- 1Department of Surgical Sciences and Urology Clinic, University of Turin and Città della Salute e della Scienza, Turin, Italy
- 2Department of Urology and Clinical Research Group on Predictive Onco-Urology, APHP, Sorbonne University Paris, Paris, France
- 3Department of Urology, Imperial College, London, United Kingdom
- 4Department of Surgical Sciences and Radiology Clinic, University of Turin and Città della Salute e della Scienza, Turin, Italy
- 5Department of Urology, Centre Hospitalier-Universitaire Vaudois, CHUV, Lausanne, Switzerland
- 6Department of Urology, McGill University, Montreal, Canada
- 7Department of Radiotherapy, Institut Gustave Roussy, Villejuif, France
- 8Department of Urology, Hospital Clínico San Carlos, Madrid, Spain
- 9Department of Nuclear Medicine, University of Turin and Città della Salute e della Scienza, Turin, Italy
- 10Division of Radiotherapy and Department of Oncology, University of Turin and Città della Salute e della Scienza, Turin, Italy
Introduction: Currently, the majority of prostate cancer (PCa) recurrences after non-surgical first-line treatment are managed with androgen-deprivation therapy (ADT). Salvage radical prostatectomy (sRP) is a curative alternative to ADT but yields significant morbidity. Preliminary evidence from focal salvage treatments shows similar oncological control but lower morbidity compared to sRP. Among available ablative focal energies, irreversible electroporation (IRE) is a treatment modality that proved promising, especially in treating apical lesions, where PCa most often recurs. Our aim is to test the safety of salvage IRE for recurrent PCa.
Methods: We performed a single-arm pilot feasibility study (IDEAL stage 2a): SAFE, SAlvage Focal irreversible Electroporation for recurrent localized PCa. Twenty patients with biopsy-proven PCa recurrence after primary non-surgical (radiation or ablation) treatment were included. All men will undergo mpMRI ± targeted biopsies, pre-operative PSMA-PET staging before inclusion and sIRE. Outcomes will be evaluated through internationally validated questionnaires and morbidity scales. All men will undergo a control biopsy at one year.
Results: Primary objectives were the evaluation of the safety of sIRE (and patients’ quality of life) after treatment. Secondary objectives were the evaluation of functional outcomes, namely, continence and erectile function changes and evaluation of short-term oncological efficacy.
Conclusions: SAFE is the second pilot study to evaluate sIRE and the first one performed according to the most recent diagnostic and staging imaging standards. sIRE may provide a curative option for recurrent PCa together with lower comorbidities compared to sRP.
Introduction
Prostate cancer (PCa) is the most frequent non-skin solid neoplasm in men (1). Approximately 80% of the 190,000 new cases diagnosed yearly in the United States are found at a stage localized to the prostate (1). These patients undergo surgery in the majority of cases, but one in four chooses non-surgical treatments including radiotherapy (RT) and brachytherapy (BT) (2). Overall, reported rates of disease recurrence after RT and/or BT range from 10% to 30% at 5 years up to 50%–60% at 10 years (3–5). If considering a middle way, this would translate into 15,000 PCa recurrences per year, making radio-recurrent PCa the fourth most commonest male genitourinary cancer (1, 6).
Radio-resistant disease natural history shows half of the men not developing metastases at 5 years if left untreated. The other half will develop systemic progression at a median of 3 years. Hence, a significant proportion remains with a relevant window for a definitive cure (7). Similar figures are confirmed by recent PET-PSMA imaging studies, as more than half of recurrences are localized to the prostatic bed only (8, 9).
However, more than 90% of patients indiscriminately undergo palliative androgen-deprivation therapy (ADT), losing the chance of a definitive cure and eventually developing a castration-resistant state at a median of 2–3 years after the start of ADT (7). Also, healthcare will be faced with significant costs (10, 11): patients with several ADT-related side effects and decreased quality of life (QoL) (12). This disturbing compromise does not come as a surprise if acknowledging the results of the historical salvage radical prostatectomy series (sRP); major complications were experienced in up to one in three men, and median blood loss was described as up to almost 2L and incontinence in up to 80% of cases (13).
Results of multicenter series involving our and other institutions, and including the robotic approach, show relevant improvement of sRP morbidity in the contemporary era. Nonetheless, this surgery remains challenging. Rectal injuries are now rare (<1%–2%), and strictures also diminished (<10%–20%). However, currently, one in ten and one in three men still experience high-grade and overall complications, respectively (14–16); despite almost 60% of men preserving their pre-operative continence status, up to one in four still has severe (>3 pads/day) incontinence.
