
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 15 July 2022
Sec. Obstetrics and Gynecological Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.902871
This article is part of the Research Topic Women in Obstetrics and Gynecology View all 32 articles
Background: Defects in the pelvic floor connective tissue may underlie the etiology of pelvic organ prolapse (POP). We hypothesized that the expression of proteins regulating extracellular matrix turnover is altered in the uterosacral ligament of women with POP. We compared the expression of CD44, transforming growth factor (TGF)-β, and matrix metalloproteinases (MMPs) 2/9 in women with and without POP.
Methods and Results: This matched case-control study included 30 postmenopausal women, with POP stage 2 and higher according to the POP quantification system, and 30 postmenopausal women without POP. Immunohistochemical analyses of the uterosacral ligament specimens obtained after hysterectomy were performed to determine CD44, TGF-β, MMP-2, and MMP-9 expression. The expression was quantified using ImageJ software, and the association between prolapse occurrence and risk factors was evaluated using Spearman's correlation analysis. CD44 expressions were significantly lower (p < 0.05), whereas MMP-2 and MMP-9 expression was higher (p < 0.0001 and p < 0.05, respectively), in the POP group than in the control group. The expression of TGF-β was similar in both groups. The occurrence of uterine prolapse was positively correlated with age, postmenopausal age, and MMP-2 and MMP-9 expression (p < 0.01) and negatively correlated with CD44 expression (p < 0.05).
Conclusion: CD44, MMP-2, and MMP-9 may play critical roles in the pathogenesis of POP and may be candidate biomarkers of POP progression.
Pelvic organ prolapse (POP) is a birth-induced joint urinary/gynecological condition that affects one in four women of all ages and more than 50% of parous women over the age of 50 (1). POP is the most prevalent among women aged 60–69 years (2). The incidence of POP is 3%–6% based on symptoms, and can reach 50% based on vaginal examination; among patients with symptoms, 6%–18% receive surgical treatment. The requirement for medical assistance to treat pelvic floor defects is growing twice as fast as the population (3). Therefore, the prevention of POP has become an important issue in obstetrics and gynecology.
Pelvic organs are supported by the pelvic floor muscle complex, main ligament, uterosacral ligament (USL), and pelvic fascia. The pelvic fascia is a connective tissue structure that covers the walls and floor of the pelvis. It consists mostly of collagen fibers interlaced with elastin, smooth muscle cells, and fibroblasts, is abundant in extracellular matrix (ECM) that envelops the USL-cardinal ligament, and is thought to provide a critical level of support. The USL is one of the major ligaments holding the uterus and, thus, is a very important component of the pelvic support system. The mechanisms underlying pelvic floor laxity are not clearly understood. Studies in humans and animals have suggested that USL repair defects are due to changes in the structure and organization of fibrous connective tissue (4), including the amount and composition of the ECM, which is a major factor affecting tissue stability (5).
Collagen and elastin are the primary constituents of the ECM that provide strength and flexibility to the USL. A decrease in collagen deposition or changes in the proportion of collagen types can affect tissue stiffness and increase the susceptibility to POP. Type I and type III collagens are responsible for the stiffness and elasticity of connective tissue, respectively (6). Collagen is degraded by enzymes of the matrix metalloproteinase (MMP) family, which have distinct activities and functions. Among the MMPs, MMP-2 and MMP-9 break down degenerated collagen and elastin peptides into smaller fragments and are important factors in ECM turnover (7).
Transforming growth factor (TGF)-β is a fibrogenic cytokine that plays a key role in ECM remodeling and regulation of tissue integrity. TGF-β activity can alter the balance between ECM synthesis and degradation and is implicated in the pathophysiology of many fibrotic disorders, including pulmonary fibrosis (8) and cardiac fibrosis associated with heart failure (9). Furthermore, some studies show that TGF-β expression is altered in fibroblasts and pubic fascia of women with POP (10, 11). However, some reports show that TGF-β is not associated with the prognosis for patients with POP. Thus, there is no consensus regarding the role of TGF-β in the pathogenesis of POP, and further investigation is required.
