
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 16 May 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.901674
This article is part of the Research Topic Aspiring for Onco-Functional Utopia via Radiosurgery View all 6 articles
Background: Therapy for large or deep cystic brain metastases is a troublesome procedure in clinical departments. Stereotactic cyst aspiration, combined with Gamma Knife radiosurgery, can be an effective treatment for cystic brain metastases. However, there is still a possibility that a reaccumulation of cystic fluid may lead to poor efficacy or even reoperation.
Case presentation: We present a case of a 67-year-old man who was diagnosed with lung cancer brain metastasis. The intracranial lesion seen on imaging appeared to be cystic and located deep inside the brain with associated limb dysfunction. The patient did not respond well to chemotherapy and underwent cyst aspiration with Ommaya reservoir implantation under neuronavigation. Repeated cystic fluid reaccumulation and exacerbation of symptoms occurred during treatment. We performed repeated aspiration via the Ommaya reservoir to control the symptoms and combined it with radiotherapy. During the follow-up period of 14 months, the intracranial tumor was effectively and satisfactorily controlled.
Conclusions: We highlight that Ommaya reservoir implantation during stereotactic cyst aspiration is necessary to prevent fluid reaccumulation, thereby avoiding the need for a second surgical procedure.
Brain metastases are the most common central nervous system tumors and confer a grave prognosis to patients with a median survival of less than 1 year (1). Brain metastases can be radiographically cystic or solid (2). Patients with cystic metastases tend to have a worse prognosis than those with solid metastases (3). Generally, radiosurgery is less effective for patients with cystic metastases, especially for tumors >3 cm in diameter (4), and surgical resection is not only traumatic but also increases the risk of leptomeningeal dissemination (5). Another method (6) is stereotactic cyst aspiration (SCA), directly followed by Gamma Knife radiosurgery (GKRS). SCA can quickly reduce the volume of lesions, making it suitable for GKRS afterward. Our center does not have the necessary infrastructure to complete this one-step approach. In this article, we report a case treated with additional Ommaya reservoir (OR) insertion after SCA, followed by radiosurgery up to one and a half months later. Repeated cystic fluid reaccumulation and exacerbation of symptoms occurred during treatment. We performed repeated aspiration via the OR to help the patient cope with this situation and combined it with radiotherapy. We emphasize the advantage of OR placement, which is an effective tool for sufficient reduction in volume before and after radiosurgery in the case of fluid reaccumulation without reoperation.
A 67-year-old man was admitted to the neurosurgery department of our hospital with a 1-month history of right upper limb weakness. He had no other medical history other than hypertension. Cranial computed tomography (Figure 1A) showed a circular focus of low density beside the left lateral ventricle. The lesion appeared hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging, and rim enhancement was seen on postcontrast T1-weighted imaging (Figures 1B–D).
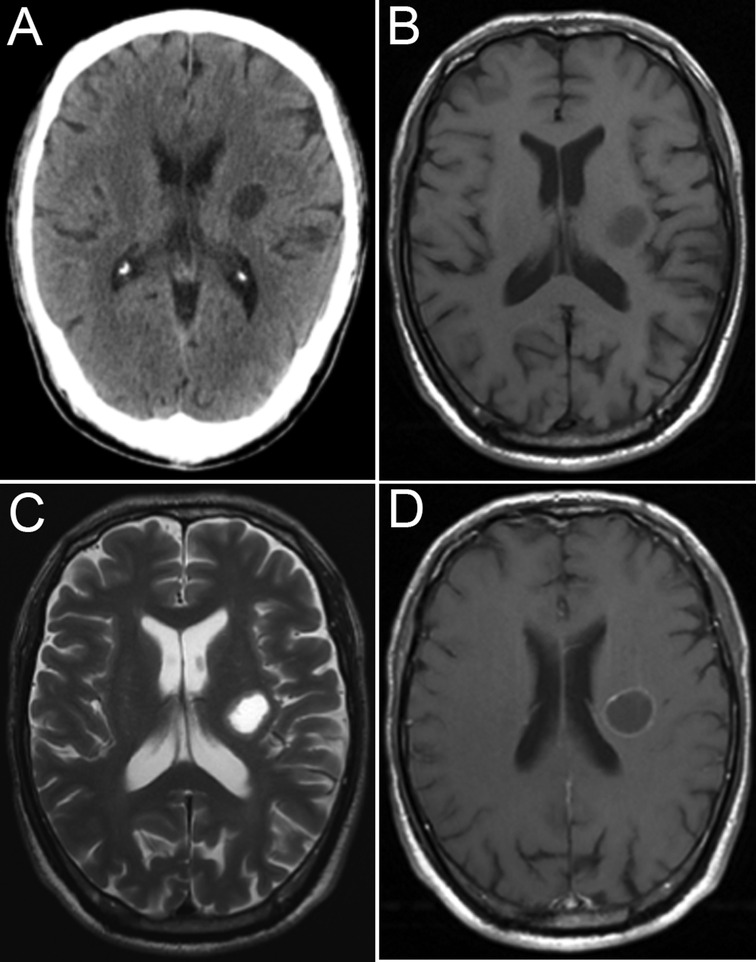
Figure 1. Image at admission. CT image (A), MR T1 image (B), MR T2 image (C), and MR T1-weighted image (D).