Similarly, oncological control is inferior to a first-line setting. Biochemical recurrence (BCR)-free survival is generally >60% at the end of a short- to intermediate-term follow-up; five-year progression-free (PFS) and cancer-specific survival (CSS) are around 50% and 90% at 5 years (17).
In recent years, there has also been an increasing interest in whole-gland and focal ablative strategies for primary PCa (18). Medium-term results of several energies have already proved promising (19). Nonetheless, on a longer follow-up, up to one in two men need some form of re-treatment due to PCa recurrence and/or persistence (20–23). Thus, recurrent PCa following non-surgical first-line treatment is further likely to grow in the near future.
Following partial ablation, sRP has proven comparable to a first-line setting rather than to surgery after radiotherapy, as complications are rare and continence is preserved in approximately 80%–90% of cases. However, erectile function preservation remains suboptimal and overall, there is still room for improvement (24, 25).
The rationale of focal treatments relies on treating the index cancer lesion, namely, the largest and more aggressive cancer focus, which likely drives PCa progression and metastatic spread, eventually leading to death (26). Contrarily, satellite non-significant lesions are unlikely to evolve and play a “clinically significant” role (19, 26). This concept has been criticized in a first-line setting and, in the presence of some contradictory evidence, likely requires further assessment (27).
Nonetheless, in a recurrent setting, PCa has been found in the same site of the original index lesion in 90% to up to 100% of the cases. First-line whole-gland treatment may definitively silence non-significant foci, while failure may be related to radio-resistant clones emerging within the index lesion (28–30). If further confirmed, this provides an evidence-based rationale for using focal strategies also in treating recurrences (30).
On the one hand, if proven to achieve adequate oncological control, focal salvage treatments (sFT) would dramatically reduce the rate of sRP-related complications. On the other hand, they would avoid ADT palliation and offer a curative option.
To date, results of more than 500 men receiving sFT have been detailed using brachytherapy, HIFU, or cryotherapy. The follow-up remains relatively short, with the majority of the series not reaching 5 years from treatment. Oncological control is promising, with 0%–20% developing metastasis at approximately 3 years and half of the men not having evidence of recurrence at 3–5 years. While results do not seem inferior to sRP, functional outcomes and complications are much improved: continence can be maintained in up to 90%; erectile function, when valid pre-operatively, generally has a slight decrease only; and complications, especially of high grade, are low, with strictures being detailed in less than 10% and most feared complications such as fistulas in less than 2% (31, 32).
Among new focal therapy energies, focal irreversible electroporation (fIRE) has proven promising due to its ability to cause direct cellular tissue damage through irreversible alterations of cell membrane permeability. Previous reports suggested only minimal damage to close structures surrounded by connective layers, including the urethral sphincter and neurovascular bundles, as IRE damages cells by disrupting the membrane equilibrium but not acellular connective tissues (33–35).
Furthermore, in a first-line setting, IRE has proven highly promising in the context of apical disease, with 90% failure-free survival at 3 years and only one in-field detailed recurrence in a series of 50 men with apical disease (36). This may represent an important advantage as, according to a recent analysis of sRP specimens, up to 90% of radio-recurrent PCa involves the prostate apex (37).
Nonetheless, the evidence in a salvage setting, where benefits in terms of morbidity and functional outcomes may be increased compared to a treatment-naïve scenario, is limited to a single-center series (38). Hence, we aim to perform a pilot single-center study to evaluate SAlvage Focal irreversible Electroporation for recurrent localized prostate cancer (SAFE) as the initial step to subsequently implement a larger phase II multicenter study.
Methods and Analyses
Study Objectives
Primary Objective
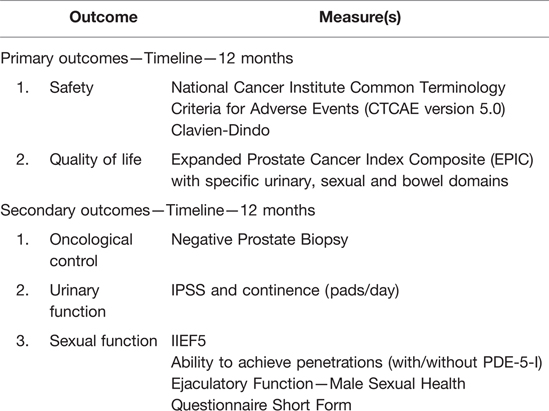
1. Evaluation of safety of focal irreversible electroporation for recurrent PCa and patients’ quality of life after treatment.
