- 1Federal Center of Neurosurgery, Tyumen, Russia
- 2Department of Neurosurgery, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
- 3Department of Neurosurgery, Federal University of Paraná, Curitiba, Brazil
- 4Division of Neurosurgery, San Filippo Neri Hospital, Roma, Italy
- 5Depatment of General Chemistry, Bashkir State Medical University, Ufa, Russia
Effective intraoperative image navigation techniques are necessary in modern neurosurgery. In the last decade, intraoperative ultrasonography (iUS), a relatively inexpensive procedure, has gained widespread acceptance.
Aim: To document and describe the neurosurgery cases, in which iUS has been employed as the primary navigational tool. This includes a discussion of the advantages that iUS may possess relative to other forms of neuronavigation.
Conclusion: The application of iUS as an intraoperative navigation tool during neurosurgery holds great potential as it has been shown, relative to other neuronavigation techniques, to be quick, repeatable, and able to provide real-time results.
Introduction
The ideal intraoperative navigational modality for neurosurgeons is the one that is accurate, user-friendly, and most of all cost-efficient (1–4). Over the last two decades, the most reliable and frequently used intraoperative navigational tools include intraoperative computed tomography (iCT) and intraoperative magnetic resonance imaging (iMRI), with the latter being deemed the “golden standard” (5–7). Despite the effectiveness of the aforesaid intraoperative tools, neurosurgeons still face challenges in the acquisition and use of iCT and iMRI. These challenges include the following: (1) Enormous costs in the acquisition of these tools, especially in low-budget neurosurgical centers and developing countries; (2) Heavy dependence on acquired pre-operative images; (3) At every stage of the procedure; post craniotomy, dural opening, tumor debulking, and resection, the element of brain shift results in varying degrees of loss of accuracy; (4) Inherent lack of soft-tissue resolution and the associated radiation exposure like in the case of iCT. To circumvent these hurdles, more innovative, convenient, and novel generation modes of Intraoperative sonography or ultrasound (iUS) systems have been developed. The iUS is comparatively inexpensive, easy to use, and requires less intraoperative preparation. The two-dimensional (2D) greyscale and three-dimensional (3D) iUS provide real-time, clear, and well-correlated images, which are easily interpreted by the neurosurgeon. Additionally, the ease and flexibility it provides the user make it possible to counter-check the location of the lesion at any stage of the surgery without prolonged workflow stoppages (8). This, therefore, solves the element of brain shift. The iUS does not require pre-existing images before surgery. However, there is a necessity to save the initial image scanned to serve as a baseline check during the surgery. In this narrative, we reclaiming these findings by outlining our center (Federal Center of Neurosurgery, Tyumen, Russia) experience in the use of iUS during brain, spine, vascular, and epilepsy surgery. We do this by highlighting technical nuances and discussing, with illustrative cases, the spectrum of applications and benefits. Finally, we descriptively evaluate neurosurgeons' experience in using iUS as a neuronavigation tool.
Methodology
We took a retrospective approach for this investigation, and it involved the extraction of data from a facility database on neurosurgical procedures that were performed between 2015 and December of 2021 at the Federal Center of Neurosurgery, Tyumen Russia. The key criterion for inclusion in the investigation was the documented intraoperative application of iUS during elective surgery. The investigators reviewed a total of 1,330 patient records, with documented brain and spinal lesions. In addition, we also assessed neurosurgeon's experience with the use of intraoperative ultrasound as a neuroimaging through the application of an online survey tool the following link: https://docs.google.com/forms/d/e/1FAIpQLSfqHcExcft3FwcTKiKb2A0b5i828UhnoXe1smoXPDIdH6_g/viewform?usp= sf_link (Table 1).
Equipment and Technical Modality
We employed a FlexFocus 800 iUS [BK Medical, Denmark for three-dimensional (3D)] iUS neuro-navigation. We applied a Linear-type and convex transducer (Figure 1B; high-frequency Linear 8870), as well as a craniotomy sensor (Craniotomy 8862). We use a frequency of 3.8–10 MHz, a contact surface of 29 × 10 mm. The iUS was done in coronal, sagittal, and axial planes.
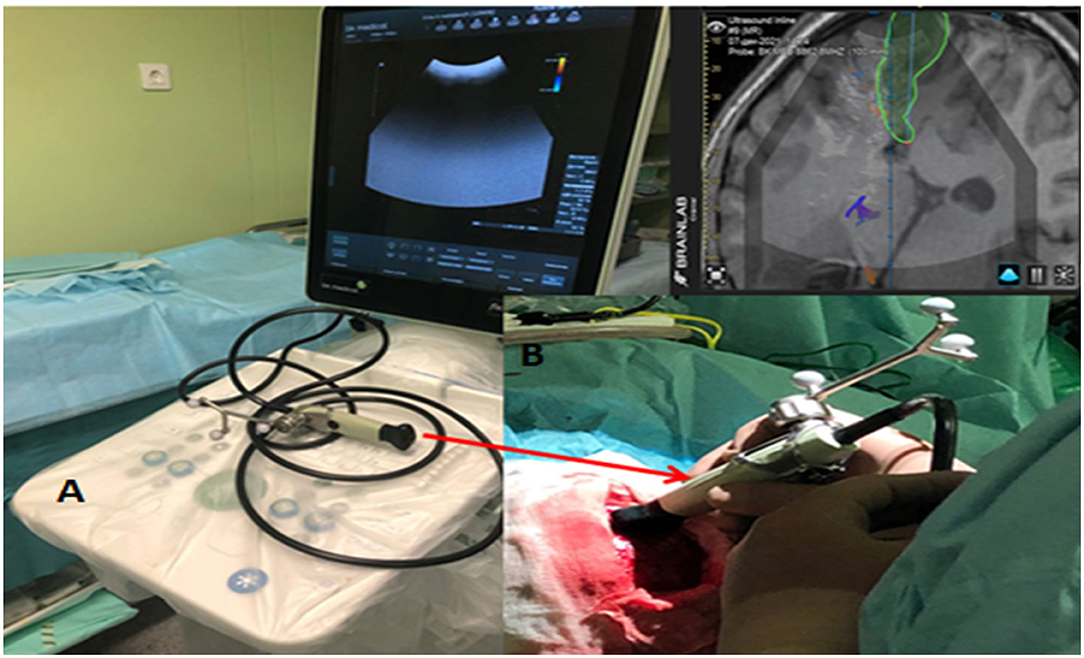
Figure 1. (A) iUS Apparatus - BK Medical flex focus 800 (Denmark) with Sterile convex transducer. (B) Integrated Brain lab neuronavigation system (Germany).