A further systemic evaluation revealed a pulmonary lesion, and transbronchial lung biopsy pathology indicated lung squamous cell carcinoma. The patient was transferred to the oncology department for chemotherapy treatment with paclitaxel plus carboplatin.
After two cycles of chemotherapy, his right muscle strength gradually decreased. Cranial computed tomography (Figure 2A) demonstrated that the intracranial cystic metastasis had progressed compared with the previous case.
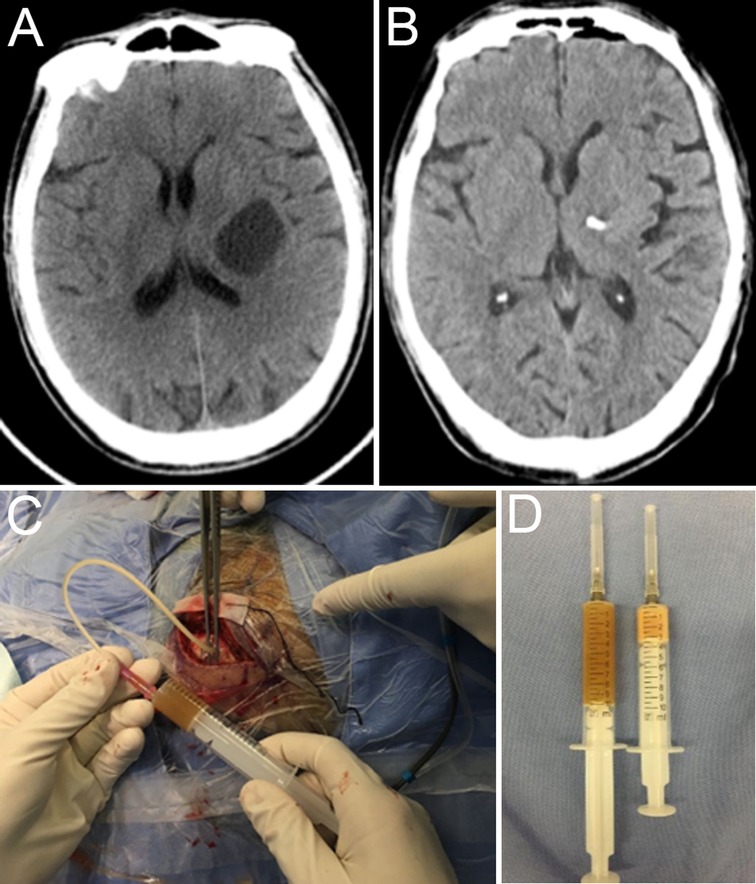
Figure 2. Preoperative CT reexamination (A), postoperative CT (B), intraoperative cystic fluid aspiration (C), and intraoperative specimen (D).
The patient underwent cyst aspiration (Figure 3C) and OR implantation under neuronavigation. A total of 13 ml of yellow viscous liquid (Figure 2D) was drawn during the operation. Postoperative computed tomography (Figure 2B) showed that the lesion had almost disappeared, and his right muscle strength had recovered.