Secondary Objectives
1. Evaluation of functional outcomes of focal irreversible electroporation for radio-recurrent PCa, namely continence and erectile function changes;
2. Evaluation of short-term oncological efficacy.
Rationale
Our aim is to confirm evidence of the preliminary data from a single-center trial (38). If proving non-inferior results, we plan to proceed with a larger phase II study.
Our work complies with the IDEAL guidelines for evaluating surgical innovation in a phased manner. Our study represents stage 2a of these guidelines (prospective development study) (39, 40).
Study Inclusion and Follow-Up
Pre-Operative Staging
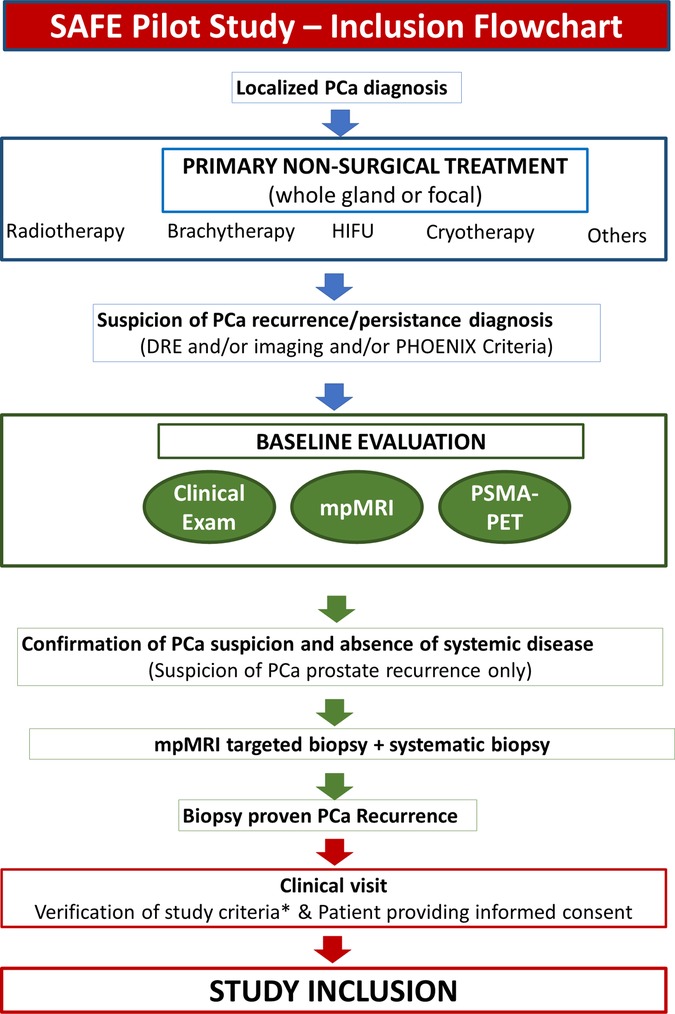
The process from patient referral to study inclusion is summarized in Figure 1. All men with clinical (DRE, mpMRI, PSMA-PET) and/or biochemical suspicion of recurrence (defined according to the PHOENIX Criteria—nadir PSA + 2 ng/ml) will undergo the following test to confirm the presence of PCa and stage the disease:
1. mpMRI and mpMRI visible lesion;
2. PSMA-PET;
3. mpMRI-targeted biopsies (in the case of a positive mpMRI) + systematic biopsies
• including 14 cores transperineal biopsies—12 cores from the posterior zone and 2 cores from the anterior prostate bilaterally),
• using a software able to provide prostate cartography (needle trajectories recorded) to be used to guide treatment needle positioning in case of subsequent treatment; and
4. Two PSA determinations in 3 months before treatment.
Study Criteria
Inclusion Criteria
• Biopsy-proven recurrent PCa—defined as recurrent after primary treatment with curative intent—including radiation therapy, HIFU, cryotherapy, and other ablation techniques. Both recurrences after focal or whole-gland primary treatment will be included;
• Clinically localized disease at mpMRI and PSMA-PET showing no extra-prostatic distant spread (pelvic nodes or other sites);
• Any Gleason score;
• Life expectancy >10 years;
• PSA≤20 ng/ml;
• Apical disease will be included;
• PCa suitable for focal salvage ablation up to hockey stick ablation, defined as treatment on three on four prostate quadrants; and
• Multifocal recurrent PCa involving more than three prostate quadrants (not suitable for hockey stick ablation).