The transmembrane glycoprotein CD44 is a major cell surface receptor for hyaluronic acid and can also interact with other ECM components, such as collagen and laminin. CD44 is implicated in the regulation of numerous attachment-dependent processes, such as cell proliferation, migration, and differentiation (12). Furthermore, extensive evidence indicates that CD44 plays a role in fibroblast invasion and progressive fibrosis of the lung and heart, not only through the establishment of specific transmembrane complexes but also through its participation in key signal transduction cascades (13–16). These data suggest that CD44 may be incorporated into the biochemical pathways responsible for the pathogenic alterations in the ECM that may promote POP. However, the role of CD44 in POP has not been investigated and, to the best of our knowledge, there have been no studies on CD44 expression in the USL of women with POP.
The main objective of this study was to compare the expression levels of CD44, TGF-β, MMP-2, and MMP-9 in the USL of patients with and without POP. We hypothesized that CD44 would be lower in the tissues of prolapse patients compared with those of the control group. We believe that this is the first report to illustrate the difference in CD44 expression between POP and non-POP women, aa well as the correlation between these indicators and uterine prolapse.
Clinical information, including follow-up data, was extracted from the Department of Gynecology database between 2020 and 2021. Ethical approval was obtained from the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine, China (approval number: IIT20210069B-R1). All procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The case group (n = 30) included postmenopausal women who had prolapse of POP stage 2 or higher (according to the POP quantification system) and underwent planned vaginal hysterectomy because of POP-related symptoms. The control group (n = 30) consisted of women without prolapse who had undergone hysterectomy for other benign diseases. The patients had no oxidative stress-related disorders, including connective tissue disease or coronary heart disease. Patients who had taken estrogen in the past 3 months were excluded from the study. Informed consent was obtained from all individual participants included in the study.
USL specimens were obtained from tissues extracted by hysterectomy. Samples (approximately 5 mm3 in size) were taken from the dorsal part of the cervix, 1 cm from the beginning of the cervix (at the left side), and verified by histological analysis.
Tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin, and treated with citric acid buffer (pH 6.0) in a microwave for antigen retrieval. Sections were then incubated in 3% hydrogen peroxide solution at room temperature in the dark for 25 min to inhibit endogenous peroxidases, then blocked in 3% bovine serum albumin at room temperature for 30 min. Reactions with primary antibodies against CD44 (dilution 1:100; GB13065), MMP-2 (dilution 1:500; GB11130), MMP-9 (dilution 1:500; GB11132-2), and TGF-β (dilution 1:100; GB13028) (all from Servicebio, Wuhan, China) were performed overnight at 4°C. After incubation with horseradish peroxidase-labeled secondary antibodies (Servicebio) at room temperature for 50 min, immune reactivity was detected with diaminobenzidine (K5007, DAKO, Wuhan, China); color development was observed under a microscope. Nuclei were counterstained with Harris hematoxylin. The percentages of positively stained areas were quantified using ImageJ software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA).
Data of at least three independent experiments were expressed as mean ± standard deviation. Patient groups were compared using two-sample t-tests, Mann–Whitney test, and Fisher’s exact test, separately for means and proportions. Spearman's test was used for the correlation analysis of non-normally distributed continuous variables. Statistical analyses were performed using SPSS (version 23.0; IBM, Armonk, NY, USA) and Prism Version 8.01 (GraphPad Software, La Jolla, CA, USA). Statistical significance was set at p < 0.05.
In total, 60 postmenopausal women who had undergone hysterectomy were analyzed: 30 with symptomatic POP (cases) and 30 without POP (controls); their clinical parameters are shown in Table 1. Women with POP were significantly older (p < 0.001), had a longer postmenopausal period (p < 0.0001), and showed a higher incidence of hypertension (p = 0.038) than women without POP. Women in the POP group had a vaginal hysterectomy as the standard surgical treatment for symptomatic POP. In the control group, hysterectomy was performed for uterine leiomyoma (n = 20), postmenopausal bleeding (n = 2), uterine adenomyosis (n = 7), and cervical intraepithelial neoplasia (n = 3).