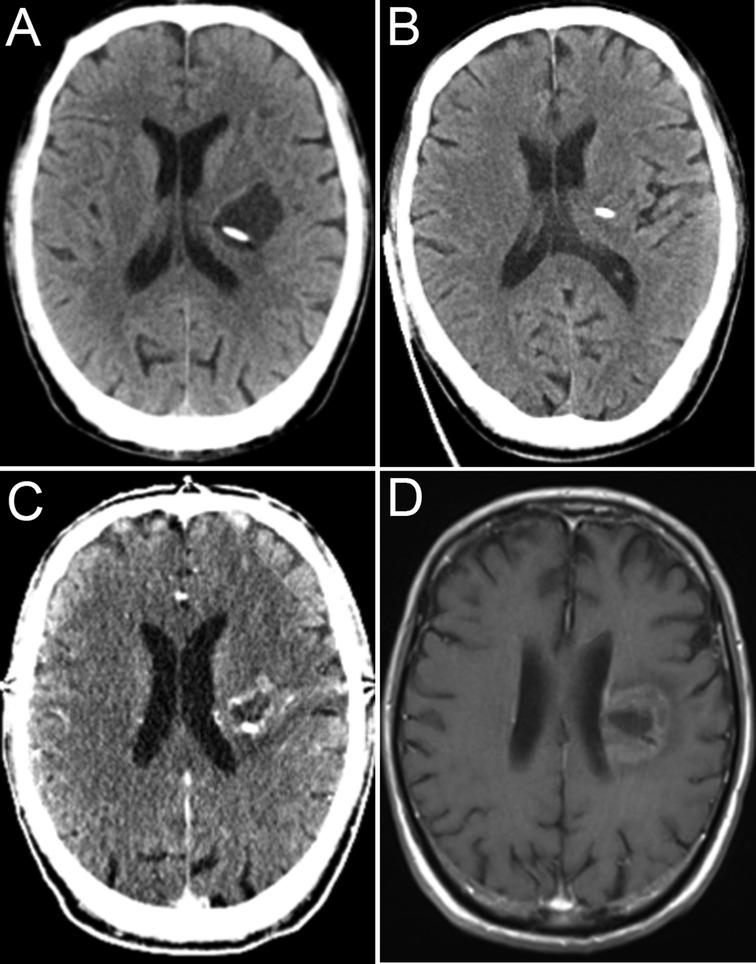
Figure 3. CT image showing fluid reaccumulation (A). CT image immediately after aspiration via the OR (B), CT image after first radiosurgery (C), and final imaging follow-up (D).
The cyst immediately (Figure 3A) returned, with a progressive decline in contralateral limb muscle strength. We promptly implemented cystic fluid aspiration (Figure 3B), and the patient’s symptoms were relieved. Our hospital is not equipped to carry out Gamma Knife therapy. Therefore, we performed stereotactic body radiation therapy (SBRT) after aspiration to prevent a recurrence. Cystic lesions can still recur and become enlarged under such combined treatment. Repeated cystic fluid reaccumulation and exacerbation of symptoms occurred during treatment. We performed repeated aspirations via the OR to help the patient cope with such a situation. We performed SBRT again approximately half a year later (Figure 3C). However, we were unable to draw out the cyst fluid due to blockage of the OR before the last radiosurgery. Throughout the treatment, we received a total of five aspirations. The patient’s last radiographic follow-up was 14 months after onset (Figure 3D). The intracranial tumor was effectively and satisfactorily controlled. Due to the loss of follow-up, the patient’s current survival status is unknown.
Brain metastases often have associated cystic components. However, the mechanism of cyst formation has not been demonstrated yet. The breakdown of the blood–brain barrier is considered the possible cause (7), and the consensus is that cystic metastases tend to be more malignant than solid metastases (8). Therefore, cystic brain metastases usually progress rapidly and lead to serious clinical consequences.
A single large cystic brain metastasis is considered the operative indication, and surgical treatment can obviously relieve the clinical symptoms (9, 10). However, if the lesions are deeply seated or located in important functional areas, surgery can cause severe neurological dysfunction. Moreover, patients in poor general condition are intolerant to craniotomy operations. Stereotactic radiosurgery (SRS) is an alternative treatment option for brain metastases (8). However, cystic brain metastases are often too large to be suitable for SRS, and these types of tumors do not respond well to SRS (11). In this situation, we can use radioenhancers to increase the efficiency of SRS in the context of brain metastasis (12, 13) and reduce the cyst volume via SCA to make the lesion suitable for SRS (6).
Effective reduction of tumor volume is the key to subsequent treatment. The volumetric decrease reported in the literature ranged from 47.8% to 77.9% (6, 9, 14–17). Akito Oshima reported that the degree of tumor volume reduction depended on the location of the puncture target (16). He suggested that the tip of the puncture needle should be placed at the center of the cyst to ensure adequate volume reduction. Although the drainage tube was off-centered in our case, the cyst was close to disappearing. We believe that this is also related to the preponderance of cystic fluid, a thin cystic wall, and less tumor parenchyma.