Exclusion Criteria
• Prior ADT alone will not be considered as a previous treatment (e.g., patients with PCa after a cycle of ADT will not be considered for the study as ADT does not have a curative intent);
• Clinical T-stage cT4 and cT3b with >1 cm seminal vesicle involvement (mpMRI);
• Less than 6 months from primary treatment (persistent PCa);
• ADT performed in the 12 months before the treatment of recurrence;
• Patient history of epilepsy or cardiac arrhythmia or cardiac pacemaker;
• Recent history of myocardial infarction;
• Cardiac pacemaker;
• Active urinary tract and or other site infections;
• Ablation of lesions in the vicinity of implanted electronic devices or implanted devices with metal parts;
• Contraindications to performing mpMRI and/or PSMA-PET;
• Patients <18 years old; and
• Patients not providing written informed consent.
Procedure
Salvage IRE will be performed using the Nanoknife system (Angiodynamics, Queensbury, NY, USA).
Patients will be placed in the lithotomy position after general anaesthesia—I.V. muscle paralysis and single-shot antibiotic prophylaxis. An indwelling urethral catheter will be placed.
A prostate biopsy using a co-axial needle will be taken at the site of needle placement before the procedure. The needle electrodes (19-gauge) will be placed through the perineum using TRUS guidance with a 5-mm brachytherapy template grid. A safety margin of 10 mm will be applied as previously described (38) based on biopsy and MRI evaluation. No Denonvilliers hydrodissection will be performed. The number of electrodes and active tip length depend on the required ablation size based on the recurrent PCa volume. The ablation template will also depend on lesion size, ranging from focal ablation to up to hockey stick ablation (Figure 2). A peri-procedural biopsy at the center of the needle placement will be performed before treatment delivery. N = 2 cores will be taken in the case of hockey stick ablation. The pulse features will be changed after a test using 20 test pulses to reach the required current for the ablative effect of IRE (20–40 A between each electrode pair). The remaining 80 treatment pulses will be then administered.
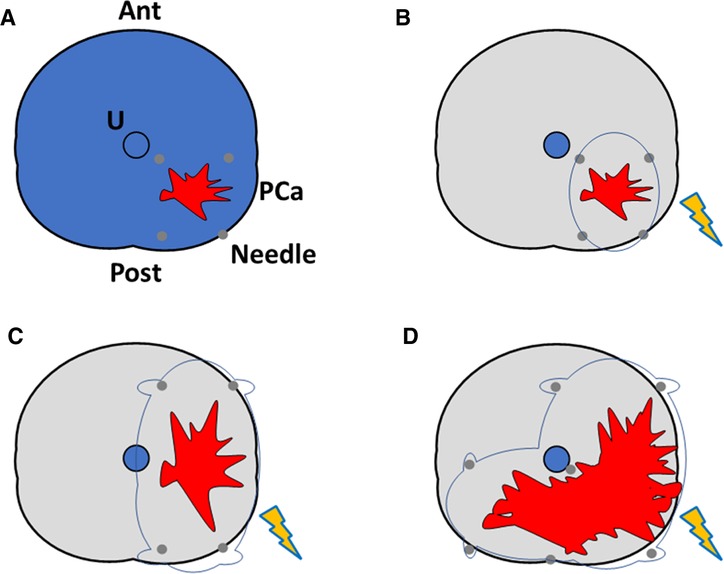
Figure 2. Different axial representations of salvage irreversible electroporation ablation schemes used in the SAFE study. Ant, anterior prostate; U, urethra; PCa, prostate cancer focus (red); Needle, irreversible electroporation needle (gray); ablation zone is displayed in orange. (A) Overall prostate view; (B) focal ablation; (C) hemi-ablation; (D) hockey stick ablation. Quadrant ablation will also be performed (not shown in the image).
Patient discharge will be attempted on day 1 after catheter removal.
Outcomes and Outcomes Measures
Follow-Up
The study flowchart from inclusion to follow-up is summarized in Figure 3. All study measures will be performed
• at 1 week (phone interview) and
• data manager for questionnaire plus clinical visit (6 weeks, 3 months, 6 months, and 12 months).