Anatomically consistent reference points, as mentioned above, were used for the localization of the lesions. The interpretation of anatomical features on iUS was described by their respective echogenicity reflections' tissue consistency. We described spaces that had no echogenicity, such as ventricles/ CSF spaces as anechogenic. Hypoechoic features were defined in spaces with less echogenicity, such as the brainstem. Isoechogenic structures included the normal brain tissue (white matter) and conversely hyperechoic in tissues with high enchogenicity, such as in gliomas, calcifications, meningiomas, and vascular anatomical structures, such as the choroid plexus (Figure 2). Imaging characteristics on iUS were compared with pre- and post-operative CT or MRI images available to assess for concordance.
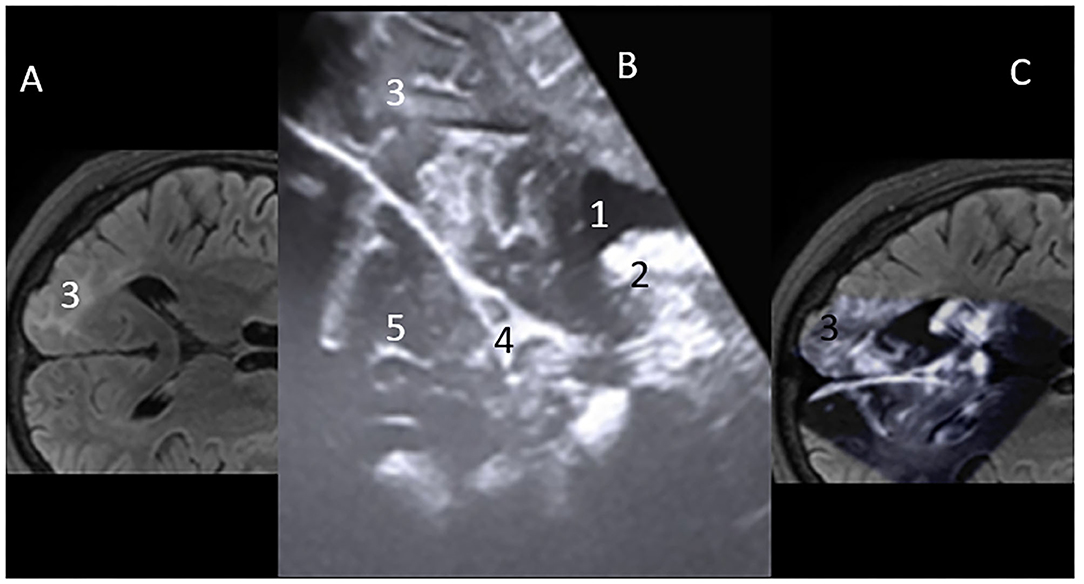
Figure 2. (A) MRI – flair axial view of Rt occipital lobe Focal cortical dysplasia (FCD) lesion-3. (B) Mirror iUS Axial view of Rt occipital lobe FCD lesion-3. Cerebral spinal fluid (CSF) in ventricle occipital horn is “Hypoechoic” on iUS-1; “Hyperechoic” futures vascular stuctures, high grade tumors. Here, the choroid plexis is hyperechoic-2. Falx- 4. Normal brain tissue on iUS is “isoechoic”-5. (C) Projection on the intraoperative Ultrasound Concordance of lesion location on MRI and iUS.
Results
Survey on the Applicability of iUS in Neurosurgery as a Neuronavigation Tool
There are 41 neurosurgeons, 33 males, and eight females, from 15 countries, 12 of whom were from low-and-middle-income countries (LMIC), while three were from middle-income (MIC) and high-income countries (HIC). Regarding years of experience, 73.8% had, at most, 5 years of working experience as neurosurgeons, being relatively junior residents, while 26.2% had at least 6 years of working experience, being individuals at the registrar to consultant professor level.
iUS-Navigated Neurosurgical Cases
All the surgeries were performed by Professor Albert Sufianov at the Federal Center of Neurosurgery, Tyumen, Russia, with the assistance of the same surgical team. Intraoperative ultrasonography was performed after the craniotomy, both before and after opening the dura mater.
Supratentorial Low-Grade Glioma
Low-grade subcortical gliomas can be difficult to locate after the dura mater has been opened. However, as shown in Figure 3, LGG are readily identified, and their margins are well-defined by intraoperative ultrasound regardless of pre-operative imaging patterns. This enhances intraoperative lesion delineation and the extent of resection. Features of lesion-vascular interactions and landscape can be also demonstrated while localizing lesions, a vital component for undesirable hemorrhage avoidance.