Figure 1 shows representative images of USL sections from the POP and control groups after staining with antibodies against CD44, TGF-β, MMP-2, and MMP-9. Quantitative analyses revealed a significant decrease in CD44 expression and an increase in MMP-2 and MMP-9 expression in the POP group compared with those in the control group (Figure. 2). The percentages of USL-positive areas stained with anti-CD44 antibodies were 11.8% in patients with and 13.5% in those without POP (p = 0.0263). The CD44 expression levels in younger and older POP patients did not differ significantly from those in younger and older control subjects (11.6% vs. 11.9% and 14.5% vs. 13.1% stained areas in younger and older patients, respectively). The expression levels of MMP-2 and MMP-9 were similar in the POP group (13.2% and 13.7% stained areas, respectively) but significantly higher than those in the control group (9.92% and 11.4%; p < 0.0001 and p = 0.0155, respectively). However, there was no significant difference in the expression of TGF-β between the POP and control groups (9.34% vs. 10.2%; p = 0.2213).
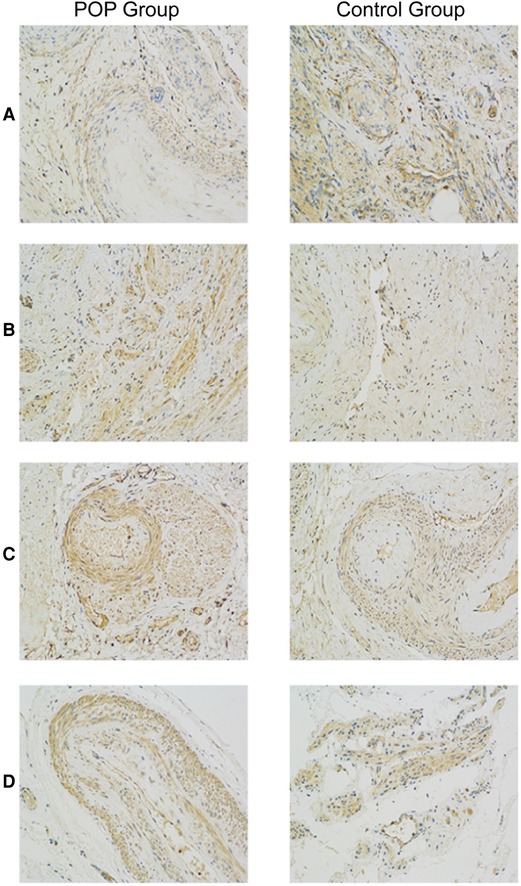
Figure 1. Immunohistochemistry analysis of uterosacral ligaments from women with and without pelvic organ prolapse. Sections of uterosacral ligaments obtained from women who had undergone hysterectomy were stained with antibodies against CD44 (A), transforming growth factor-β (B), matrix metalloproteinase (MMP)-2 (C), and MMP-9 (D).

Figure 2. Quantification of matrix metalloproteinase (MMP)-2 (A), MMP-9 (B), transforming growth factor (TGF)-β (C), and CD44 (D) expression in uterosacral ligaments. Sections of uterosacral ligaments were stained with antibodies against CD44, TGF-β, MMP-2, and MMP-9, and counterstained with Harris hematoxylin. The percentages of positively stained areas were quantified using ImageJ software and are expressed as means ± standard deviations. POP, pelvic organ prolapse.
Spearman's correlation analyses were performed to determine the associations of various risk factors with POP. The results indicated that the occurrence of uterine prolapse was positively correlated with age, postmenopausal age, and MMP-2 and MMP-9 expression (p < 0.01), and negatively correlated with CD44 expression (p < 0.05) (Table 2).
In this study, we showed that postmenopausal women with symptomatic POP, and those without POP, differed in the expression of factors involved in the synthesis and turnover of the ECM. Specifically, the levels of CD44 were significantly lower, and those of MMP-2 and MMP-9 were higher in the USLs of the POP group than in the USLs of the control group. Furthermore, CD44 levels were negatively correlated and MMP-2/9 levels positively correlated with prolapse. However, there was no difference in the expression of TGF-β between women with and without POP. These results support our hypothesis that CD44 could be involved in the pathogenesis of POP and are consistent with roles for CD44, MMP-2, and MMP-9 in the mechanisms underlying the balance between the production and degradation of the ECM.