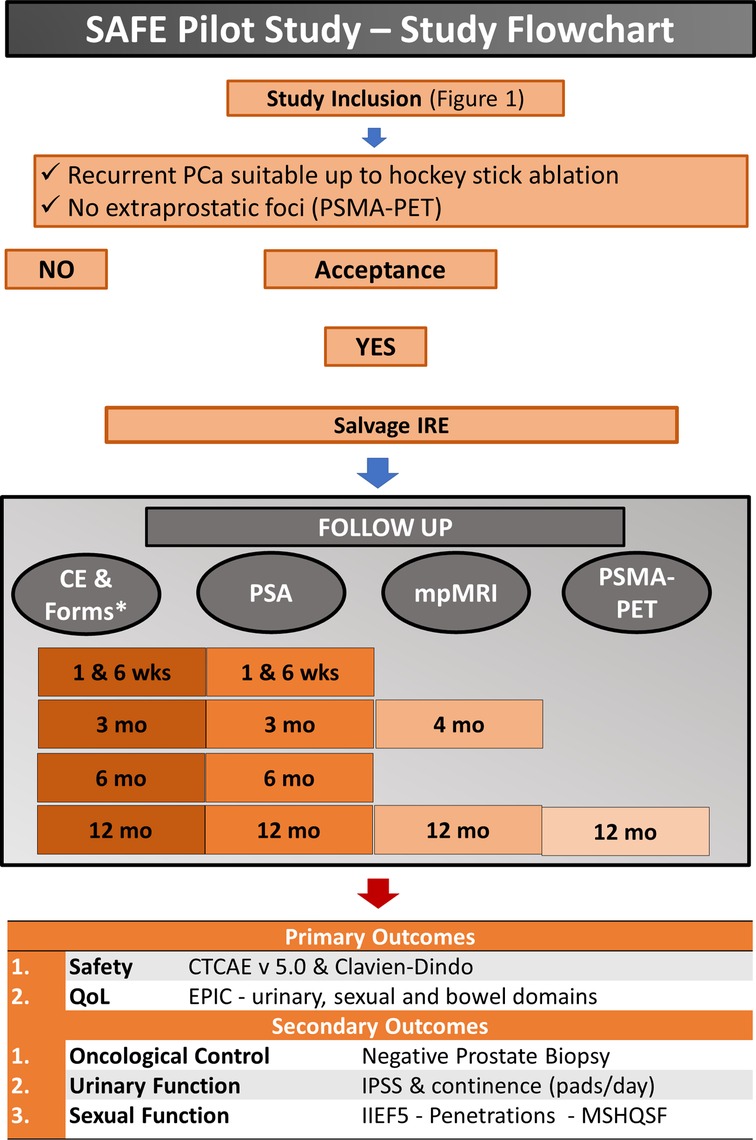
Figure 3. Flowchart illustrating the different steps of the study after patient inclusion. *=Clinical examination and questionnaires; at 1 week, a phone interview will be performed instead of a clinical visit; PCa, prostate cancer; IRE, irreversible electroporation; TCAE v 5.0, National Cancer Institute Common Terminology Criteria for Adverse Events; QoL, quality of life; EPIC, Expanded Prostate Cancer Index Composite—with specific urinary, sexual and bowel domains; IPSS, International Prostate Symptoms Score; IIEF-5, International Index of Erectile Function version 5.
Additional Oncological Assessment
• PSA at 1 week, 6 weeks, and 3, 6, and 12 months.
As per the recommended standards according to clinical practice and the latest evidence to maximize oncological results and PCa assessment and detection:
• mpMRI at 4 months (29, 38, 41, 42) and
• mpMRI (29, 38, 41, 42), PSMA-PET (43), and prostate biopsy (14-core systematic biopsy in the case of negative mpMRI) at 12 months—earlier in the case of PSA rise.
Statistical Analysis
Our work corresponds to a pilot development study (stage IDEAL 2a).
As the primary objective of the study is to determine the safety profile of fsIRE, the sample size was calculated on the basis of complications. Previous work on fsIRE detailed two patients having CTCAE grade 2 adverse events (11%), five patients having grade 1 adverse events (27%), and no major complications (0%) (38). More recent sRP series detailed overall complications in approximately 30% and high-grade complications in 10% (44).
Considering an expected proportion of overall complications of 30%, the ±95% CIs around such proportions with n = 10, n = 20, and n = 25 would be at 62.6%, 45.6%, and 40.9%, respectively.
We have therefore chosen to set the sample size at n = 20, as there is significantly increased precision from n = 10 (Δ = 17.0%), but little improvement is achieved if more patients are included (Δ = 4.6%).
Analysis Plan
The study is supposed to start in February 2022 and finish recruitment in August 2023 (18 months). Preliminary evaluation of the safety outcome will be evaluated at 6 months and 12 months. All primary and secondary outcomes will also be assessed on the first 10 patients in a 12-month follow-up.
Discussion
Summary and Strengths
SAFE is the second pilot study to evaluate safety and short-term oncological control of sIRE prospectively. Previous studies on focal salvage treatment did not include systematic staging and/or more recent diagnostic modalities (11, 31). In our study, all men will undergo mpMRI and, more importantly, PSMA-PET, which recently proved superior to conventional imaging by level 1 evidence in a staging setting (45). The use of pre-procedural PSMA-PET may help in excluding men with micro-metastases at the time of recurrence, potentially improving the overall treatment success rate and decreasing the rate of treatment with an unproven benefit (micro-metastatic patients). Also, the performance of mpMRI-targeted biopsies through elastic fusion software with the possibility of storing needle trajectories and outcomes will allow accurate treatment planning and electroporation needle positioning (46, 47).