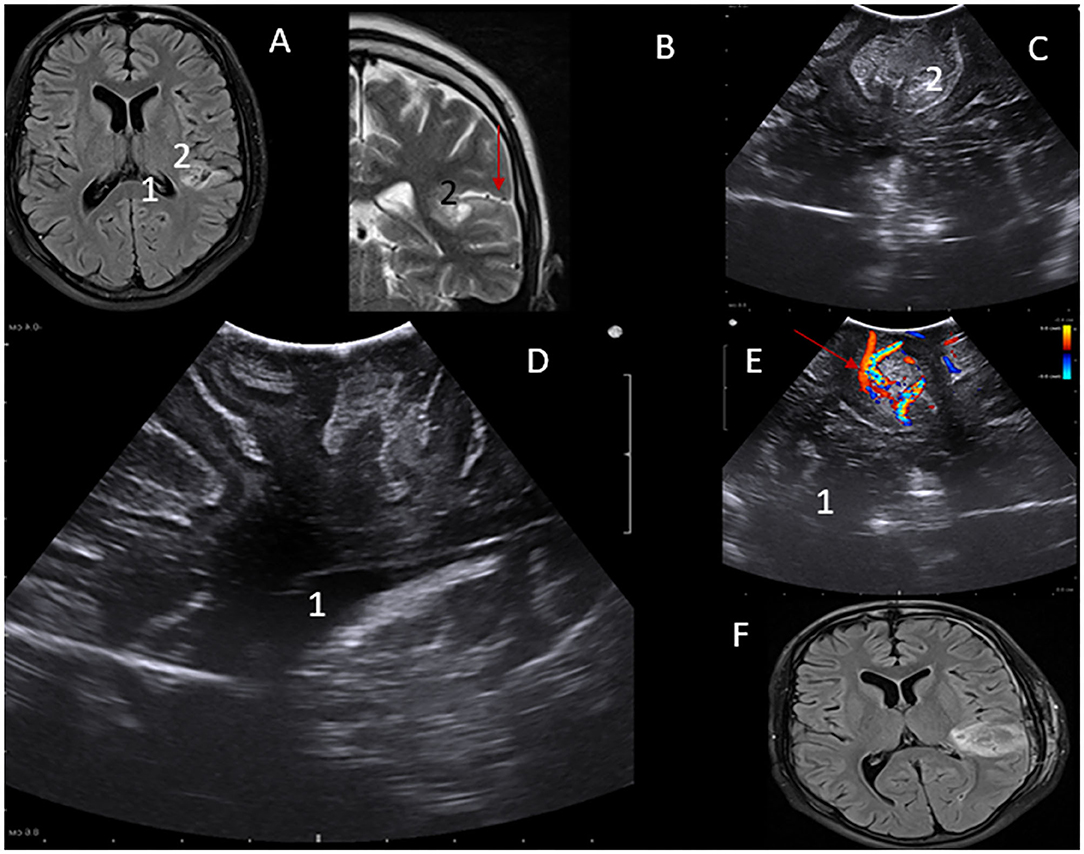
Figure 3. (A) MRI – flair axial view of Lt parital lobe lesion- 1. (B) MRI – T2WI MRI coronal view of Lt parietal lobe lesion- 2. iUS depiction of low-grade lesion in axial. (C,D) Saggital views Perilesional vascular structures can be visualized using Color Doppler function-Red arrow (A,E). Post-operative MRI showing gross total resection of the lesion (F).
Deep High-Grade Gliomas
One of the key challenges during the surgical treatment of high-grade gliomas is the achievement of the optimum balance between maximizing the resection of the lesion and minimizing the effect on healthy tissue (and any potential neurological impairment). It is in this regard that iUS demonstrates one of its key strengths in being able to provide real-time information on the lesion's location and boundaries. On iUS, they demonstrate varying levels of echoicity and are normally heterogenic. Despite being able to detect deep HGGs, intraoperative evaluation of the extent of resection of deep high-grade gliomas may be challenging when in B-mode iUS. This is because both malignant tumor tissue and peritumoral edema are hyperechoic (Figure 4F). This poses a notable disadvantage compared to iMRI. It is for this reason that we still advocate for early post-operative MRI images and clinical (at 3 and 6 months, then once a year) follow-up to rule out a residual tumor.
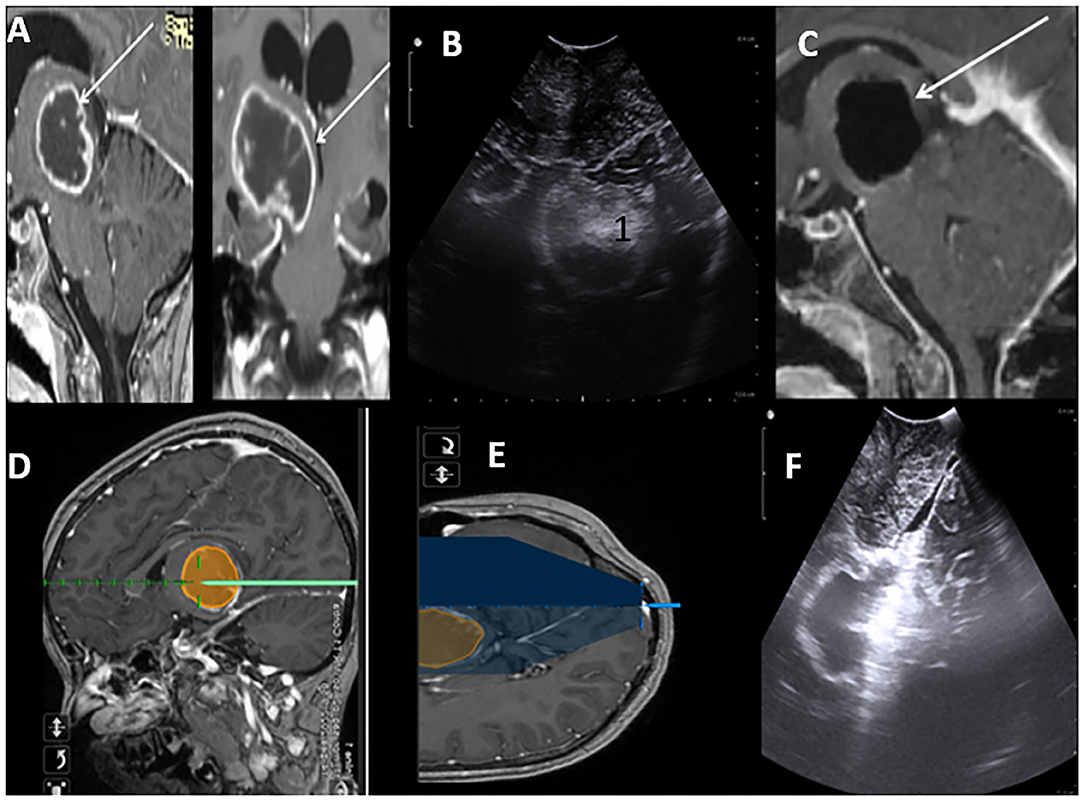
Figure 4. Post Contrast MRI T1WI Sagittal and Coronal sections suggestive of high-grade thalamic glioma (HGG) with cystic central cystic component (A). Brain lab navigation system depicting trajectory to the lesion (D,E). iUS Axil section depicting thalamic hyperechoic lesion (B). Post-resection iUS Sagital section depicting wall of the cavity after complete resection (F). MRI in concordance with ius showing gross total resection of the HGG (C).