POP is commonly considered as a condition caused by the weakness of pelvic floor muscles and fascia due to childbirth, age, and abnormalities in connective tissue. The ECM is a key component of connective tissue that provides a framework for pelvic support; therefore, it may play a critical role in the occurrence of POP (17). The ECM is dynamically reshaped through quantitative and qualitative changes in cellular and non-cellular components, including enzymes such as collagenase and MMPs that regulate ECM turnover (18). MMPs are closely involved in ECM remodeling in normal and pathological conditions as they can cleave most ECM proteins (19). One of the major functions of MMP-2 is to degrade type IV collagen, elastin, and fibronectin. It is regulated by tissue inhibitor of MMP-2 (TIMP-2), which irreversibly inactivates the enzyme, preventing ECM degradation. MMP-9 is also involved in ECM protein degradation and is implicated in the development of several diseases (20). Previous studies revealed higher expression of MMPs, including MMP-2 and MMP-9, and lower expression of TIMP-2, in USL and vaginal wall biopsies from patients with POP (21, 22). These data, together with our results, suggest that the increased expression of MMP-2 and MMP-9 can accelerate ECM breakdown and promote POP.
TGF-β is considered a critical fibrogenic cytokine that plays a role in myofibroblast differentiation in different tissues (23). The TGF-β/Smad signaling pathway regulates the activity of MMPs and promotes stress-related urinary incontinence after natural birth in animal experiments, whereas TGF-β is downregulated after excessive mechanical strain or oxidative stress (17, 20). However, the role of TGF-β in POP is controversial. For example, a study reports that TGF-β1 expression in patients with POP is significantly reduced (22), whereas another study did not detected any difference in TGF-β1 levels in the USL of women with and without POP (24). In our study, we also did not observe a significant difference in TGF-β expression between the POP and control groups. However, the TGF-β1 pathway is known as a signaling mechanism critical for the cellular response to mechanical stress (17) and, as such, may be related to the pathophysiology of uterine prolapse in postmenopausal women.
To the best of our knowledge, this is the first report on the expression of CD44 in the USL of women with POP. CD44, a cell surface glycoprotein, participates in cell proliferation, adhesion, and migration, as well as in hematopoiesis and lymphocyte activation (25), through binding to its ligands including hyaluronic acid, MMP-9, vascular endothelial growth factor, epidermal growth factor, fibronectin, type I collagen, and osteopontin (11, 26). It has also been shown that fibril-associated collagen XIV can directly interact with CD44 (27). However, the role of CD44 in ECM deposition and fibrogenesis is unclear. On the one hand, some reports indicate that CD44 may promote the synthesis of ECM constituents. For example, it has been reported that CD44-deficient mice show reduced fibroblast infiltration and collagen deposition but increased proliferative activity of fibroblasts (28), and that they also have decreased collagen breakdown and fibrous collagen deposition (29). However, CD44 knockout mice with acute lung injury do not show significant changes in the accumulation of lung interstitial collagen (14). An interesting novel role of CD44 was observed in an in vitro study showing that CD44 may be involved in the coordination of cell motility and proliferative responses to ECM stiffening (30). These data suggest that the effects of CD44 on ECM-related processes may depend on the specific environment. Our results, that CD44 expression was significantly associated with POP, may indicate the involvement of CD44 in the pathophysiology of prolapse.
The mechanism of uterine prolapse is complex and not well-understood. Our results implicate CD44, along with MMP-2 and MMP-9, in the development of POP. This is consistent with a previous report on the synergy between CD44, MMP-2, MMP-9, and TGF-β in the physiological mechanism of tissue remodeling (26). Our results can further improve our understanding of the molecular mechanisms underlying POP pathology and contribute to the establishment of novel therapeutic targets in POP.