The use of mpMRI targeted biopsies in a radio-recurrent setting is supported by the recent preliminary results of the FORECAST study, showing good accuracy of targeted compared to transperineal template mapping biopsies (48). Furthermore, we will add two cores to our previously published biopsy protocol (49), including systematic anterior zone sampling. In the absence of mpMRI and PSMA-PET suspicion and considering the generally low volume of prostate glands that previously received non-surgical treatment, this should allow to safely reduce the ablation zone, avoiding the anterior gland and, potentially, reducing morbidity (50, 51).
Finally, previous suggestions by experts agree with an “à la carte” approach, favoring the use of different energies depending on cancer features and location to maximize ablation efficacy (52–54). In this context, sIRE may potentially allow an overall optimal disease control in the posterior gland, in the anterior gland as the needles are positioned through perineal access (54), and in the apical prostate segments, where radio-recurrent disease frequently lies (37), as suggested by first-line fIRE series (36, 55, 56). If SAFE confirms safety and oncological control of other ablative energies and the FIRE trial, this will justify carrying out a larger trial looking at longer-term results of fsIRE in the context of a multicenter collaboration.
Limitations
There are some relevant study limitations.
First, SAFE is a pilot trial. By definition, it aims to provide sufficient evidence favoring larger trials; however, it will not provide definitive evidence per se.
Second, the 12-month follow-up is sufficient to cover eventual toxicity. Nonetheless, it is too short to accurately record an estimate of stronger oncological endpoints such as metastatic progression, as this tends to occur at a median of 2–3 years from recurrence (7).
Third, we did not contemplate randomization. Although this has been recently proven feasible in a first-line setting (57), randomization may be even more difficult in a salvage context where one option, i.e., radical treatment, has potentially much higher morbidities and another, i.e., ADT, does not offer a curative chance. Nonetheless, we are aiming to prospectively collect data for men eventually preferring other treatment modalities/refusing sIRE off trial. These results may help in planning future sample size calculations in the case of randomized and/or non-randomized prospective cohort studies.
Conclusions
Recurrence of PCa after non-surgical first-line treatment is not infrequent and likely to increase in absolute numbers. Currently, the majority of men undergo palliative ADT without being offered a curative chance. The main alternative is constituted by sRP, which, however, yields significant morbidities. In this setting, focal salvage treatment may potentially allow the same oncological control of radical treatment while significantly reducing its morbidity. Among ablative energies, sIRE may be advantageous as it has promising PCa control at the apex, where recurrent PCa is more frequently located. SAFE would be the second pilot study to evaluate sIRE and the first one performed according to the most recent diagnostic and staging imaging standards.
Ethics Statement
This study will be conducted according to Good Clinical Practice (GCP) once the approval of the Institutional Ethics Committee is available.
The data property of this non-commercial study belongs to the sponsor (a public health Institution) and will be published after the end of the study in a peer-reviewed journal.
The product manufacturer (AngioDynamics, Latham, New York, USA) will provide the NanoKnife device free of charge and cover the protocol publication costs.
Author Contributions
Manuscript draft preparation: GM, DDA, and GC; study design: GM, DDA, GC, and PG; critical revision of the protocol and manuscript for important intellectual content: all authors; statistical analysis: CF. All authors contributed to the article and approved the submitted version.
Funding
There will be no economic contribution from AngioDynamic or other third parties to the study described in this protocol, which is a spontaneous, non-commercial trial. The costs of the submission open-access will be covered by AngioDynamics. AngioDynamics will also provide device NanoKnife free of charge.