Intraventricular Lesions
In Figures 5, 6, we illustrate two cases of lateral and fourth ventricular lesions resected with iUS guidance along with Bain lab neuronavigation. As illustrated, iUS can be used to highlight even deep-seated brain lesions, to show their relationships with surrounding neural and vascular structures, and to provide real-time and dynamic imaging. As in the previously described cases, the probe is placed over the dura to acquire standard B-mode imaging scans. The lesion is identified on the two axes and is measured. The iUS with brain lab integration, gives greater details of the dimensions of the lesion, about the normal cortical structure during each stage of the procedure, and, therefore, abetting the element of brain shift (Figures 5C–E). Even within narrow and deep corridors to the fourth ventricle, lesion dimensions can be captured on the coronal and sagittal axis. Intricate lesion-anatomical structure interrelations of the fourth ventricle can be clearly defined (Figures 6, 7).
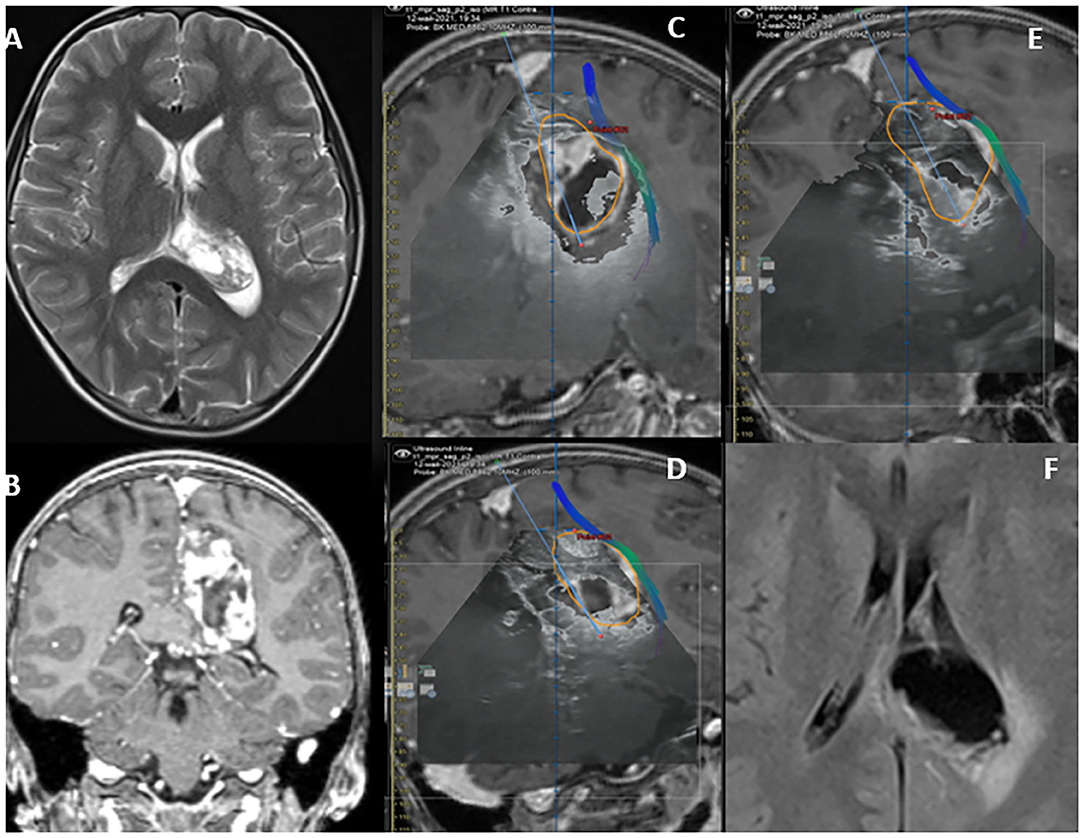
Figure 5. MRI T2 WI Axial parietal- intraventricular cystic lesion (A). Heterogeneous enhancing on T1WI-C as depicted on its coronal section (B). The lesion boundaries are depicted in orange on (C–E), illustrating “Brain shift.” Lesion in close proximity with the cortical spinal tracts. Plain axial post-operative TIWI depicting complete resection of the lesion.