Our study had some limitations. As it was a case-control investigation, it was not possible to determine the cause-and-effect relationship between POP and protein expression, and we can only refer to the correlation between the occurrence of prolapse and protein expression detected by immunohistochemistry. Furthermore, additional studies involving young patients are needed to establish the role of CD44 in the etiology of POP. Nevertheless, our results suggest an interesting link between CD44 expression and POP in postmenopausal women that has not been reported previously.
In summary, this study provides evidence that CD44 may be a risk factor for uterine prolapse and a novel candidate biomarker of POP. As the prevalence of POP is rapidly increasing with the increase in the aging population, the identification of new biomarkers offers a foundation for the development of improved therapeutic interventions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HZ, WWY, and YPH. The first draft of the manuscript was written by WWY and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
We would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dietz HP. Pelvic floor trauma in childbirth. Aust N Z J Obstet Gynaecol. (2013) 53(3):220–30. doi: 10.1111/ajo.12059
2. Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. (2013) 24(11):1783–90. doi: 10.1007/s00192-013-2169-9
3. Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. Women. Obstet Gynecol. (2014) 123(1):141–8. doi: 10.1097/AOG.0000000000000057
4. Kerkhof MH, Hendriks L, Brolmann HA. Changes in connective tissue in patients with pelvic organ prolapse–a review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct. (2009) 20(4):461–74. doi: 10.1007/s00192-008-0737-1
5. Gong R, Xia Z. Collagen changes in pelvic support tissues in women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. (2019) 234:185–9. doi: 10.1016/j.ejogrb.2019.01.012
6. Leegant A, Zuckerwise LC, Downing K, Brouwer-Visser J, Zhu C, Cossio MJ, et al. Transforming growth factor Beta1 and extracellular matrix protease expression in the uterosacral ligaments of patients with and without pelvic organ prolapse. Female Pelvic Med Reconstr Surg. (2015) 21(1):53–8. doi: 10.1097/SPV.0000000000000130
7. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and timps. Cardiovasc Res. (2006) 69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002
8. Li FZ, Cai PC, Song LJ, Zhou LL, Zhang Q, Rao SS, et al. Crosstalk between calpain activation and Tgf-Beta1 augments collagen-I synthesis in pulmonary fibrosis. Biochim Biophys Acta. (2015) 1852(9):1796–804. doi: 10.1016/j.bbadis.2015.06.008
9. Huang XR, Chung AC, Yang F, Yue W, Deng C, Lau CP, et al. Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling. Hypertension. (2010) 55(5):1165–71. doi: 10.1161/HYPERTENSIONAHA.109.147611
10. Li BS, Hong L, Min J, Wu DB, Hu M, Guo WJ. The expression of glutathione peroxidase-1 and the anabolism of collagen regulation pathway transforming growth factor-beta1-connective tissue growth factor in women with uterine prolapse and the clinic significance. Clin Exp Obstet Gynecol. (2013) 40(4):586–90.
11. Midgley AC, Rogers M, Hallett MB, Clayton A, Bowen T, Phillips AO, et al. Transforming growth factor-beta1 (Tgf-beta1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (Ha)-facilitated epidermal growth factor receptor (Egfr) and Cd44 co-localization in lipid rafts. J Biol Chem. (2013) 288(21):14824–38. doi: 10.1074/jbc.M113.451336
12. Suleiman M, Abdulrahman N, Yalcin H, Mraiche F. The role of Cd44, hyaluronan and Nhe1 in cardiac remodeling. Life Sci. (2018) 209:197–201. doi: 10.1016/j.lfs.2018.08.009
13. Ponta H, Sherman L, Herrlich PA. Cd44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. (2003) 4(1):33–45. doi: 10.1038/nrm1004