SCA is a relatively safe procedure, and related complications are rare. Its complications include infection, hemorrhaging, neurological symptoms, and seizures, similar to stereotactic biopsy. One study reported that the SCA-related complication rate was 5.8%, and the mortality rate was 3.8% (6). The article attributed the higher mortality rate to the fact that the study population was all ASA IV. Another theoretical complication after SCA is cancer cells spreading through the aspiration needle tract. A few such complications have been reported. However, the literature reports only a 9% risk of needle biopsies leading to tumor cell seeding (18).
It is debatable whether additional ORs are inserted after SCA. Some studies have suggested that SCA without Ommaya insertion and directly followed by Gamma Knife therapy is an effective and time-efficient treatment (6, 14). However, there are many limitations to this one-step approach. First, most centers do not have the necessary infrastructure for a one-step approach to be a regular process. In our hospital, surgery and SRS are performed in two different departments. It is not easy to guarantee that Gamma Knife therapy can be performed immediately after SCA. If the interval between the two procedures is too long, the risk of fluid reaccumulation will increase. Second, the reaccumulation of cystic fluid can still occur after aspiration and GKRS. The local control rates ranged from 54.2% to 91.3% (6, 9, 15, 17, 19). OR insertion during SCA can be an additional tool for subcutaneous cyst aspiration in the case of fluid reaccumulation. In our case, the cystic fluid accumulated repeatedly. Although the fluid type was turbid (Figure 2D), we could still take subcutaneous cyst aspiration via the OR multiple times and avoided reoperation. In my opinion, the OR should be inserted to reduce the chances of repeated SCA.
In our case, we selected SCA, combined with radiosurgery, which is less invasive than resection, to treat a deeply located cystic lesion. Although the cystic fluid was found to be turbid during surgery, we still implanted an OR during SCA. Because of the implanted OR, we solved the problem of cystic fluid reaccumulation in subsequent treatment.
We believe that OR implantation during cyst aspiration is necessary to prevent fluid reaccumulation, avoiding the need for a second surgical procedure. Although turbid cystic fluid may lead to tube blockage, OR implantation is still worth giving a try.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JL, KW, YW, and SY were the team responsible for the surgical treatment of this patient. ZW and WH were the team responsible for the medical treatment of this patient. JL participated in the perioperative management throughout the process and was responsible for collating data and writing papers. WZ participated in the whole process of medical antitumor treatment and assisted in collating data and writing papers. KW participated in the management of the perioperative period. YW assisted in revising the paper. SY and WH jointly formulated the treatment plan and handled the research design. WH also directed the writing and submission of the article. All authors contributed to the article and approved the submitted version.
This work was supported by the Youth Fund Project of the Zhejiang Natural Science Foundation (project number LQ20H160042).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Doron H, Pukrop T, Erez N. A blazing landscape: neuroinflammation shapes brain metastasis. Cancer Res. (2019) 79(3):423–36. doi: 10.1158/0008-5472.CAN-18-1805
2. Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. (2013) 4(Suppl 4):S209–19. doi: 10.4103/2152-7806.111298
3. Sun B, Huang Z, Wu S, Ding L, Shen G, Cha L, et al. Cystic brain metastasis is associated with poor prognosis in patients with advanced breast cancer. Oncotarget. (2016) 7(45):74006–14. doi: 10.18632/oncotarget.12176
4. Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. (2010) 96(1): 45–68. doi: 10.1007/s11060-009-0073-4