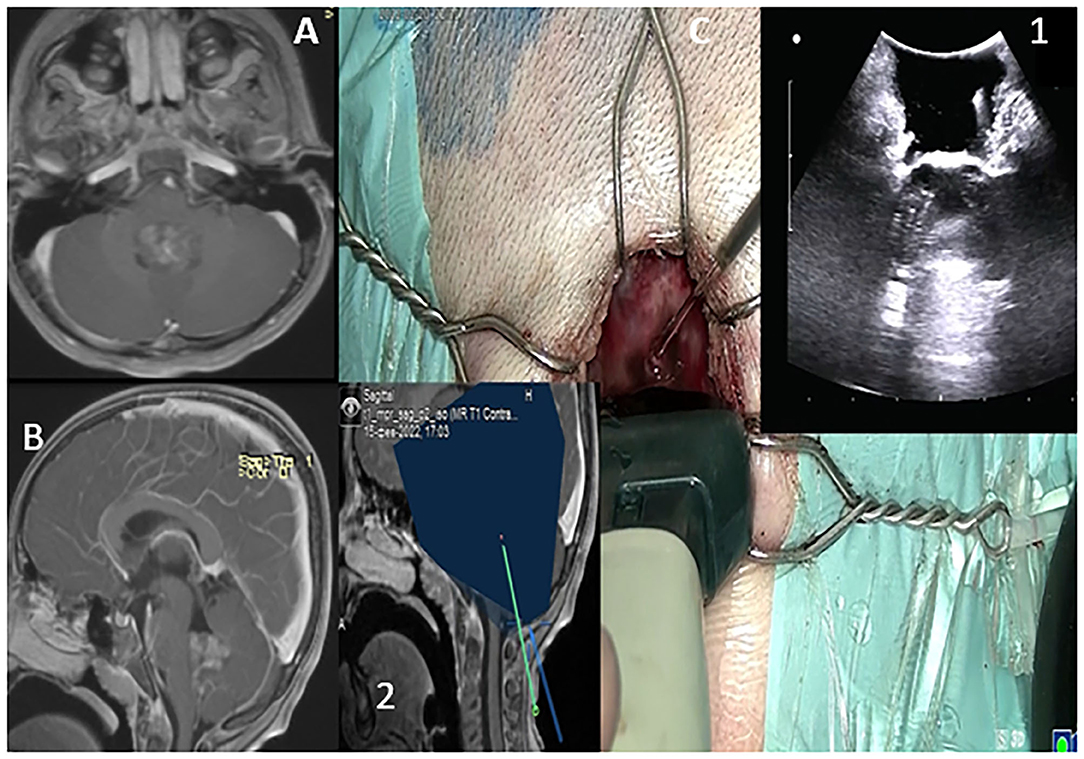
Figure 6. T1WI in sagittal and axial sections depicting 4th ventricular lesion (A,B). Probe is placed over the dura to acquire standard B-mode imaging scans via a sub occipital approach. The lesion is depicted as hyperechoic incaved between cerebellar tonsils (C,1). The trajectory to the lesion is also depicted (C,2).
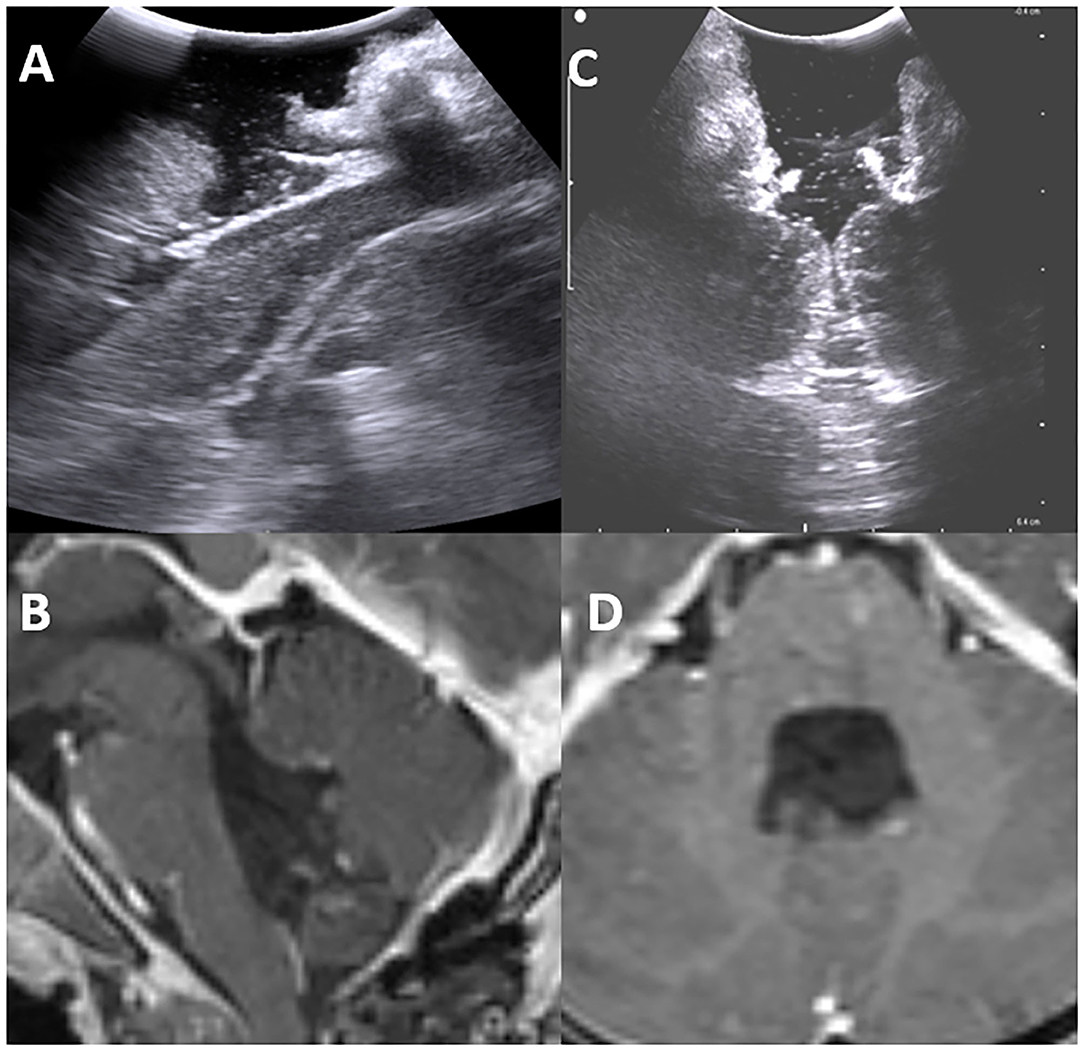
Figure 7. Post-operative intraoperative ultrasound images in sagittal and axial view showing total resection of the lesion (A,C) in concordance with postoperative MRI -TIWI in sagittal and axial views (B,D).
