Unresponsive to Bacillus Calmette-Guerin (CORE-001) (2020). Available from: https://clinicaltrials.gov/ct2/show/NCT04387461
99. Li R, Steinberg G, Lamm D, Uchio E, Packiam V, Kamat A, et al. 955 CORE1: phase 2, single arm study of CG0070 combined with pembrolizumab in patients with non muscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guerin (BCG). J Immunother Cancer. (2021) 9(Suppl 2):A1005-A. doi: 10.1136/jitc-2021-SITC2021.955
100. Smaldone MC, Davies BJ. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther. (2010) 12(5):607–16. PMID: 20886393
101. Matouk I, Evantal N, Amit D, Ohana P, Gofrit O, Sorin V, et al. The H19-IGF2 role in bladder cancer biology and DNA-based therapy. In: Bladder cancer-from basic science to robotic surgery: citeseer. (2012). Available from: https://www.intechopen.com/chapters/27331. doi: 10.5772/29236
102. Gofrit ON, Benjamin S, Halachmi S, Leibovitch I, Dotan Z, Lamm DL, et al. DNA Based therapy with diphtheria toxin-A BC-819: a phase 2b marker lesion trial in patients with intermediate risk nonmuscle invasive bladder cancer. J Urol. (2014) 191(6):1697–702. doi: 10.1016/j.juro.2013.12.011
103. U.S National Library of Clinical Medicine. Codex: Study of Inodiftagene Vixteplasmid (BC-819) in Unresponsive NMIBC (2018). Available from: https://clinicaltrials.gov/ct2/show/NCT03719300
104. GuhaSarkar S, Banerjee R. Intravesical drug delivery: challenges, current status, opportunities and novel strategies. J Controlled Release. (2010) 148(2):147–59. doi: 10.1016/j.jconrel.2010.08.031
105. Jung JH, Gudeloglu A, Kiziloz H, Kuntz GM, Miller A, Konety BR, et al. Intravesical electromotive drug administration for non-muscle invasive bladder cancer. Cochrane Database Syst Rev. (2017) 9(9):CD011864. doi: 10.1002/14651858.CD011864.pub2.28898400
106. National Institute for Clinical Excellence. Interventional procedure overview of intravesical microwave hyperthermia and chemotherapy for nonmuscle-invasive bladder cancer (2018). Available from: https://www.nice.org.uk/guidance/ipg628/evidence/overview-final-pdf-6539170141
107. Slater S, Patel P, Viney R, Foster M, Porfiri E, James ND, et al. The effects and effectiveness of electromotive drug administration and chemohyperthermia for treating non-muscle invasive bladder cancer. Ann R Coll Surg Engl. (2014) 96(6):415–9. doi: 10.1308/003588414X13946184901001
108. Zhao H, Chan VW-S, Castellani D, Chan EO-T, Ong WLK, Peng Q, et al. Intravesical chemohyperthermia vs. Bacillus Calmette-Guerin instillation for intermediate-and high-risk non-muscle invasive bladder cancer: a systematic review and meta-analysis. Front Surg. (2021) 23(8):775527. doi: 10.3389/fsurg.2021.775527
109. Liem EI, Crezee H, de la Rosette JJ, de Reijke TM. Chemohyperthermia in non-muscle-invasive bladder cancer: an overview of the literature and recommendations. Int J Hyperthermia. (2016) 32(4):363–73. doi: 10.3109/02656736.2016.1155760
110. Tan WS, Panchal A, Buckley L, Devall AJ, Loubière LS, Pope AM, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus Calmette-Guérin or institutional standard in patients with recurrence of non–muscle-invasive bladder cancer following induction or maintenance bacillus Calmette-Guérin therapy (HYMN): a phase III, open-label, randomised controlled trial. Eur Urol. (2019) 75(1):63–71. doi: 10.1016/j.eururo.2018.09.005
111. Daneshmand S, Pohar KS, Steinberg GD, Aron M, Cutie C. Effect of GemRIS (gemcitabine-releasing intravesical system, TAR-200) on antitumor activity in muscle-invasive bladder cancer (MIBC). Am J Clin Oncol. (2017) 35(15_Supplement):e16000. doi: 10.1200/JCO.2017.35.15_suppl.e16000
112. U.S National LIbrary of Medicine. Safety and Tolerability of TAR-200 mg in Subjects With Non-Muscle-Invasive Bladder Cancer (2016). Available from:https://clinicaltrials.gov/ct2/show/NCT02720367
113. Grimberg DC, Shah A, Inman BA. Overview of taris GemRIS, a novel drug delivery system for bladder cancer. Eur Urol Focus. (2020) 6(4):620–2. doi: 10.1016/j.euf.2019.09.006
114. Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. (2019) 4(3):e10143. doi: 10.1002/btm2.10143
115. Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Controlled Release. (2013) 170(3):365–72. doi: 10.1016/j.jconrel.2013.05.041
116. McKiernan JM, Holder DD, Ghandour RA, Barlow LJ, Ahn JJ, Kates M, et al. Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guérin treatment failure. J Urol. (2014) 192(6):1633–8. doi: 10.1016/j.juro.2014.06.084