5. Press RH, Zhang C, Chowdhary M, Prabhu RS, Ferris MJ, Xu KM, et al. Hemorrhagic and cystic brain metastases are associated with an increased risk of leptomeningeal dissemination after surgical resection and adjuvant stereotactic radiosurgery. Neurosurgery. (2019) 85(5):632–41. doi: 10.1093/neuros/nyy436
6. Sadik ZHA, Hanssens PEJ, Verheul JB, Ardon H, Lie ST, van der Pol B, et al. Stereotactic cyst aspiration directly followed by Gamma Knife radiosurgery for large cystic brain metastases. Acta Neurochir (Wien). (2021) 163(2): 343–50. doi: 10.1007/s00701-020-04273-1
7. Gardner WJ, Collis JS Jr, Lewis LA. Cystic brain tumors and the blood-brain barrier. Comparison of protein fractions in cyst fluids and sera. Trans Am Neurol Assoc. (1963) 8:291–8. doi: 10.1001/archneur.1963.00460030075007
8. Xu YB, Zhang Y, Song Z, Wang W, Shao L. Treatment and prognosis of solid and cystic brain metastases in patients with non-small-cell lung cancer. Cancer Manag Res. (2021) 13:6309–17. doi: 10.2147/CMAR.S314060
9. Franzin A, Vimercati A, Picozzi P, Serra C, Snider S, Gioia L, et al. Stereotactic drainage and Gamma Knife radiosurgery of cystic brain metastasis. J Neurosurg. (2008) 109(2):259–67. doi: 10.3171/JNS/2008/109/8/0259
10. Yoshida S, Morii K. The role of surgery in the treatment of brain metastasis: a retrospective review. Acta Neurochir (Wien). (2004) 146(8):767–70. doi: 10.1007/s00701-004-0228-1
11. Pan HC, Sheehan J, Stroila M, Steiner M, Steiner L. Gamma Knife surgery for brain metastases from lung cancer. J Neurosurg. (2005) 102(Suppl):128–33. doi: 10.3171/sup.2005.102.s_supplement.0128
12. Ganau M, Foroni RI, Gerosa M, Zivelonghi E, Longhi M, Nicolato A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part I: therapeutic strategies. Tumori. (2014) 100(4): 459–65. doi: 10.1177/1636.17912
13. Ganau M, Foroni RI, Gerosa M, Ricciardi GK, Longhi M, Nicolato A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part II: adjuvant radiobiological tools. Tumori. (2015) 101(1):57–63. doi: 10.5301/tj.5000215
14. Higuchi F, Kawamoto S, Abe Y, Kim P, Ueki K. Effectiveness of a 1-day aspiration plus Gamma Knife surgery procedure for metastatic brain tumor with a cystic component. J Neurosurg. (2012) 117(Suppl):17–22. doi: 10.3171/2012.7.GKS121001
15. Jung TY, Kim IY, Jung S, Jang WY, Moon KS, Park SJ, et al. Alternative treatment of stereotactic cyst aspiration and radiosurgery for cystic brain metastases. Stereotact Funct Neurosurg. (2014) 92(4):234–41. doi: 10.1159/000362935
16. Oshima A, Kimura T, Akabane A, Kawai K. Optimal implantation of Ommaya reservoirs for cystic metastatic brain tumors preceding Gamma Knife radiosurgery. J Clin Neurosci. (2017) 39:199–202. doi: 10.1016/j.jocn.2016.12.042
17. Park WH, Jang IS, Kim CJ, Kwon DH. Gamma Knife radiosurgery after stereotactic aspiration for large cystic brain metastases. J Korean Neurosurg Soc. (2009) 46(4):360–4. doi: 10.3340/jkns.2009.46.4.360
18. Karlsson B, Ericson K, Kihlstrom L, Grane P. Tumor seeding following stereotactic biopsy of brain metastases. Report of two cases. J Neurosurg. (1997) 87(2):327–30. doi: 10.3171/jns.1997.87.2.0327
Keywords: cystic brain metastases, stereotactic cyst aspiration, Gamma Knife radiosurgery, Ommaya reservoir, craniotomy
Citation: Lv J, Wu Z, Wang K, Wang Y, Yang S and Han W (2022) Case Report: Clinical and Procedural Implications of Ommaya Reservoir Implantation in Cystic Brain Metastases Followed by Radiosurgery Treatment. Front. Surg. 9:901674. doi: 10.3389/fsurg.2022.901674
Received: 22 March 2022; Accepted: 22 April 2022;
Published: 16 May 2022.
Edited by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Nikolaos CH. Syrmos, Aristotle University of Thessaloniki, GreeceCopyright © 2022 Lv, Wu, Wang, Wang, Yang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weidong Han aGFud2RAemp1LmVkdS5jbg== ShuXu Yang MzE5NTAxOUB6anUuZWR1LmNu
†These authors have contributed equally to this work
Specialty section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Abbreviations: GKRS, Gamma Knife radiosurgery; OR, Ommaya reservoir; SBRT, stereotactic body radiation therapy; SCA, stereotactic cyst aspiration; SRS, stereotactic radiosurgery.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.