Vascular Lesions
Arteriovenous Malformation
The iUS can be used to visualize a range of vascular abnormalities, including arteriovenous malformations (AVM). We illustrate our experience in a case of a 17-year-old female who presented with seizures and worsening headaches. Preliminary CT and MRI were suggestive of a 4-cm left occipital AVM (Spetzer Martin Grade 3). The iUS's Doppler mode allows for the precise imaging of arterial feeders and venous drainage. This mode is extremely valuable in AVM surgery because it depicts vascular flow in real-time. Locating the main arteries and veins of a tumor, as well as the patency of the venous sinuses and proof of the tumor's vascularity, aids in safe removal (Figure 8).
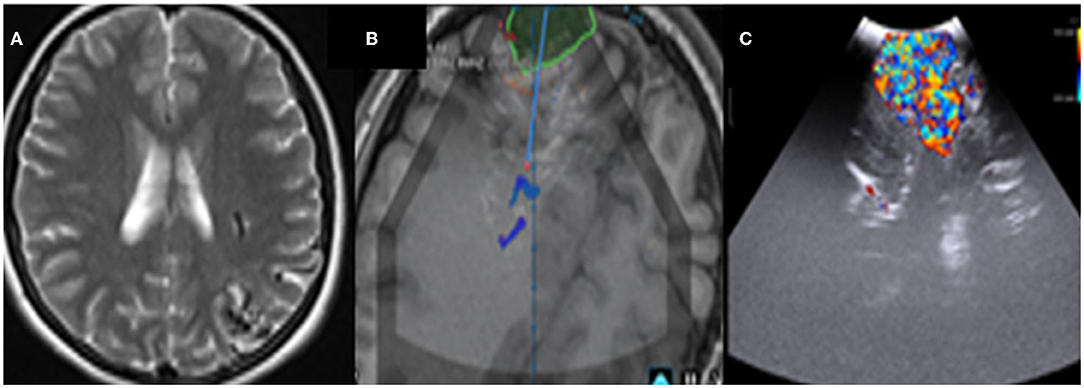
Figure 8. (A) Pre-operative MRI depicting an occipital arteriovenous-malformation (AVM). (B) Intraoperative ultrasound integrated with brain lab system clearly identifying the AVM. (C) Doppler mode.
Spinal Lesions
The iUS is fairly useful for determining the level and localizing the lesion during spine surgery. We have had remarkable success using iUS for both extramedullary and intramedullary lesions, such as neurofibromas, ependymomas, and astrocytomas, in both extramedullary and intramedullary settings. The iUS can be used to safely aid and remove tumor successfully through the surgery, without disturbing the flow of surgery. The iUS made it easier to confirm tumor location and extension, plan myelotomy, and estimate the extent of the lesion (Figure 9).
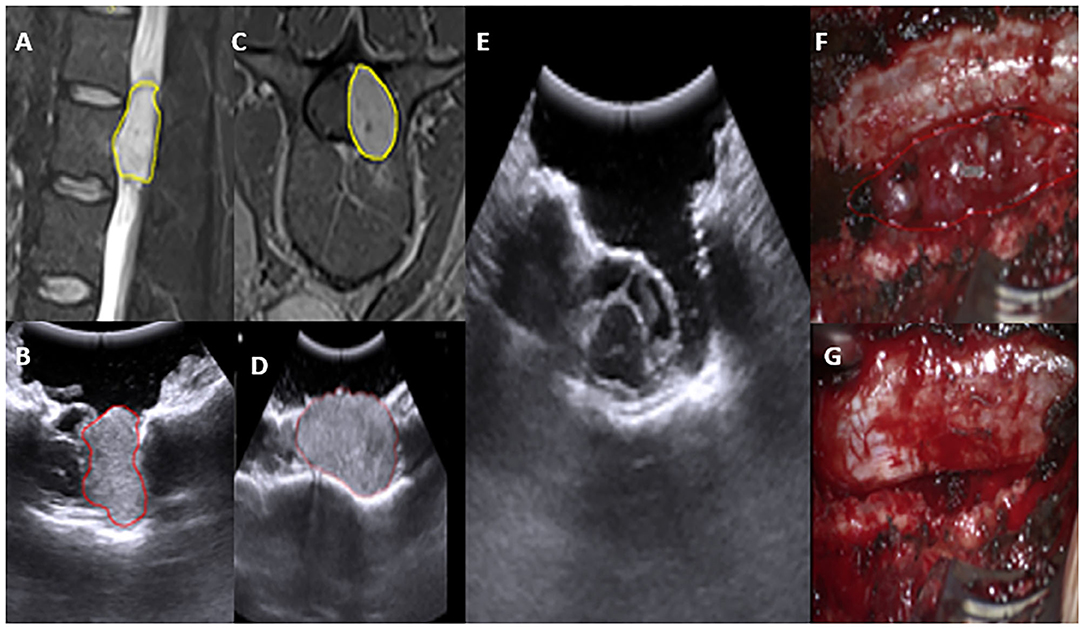
Figure 9. Preoperative MRI T2WI sagittal and axial view depicting an extradural L2–3 lesion (A,C) yellow. In concordance, IUS depicting the lesion-location with spinal cord boundary (B,D). Post-operative IUS Clearly shows total resection (E). Intraoperative images, prior and post-resection (F,G).