Conflict of Interest
Luca Lunelli and Olivier Cussenot have worked as consultants and proctors for Angiodynamics, Latham, NY. The publication costs of this study protocol are covered by Angiodynamics, Latham, NY.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. (2010) 28:1117–23. doi: 10.1200/JCO.2009.26.0133
3. Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomized study. Lancet Oncol. (2010) 11:1066–73. doi: 10.1016/S1470-2045(10)70223-0
4. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomized, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. (2016) 17:1047–60. doi: 10.1016/S1470-2045(16)30102-4
5. Michalski JM, Moughan J, Purdy J, Bosch W, Bruner DW, Bahary JP, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. (2018) 4:e180039. doi: 10.1001/JAMAONCOL.2018.0039
6. Jones JS. Radiorecurrent prostate cancer: an emerging and largely mismanaged epidemic. Eur Urol. (2011) 60:411–2. doi: 10.1016/j.eururo.2011.01.007
7. Lee WR, Hanks GE, Hanlon A. Increasing prostate-specific antigen profile following definitive radiation therapy for localized prostate cancer: Clinical observations. J Clin Oncol. (1997) 15:230–8. doi: 10.1200/JCO.1997.15.1.230
8. Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. (2019) 77:403–17. doi: 10.1016/j.eururo.2019.01.049
9. Lawal IO, Lengana T, Popoola GO, Orunmuyi AT, Kgatle MM, Mokoala KMG, et al. Pattern of prostate cancer recurrence assessed by68 Ga-PSMA-11 PET/CT in men treated with primary local therapy. J Clin Med. (2021) 10:3883. doi: 10.3390/jcm10173883
10. Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst. (2000) 92:1731–9. doi: 10.1093/JNCI/92.21.1731
11. Kanthabalan A, Shah T, Arya M, Punwani S, Bomanji J, Haroon A, et al. The FORECAST study - focal recurrent assessment and salvage treatment for radiorecurrent prostate cancer. Contemp Clin Trials. (2015) 44:175–86. doi: 10.1016/j.cct.2015.07.004
12. Alibhai SMH, Breunis H, Timilshina N, Johnston C, Tomlinson G, Tannock I, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. (2010) 28:5038–45. doi: 10.1200/JCO.2010.29.8091
13. Chade DC, Eastham J, Graefen M, Hu JC, Karnes RJ, Klotz L, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol. (2012) 61:961–71. doi: 10.1016/j.eururo.2012.01.022
14. Gontero P, Marra G, Alessio P, Filippini C, Oderda M, Munoz F, et al. Salvage radical prostatectomy for recurrent prostate cancer: morbidity and functional outcomes from a large multicenter series of open versus robotic approaches. J Urol. (2019) 202:725–31. doi: 10.1097/JU.0000000000000327
15. Zargar H, Lamb AD, Rocco B, Porpiglia F, Liatsikos E, Davis J, et al. Salvage robotic prostatectomy for radio recurrent prostate cancer: technical challenges and outcome analysis. Minerva Urol e Nefrol. (2017) 69:26–37. doi: 10.23736/S0393-2249.16.02797-1
16. Marra G, Karnes RJ, Calleris G, Oderda M, Alessio P, Palazzetti A, et al. Oncological outcomes of salvage radical prostatectomy for recurrent prostate cancer in the contemporary era: a multicenter retrospective study. Urol Oncol Semin Orig Investig. (2021) 39:296.e21–29. doi: 10.1016/j.urolonc.2020.11.002
17. Calleris G, Marra G, Dalmasso E, Falcone M, Karnes RJ, Morlacco A, et al. Is it worth to perform salvage radical prostatectomy for radio-recurrent prostate cancer? a literature review. World J Urol. (2019) 37:1469–83. doi: 10.1007/s00345-019-02749-z
18. Marra G, Ploussard G, Ost P, De Visschere PJL, Briganti A, Gandaglia G, et al. Focal therapy in localized prostate cancer: real-world urological perspective explored in a cross-sectional European survey. Urol Oncol Semin Orig Investig. (2018) 36:529.e11–22. doi: 10.1016/j.urolonc.2018.08.013
19. Marra G, Gontero P, Valerio M. Changing the prostate cancer management pathway: why Focal Therapy is a step forward. Arch Esp Urol. (2016) 69:271–80.27416630
20. Stabile A, Orczyk C, Hosking-Jervis F, Giganti F, Arya M, Hindley RG, et al. Medium-term oncological outcomes in a large cohort of men treated with either focal or hemi-ablation using high-intensity focused ultrasonography for primary localized prostate cancer. BJU Int. (2019) 124:431–40. doi: 10.1111/bju.14710
21. Shah TT, Peters M, Eldred-Evans D, Miah S, Yap T, Faure-Walker NA, et al. Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol. (2019) 76:98–105. doi: 10.1016/j.eururo.2018.12.030