14. Chang SH, Yeh YH, Lee JL, Hsu YJ, Kuo CT, Chen WJ. Transforming growth factor-Beta-mediated Cd44/Stat3 signaling contributes to the development of atrial fibrosis and fibrillation. Basic Res Cardiol. (2017) 112(5):58. doi: 10.1007/s00395-017-0647-9
15. Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and Cd44. J Exp Med. (2011) 208(7):1459–71. doi: 10.1084/jem.20102510
16. Muller J, Gorressen S, Grandoch M, Feldmann K, Kretschmer I, Lehr S, et al. Interleukin-6-dependent phenotypic modulation of cardiac fibroblasts after acute myocardial infarction. Basic Res Cardiol. (2014) 109(6):440. doi: 10.1007/s00395-014-0440-y
17. Zhang Q, Liu C, Hong S, Min J, Yang Q, Hu M, et al. Excess mechanical stress and hydrogen peroxide remodel extracellular matrix of cultured human uterosacral ligament fibroblasts by disturbing the balance of mmps/timps via the regulation of Tgfbeta1 signaling pathway. Mol Med Rep. (2017) 15(1):423–30. doi: 10.3892/mmr.2016.5994
18. Manou D, Caon I, Bouris P, Triantaphyllidou IE, Giaroni C, Passi A, et al. The Complex interplay between extracellular matrix and cells in tissues. Methods Mol Biol. (2019) 1952:1–20. doi: 10.1007/978-1-4939-9133-4_1
19. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. (2016) 97:4–27. doi: 10.1016/j.addr.2015.11.001
20. Malemud CJ. Matrix metalloproteinases and synovial joint pathology. Prog Mol Biol Transl Sci. (2017) 148:305–25. doi: 10.1016/bs.pmbts.2017.03.003
21. Dviri M, Leron E, Dreiher J, Mazor M, Shaco-Levy R. Increased matrix metalloproteinases-1,-9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. (2011) 156(1):113–7. doi: 10.1016/j.ejogrb.2010.12.043
22. Liu C, Wang Y, Li BS, Yang Q, Tang JM, Min J, et al. Role of transforming growth factor beta1 in the pathogenesis of pelvic organ prolapse: a potential therapeutic target. Int J Mol Med. (2017) 40(2):347–56. doi: 10.3892/ijmm.2017.3042
23. Sampson N, Berger P, Zenzmaier C. Redox signaling as a therapeutic target to inhibit myofibroblast activation in degenerative fibrotic disease. Biomed Res Int. (2014) 2014:131737. doi: 10.1155/2014/131737
24. Carlin GL, Bodner K, Kimberger O, Haslinger P, Schneeberger C, Horvat R, et al. The role of transforming growth factor-ss (Tgf-Ss1) in postmenopausal women with pelvic organ prolapse: an immunohistochemical study. Eur J Obstet Gynecol Reprod Biol X. (2020) 7:100111. doi: 10.1016/j.eurox.2020.100111
25. Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. The role of Cd44 in disease pathophysiology and targeted treatment. Front Immunol. (2015) 6:182. doi: 10.3389/fimmu.2015.00182
26. Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates tgf-beta and promotes tumor invasion and angiogenesis. Genes Dev. (2000) 14(2):163–76. doi: 10.1101/gad.14.2.163
27. Ehnis T, Dieterich W, Bauer M, Lampe B, Schuppan D. A chondroitin/dermatan sulfate form of Cd44 is a receptor for collagen Xiv (Undulin). Exp Cell Res. (1996) 229(2):388–97. doi: 10.1006/excr.1996.0384
28. Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, et al. Cd44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. (2008) 180(4):2625–33. doi: 10.4049/jimmunol.180.4.2625
29. Govindaraju P, Todd L, Shetye S, Monslow J, Pure E. Cd44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix Biol. (2019) 75-76:314–30. doi: 10.1016/j.matbio.2018.06.004
Keywords: CD44, extracellular matrix, matrix metalloproteinase, pelvic organ prolapse, transforming growth factor-β
Citation: Ying W, Hu Y and Zhu H (2022) Expression of CD44, Transforming Growth Factor-β, and Matrix Metalloproteinases in Women With Pelvic Organ Prolapse. Front. Surg. 9:902871. doi: 10.3389/fsurg.2022.902871
Received: 23 March 2022; Accepted: 20 June 2022;
Published: 15 July 2022.
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Beng Kwang Ng, National University of Malaysia, MalaysiaCopyright © 2022 Ying, Hu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: HaiBin Zhu emh1aGJAemp1LmVkdS5jbg==
Specialty section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.