Epilepsy Neurosurgery
One of the most common causes of drug intractable epilepsy is focal cortical dysplasia (FCD). In epilepsy surgery, considering that normal and dysplastic brain tissues are often indistinguishable, we have utilized iUS to localize epileptogenic lesions and, thereby, improve surgical outcomes. Cortical characteristics demonstrated perfect concordance between iUS (thickness and hyper-echoic of cortex, subcortical white matter) and MRI T2-weighted/FLAIR images (hyperintense cortical and subcortical changes), especially in Focal cortical type II. The lesion margins on IUS pictures are even clearer than on MRI images. As a result, we believe that iUS is a valuable tool for epilepsy surgery (Figure 10).
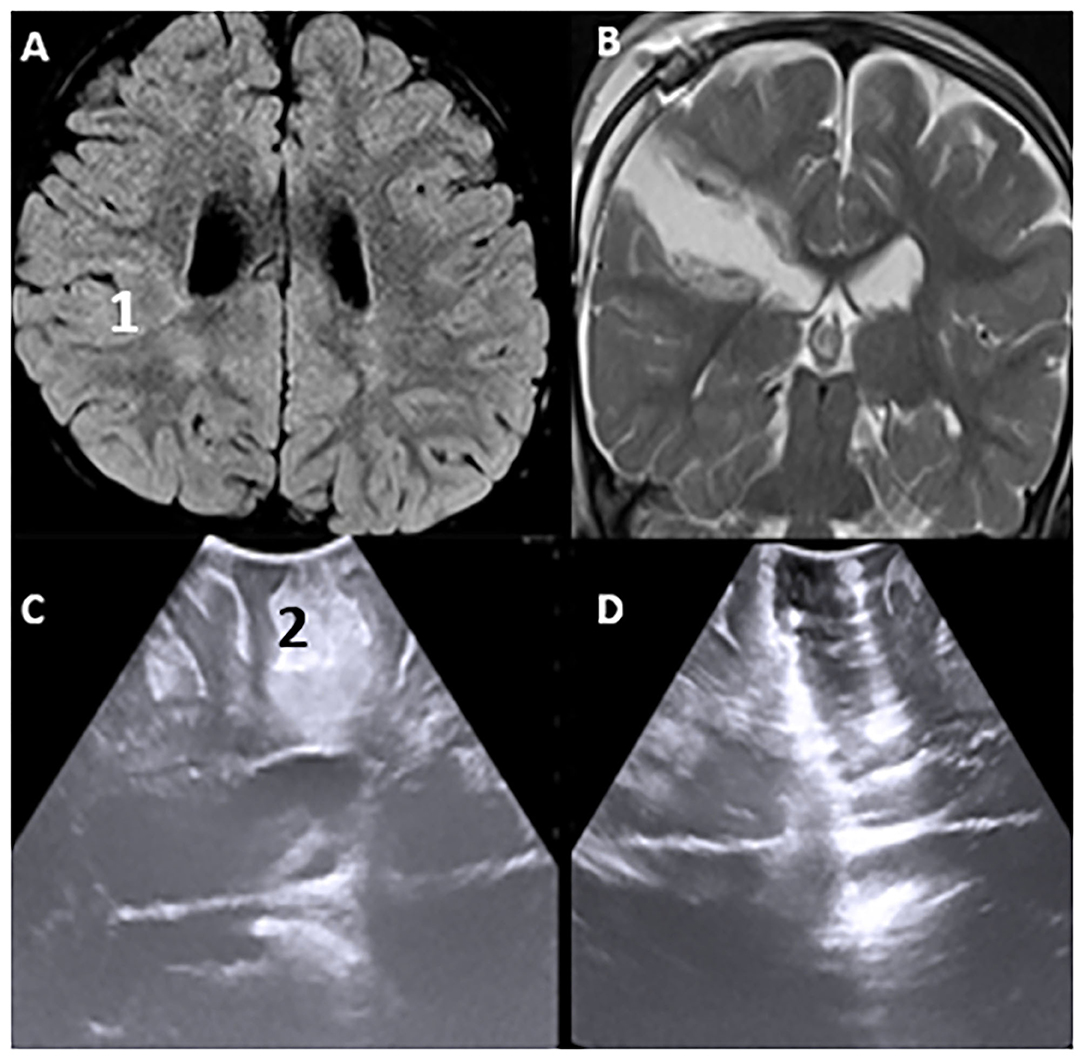
Figure 10. Pre-operative Hyperintence axil MRI FLAIR rt FCD type IIb (A,1) in concordance with pre-resection intraoperative hyper echoic cortical and subcortical IUS features (C,2). Post-operative coronal T2WI (B) and intraoperative post-resection IUS showing complete resection of the lesion (D).
Discussion
As a neuro-navigation modality, iUS navigation in neurosurgery requires a review in terms of utility and user-friendliness, assisting resection extent, and financial burden in terms of purchasing it in neurosurgical centers. The survey conducted suggested there appears to be widespread global availability and familiarity with iUS. Additionally, the general opinion appears to be that the application of iUS during neurosurgery provides relatively quick and accurate information on lesion location and boundaries without negatively affecting intraoperative workflow. The investigators have observed that the introduction of high-resolution iUS during neurosurgery at the study site has provided a relatively convenient and user-friendly modality for intraoperative identification, localization, and characterization of neurosurgical lesions.
Relative to other modes of intraoperative neuronavigation such as MRI and CT-scan, some of the key features and potential advantages of iUS that the investigators have noted include:
• Provision of real-time information on lesion location and extent even in the face of “brain shift”; This maximizes lesion resection while minimizing the negative effects on surrounding healthy tissue.