117. Robins DJ, Sui W, Matulay JT, Ghandour R, Anderson CB, DeCastro GJ, et al. Long-term survival outcomes with intravesical nanoparticle albumin-bound paclitaxel for recurrent non–muscle-invasive bladder cancer after previous bacillus Calmette-Guérin therapy. Urology. (2017) 103:149–53. doi: 10.1016/j.urology.2017.01.018
118. Seager CM, Puzio-Kuter AM, Patel T, Jain S, Cordon-Cardo C, Mc Kiernan J, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res. (2009) 2(12):1008–14. doi: 10.1158/1940-6207.CAPR-09-0169
119. McKiernan JM, Onyeji I, Lascano D, Ahn J, Desai N, Abate-Shen C, et al. A phase I/II study of albumin-bound rapamycin nanoparticles in the treatment of bacillus calmette-guerin refractory non-muscle invasive transitional cell bladder cancer. Am J Clin Oncol. (2016) 34(15_Supplement):e16008. doi: 10.1200/JCO.2016.34.15_suppl.e16008
120. Qiu H, Guo H, Li D, Hou Y, Kuang T, Ding J. Intravesical hydrogels as drug reservoirs. Trends Biotechnol. (2020) 38(6):579–83. doi: 10.1016/j.tibtech.2019.12.012
121. Mostafid AH, Porta N, Cresswell J, Griffiths TR, Kelly JD, Penegar SR, et al. CALIBER: a phase II randomized feasibility trial of chemoablation with mitomycin-C vs surgical management in low-risk non-muscle-invasive bladder cancer. BJU Int. (2020) 125(6):817. doi: 10.1111/bju.15038
122. Chevli KK, Shore ND, Trainer A, Smith AB, Saltzstein D, Ehrlich Y, et al. Primary chemoablation of low-grade intermediate-risk nonmuscle-invasive bladder cancer using UGN-102, a mitomycin-containing reverse thermal gel (Optima II): a phase 2b, open-label, single-arm trial. J Urol. (2022) 207(1):61–9. doi: 10.1097/JU.0000000000002186
123. U.S. National Library of Medicine. A Phase 3 Study of UGN-102 for Low Grade Intermediate Risk Non-Muscle-Invasive Bladder Cancer (ATLAS) 2020 [cited 2022 13th January 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT04688931
124. Yang LS, Shan BL, Shan LL, Chin P, Murray S, Ahmadi N, et al. A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg Oncol. (2016) 25(3):281–97. doi: 10.1016/j.suronc.2016.05.027
125. Mayor N, Fankhauser C, Sangar V, Mostafid H. Management of NMIBC during BCG shortage and COVID-19. Trends Urol. Men's Health. (2021) 12(1):7–11. doi: 10.1002/tre.783
126. Balar AV, Kulkarni GS, Uchio EM, Boormans J, Mourey L, Krieger LEM, et al. Keynote 057: phase II trial of pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guérin (BCG). Am J Clin Oncol. (2019) 37(7_Supplement):350. doi: 10.1200/JCO.2019.37.7_suppl.350
127. Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. (2017) 8(23):38022. doi: 10.18632/oncotarget.16723
128. U.S. National Library of Medicine. Testing the Addition of an Anti-cancer Drug, Pembrolizumab, to the Usual Intravesical Chemotherapy Treatment (Gemcitabine) for the Treatment of BCG-Unresponsive Non-muscle Invasive Bladder Cancer (2019). Available from: https://clinicaltrials.gov/ct2/show/NCT04164082?term = intravesical + gemcitabine&draw=2&rank=10
129. Simm C, Caridis A, Di Maio A, Knowles P, Gorman B, Jones R, et al. Targeting of BRD4 with JQ1, combined with mitomycin C as a novel combination therapy for non-muscle invasive bladder cancer. Eur Urol Suppl. (2018) 17(2):e663. doi: 10.1016/S1569-9056(18)31300-9
130. Caridis A PC, Di Maio A, Ward D, Oppermann U, Bountra C, Khanim F, et al. Front. Oncol. Conference Abstract: Bladder Cancer Translational Research Meeting. doi: 10.3389/conf.fonc.2019.01.00010, editor GSK-J4 combined with mitomycin C as a novel combination therapy for non-muscle invasive bladder cancer. Bladder Cancer Translational Research Meeting; 2019; London: Front. Oncol. (2019).
Keywords: intravesical, non-muscle invasive bladder cancer, gemcitabine, immune checkpoint inhibitors, monoclonal antibodies, gene therapy, electromotive therapy, hydrogels
Citation: Ward K, Kitchen Mark O, Mathias S, Khanim F and Bryan RT (2022) Novel intravesical therapeutics in the treatment of non-muscle invasive bladder cancer: Horizon scanning. Front. Surg. 9:912438. doi: 10.3389/fsurg.2022.912438
Received: 5 April 2022; Accepted: 7 July 2022;
Published: 26 July 2022.
Edited by:
Jeffrey J. Leow, Tan Tock Seng Hospital, SingaporeReviewed by:
Alexandre Zlotta, University of Toronto, Canada© 2022 Ward, Kitchen, Mathias, Khanim and Bryan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly Ward a2VsbHl3YXJkOTNAZG9jdG9ycy5vcmcudWs=
†ORCID Mark O Kitchen orcid.org/0000-0002-1124-2940 Richard Bryan orcid.org/0000-0003-2853-4293
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.