• Significant reduction in surgical time. A time duration evaluation use of intraoperative MRI was evaluated in a study conducted by Sacino et al. Their study observed that they had an operative time range of 1.5–3 h of additional surgery as a result of the technicalities of performing iMRI. The preparation and transportation of the patient to the MRI cabinet, conducting the MRI, and returning to the operation room were major factors that contributed to additional operative time. Additionally, when back in the theater, re-sterilization, and re-gowning of the patient, nurses, and surgeon compounded into the time factor (7). This was in sharp contrast to our experience in which using iUS did not influence the operational flow. The approximate time taken for each IUS navigation screening session was as follows: (1) prior to dura opening-−1–2 min; (2) after dura opening, lesion localization and delineation are 1–2 min. Control for residual lesion post-resection is 1–2 min.
• Cost-effectiveness about acquisition, running, and maintenance of iUS apparatus – It is important to appreciate that the prohibitively high costs associated with CT- and MRI-based neuronavigation techniques limit their availability and use in LMICs (9).
• Non-invasive nature and associated safety for both patient and neurosurgeon.
• Intraoperative repeatability.
Unfortunately, as is the case with any technique applied in many fields, iUS has some disadvantages, key of which is the steep learning curve. However, the investigators are of the opinion that the rate at which different surgeons will acquire the skill to effectively employ iUS is largely a function of their years of surgical experience (2, 9).
Conclusion
The application of iUS in neurosurgical practice will continue to evolve in the face of improvements in surgical techniques, as well as new and improved technologies.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KS, RS, II, and AS: conceptualization. KS, LB, and LM: data curation. KS, AS, and RS: formal analysis. KS: investigation and project administration. RS and LB: resources. LB, RS, II, and LM: software. LB and LM: supervision. KS, RS, and II: validation. KS and RS: roles/writing—original draft and writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
iUS, intraoperative ultrasonography; iMRI, intraoperative magnetic resonance imaging; iCT, intraoperative computed tomography; LMIC, low-&-middle-income countries; MIC, middle-income countries; HIC, high-income countries; CSF, cerebral spinal fluid; FCD, focal cortical dysplasia; AVM, arteriovenous malformation; LGG, low grade glioma; HGG, high-grade gliomas.
References
1. Akeret K, Bellut D, Huppertz HJ, Ramantani G, König K, Serra C, et al. Ultrasonographic features of focal cortical dysplasia and their relevance for epilepsy surgery. Neurosurg Focus. (2018) 45:E5. doi: 10.3171/2018.6.FOCUS18221
2. Sastry R, Bi WL, Pieper S, Frisken S, Kapur T, Wells W 3rd, et al. Applications of ultrasound in the resection of brain tumors. J Neuroimaging. (2017) 27:5–15. doi: 10.1111/jon.12382
3. Prada F, Gennari AG, Quaia E, D'Incerti L, de Curtis M, DiMeco F, et al. Advanced intraoperative ultrasound (ioUS) techniques in focal cortical dysplasia (FCD) surgery: a preliminary experience on a case series. Clin Neurol Neurosurg. (2020) 198:106188. doi: 10.1016/j.clineuro.2020.106188
4. Tringali G, Bono B, Dones I, Cordella R, Didato G, Villani F, et al. Multimodal approach for radical excision of focal cortical dysplasia by combining advanced magnetic resonance imaging data to intraoperative ultrasound, electrocorticography, and cortical stimulation: a preliminary experience. World Neurosurg. (2018) 113:e738–46. doi: 10.1016/j.wneu.2018.02.141
5. Goren O, Monteith SJ, Hadani M, Bakon M, Harnof S. Modern intraoperative imaging modalities for the vascular neurosurgeon treating intracerebral hemorrhage. Neurosurg Focus. (2013) 34:E2. doi: 10.3171/2013.2.FOCUS1324
6. Ghinda D, Zhang N, Lu J, Yao CJ, Yuan S, Wu JS. Contribution of combined intraoperative electrophysiological investigation with 3-T intraoperative MRI for awake cerebral glioma surgery: comprehensive review of the clinical implications and radiological outcomes. Neurosurg Focus. (2016) 40:E14. doi: 10.3171/2015.12.FOCUS15572
7. Sacino MF, Ho CY, Murnick J, Tsuchida T, Magge SN, Keating RF, et al. Intraoperative MRI-guided resection of focal cortical dysplasia in pediatric patients: technique and outcomes. J Neurosurg Pediatr. (2016) 17:672–8. doi: 10.3171/2015.10.PEDS15512
8. Bø HK, Solheim O, Kvistad KA, Berntsen EM, Torp SH, Skjulsvik AJ, et al. Intraoperative 3D ultrasound-guided resection of diffuse low-grade gliomas: radiological and clinical results. J Neurosurg. (2019) 132:518–29. doi: 10.3171/2018.10.JNS181290
Keywords: ultrasonography, navigation, neurosurgery, epilepsy, glioma
Citation: Simfukwe K, Iakimov I, Sufianov R, Borba L, Mastronardi L and Shumadalova A (2022) Application of Intraoperative Ultrasound Navigation in Neurosurgery. Front. Surg. 9:900986. doi: 10.3389/fsurg.2022.900986
Received: 21 March 2022; Accepted: 04 April 2022;
Published: 10 May 2022.
Edited by:
Ozal Beylerli, Federal Center of Neurosurgery, RussiaReviewed by:
Bipin Chaurasia, Neurosurgery Clinic, NepalAndrey Tulupov, International Tomography Center (RAS), Russia
Copyright © 2022 Simfukwe, Iakimov, Sufianov, Borba, Mastronardi and Shumadalova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keith Simfukwe, a2VpdGhzaW1mdWt3ZUBnbWFpbC5jb20=
 Keith Simfukwe
Keith Simfukwe Iurii Iakimov1,2
Iurii Iakimov1,2 Rinat Sufianov
Rinat Sufianov Luís Borba
Luís Borba Luciano Mastronardi
Luciano Mastronardi Alina Shumadalova
Alina Shumadalova