22. Marra G, Soeterik T, Oreggia D, Tourinho-Barbosa R, Moschini M, Filippini C, et al. Long-term outcomes of focal cryotherapy for low- to intermediate-risk prostate cancer: results and matched pair analysis with active surveillance. Eur Urol Focus. (2021) 39:1–9. doi: 10.1016/j.euf.2021.04.008
23. Gontero P, Marra G, Teber D, Shariat S, Albayrak S, Coelho R, et al. Making a case “against” focal therapy for intermediate-risk prostate cancer. World J Urol. (2020) 39:719–28. doi: 10.1007/s00345-020-03303-y
24. Marra G, Valerio M, Emberton M, Heidenreich A, Crook JM, Bossi A, et al. Salvage local treatments after focal therapy for prostate cancer. Eur Urol Oncol. (2019) 2:539–40. doi: 10.1016/j.euo.2019.03.008
25. Marra G, Gontero P, Walz JC, Sivaraman A, Tourinho-Barbosa R, Cathelineau X, et al. Complications, oncological and functional outcomes of salvage treatment options following focal therapy for localized prostate cancer: a systematic review and a comprehensive narrative review. World J Urol. (2019) 37:1517–34. doi: 10.1007/s00345-019-02642-9
26. Ahmed HU. The index lesion and the origin of prostate cancer. N Engl J Med. (2009) 361:1704. doi: 10.1056/NEJMcibr0905562
27. Kneppers J, Krijgsman O, Melis M, de Jong J, Peeper DS, Bekers E, et al. Frequent clonal relations between metastases and non-index prostate cancer lesions. JCI Insight. (2019) 4(2):e124756. doi: 10.1172/jci.insight.124756
28. Jalloh M, Leapman MS, Cowan JE, Shinohara K, Greene KL, Roach M, et al. Patterns of local failure following radiation therapy for prostate cancer. J Urol. (2015) 194:977–82. doi: 10.1016/j.juro.2015.04.111
29. Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M, Jung AJ, Carroll PR, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. (2012) 82:e787–93. doi: 10.1016/j.ijrobp.2011.11.030
30. Van Son M, Peters M, Moerland M, Kerkmeijer L, Lagendijk J, Van der Voort van Zyp J. Focal salvage treatment of radiorecurrent prostate cancer: a narrative review of current strategies and future perspectives. Cancers (Basel). (2018) 10:1–18. doi: 10.3390/cancers10120480
31. Khoo CC, Miah S, Connor MJ, Tam J, Winkler M, Ahmed HU, et al. A systematic review of salvage focal therapies for localized non-metastatic radiorecurrent prostate cancer. Transl Androl Urol:9:1535–45. doi: 10.21037/tau.2019.08.21
32. Valle LF, Lehrer EJ, Markovic D, Elashoff D, Levin-Epstein R, Karnes RJ, et al. A systematic review and meta-analysis of local salvage therapies after radiotherapy for prostate cancer (MASTER)[Formula presented]. Eur Urol. (2021) 80:280–92. doi: 10.1016/j.eururo.2020.11.010
33. Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. (2007) 6:295–300. doi: 10.1177/153303460700600405
34. Sirois J, Albadine R, Latour M, Trinh VQ, Benzerdjeb N, Grosset A-A, et al. The impact of intraductal carcinoma of the prostate on the site and timing of recurrence and cancer-specific survival. Prostate. (2018) 78:697–706. doi: 10.1002/pros.23513
35. Martin RCG. Use of irreversible electroporation in unresectable pancreatic cancer. Hepatobiliary Surg Nutr. (2015) 4:211–5. doi: 10.3978/j.issn.2304-3881.2015.01.10
36. Blazevski A, Amin A, Scheltema MJ, Balakrishnan A. Focal ablation of apical prostate cancer lesions with irreversible electroporation (IRE). World J Urol. (2020) 39:1107–14. doi: 10.1007/s00345-020-03275-z
37. Takeda T, Tin AL, Corradi RB, Mamoor M, Benfante NE, Sjoberg DD, et al. Topography of prostate cancer recurrence after radiation therapy: a detailed mapping study of salvage radical prostatectomy specimens. Eur Urol. (2018) 73:488–90. doi: 10.1016/j.eururo.2017.08.005
38. Scheltema MJ, van den Bos W, Siriwardana AR, Kalsbeek AMF, Thompson JE, Ting F, et al. Feasibility and safety of focal irreversible electroporation as salvage treatment for localized radio-recurrent prostate cancer. BJU Int. (2017) 120:51–8. doi: 10.1111/bju.13991
39. Ergina PL, Barkun JS, McCulloch P, Cook JA, Altman DG, Group IDEAL. IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ. (2013) 346:1–5. doi: 10.1136/bmj.f3011
40. Pennell CP, Hirst AD, Campbell WB, Sood A, Agha RA, Barkun JST, et al. Practical guide to the Idea, Development and Exploration stages of the IDEAL framework and recommendations. Br J Surg. (2016) 103:607–15. doi: 10.1002/bjs.10115
41. Barret E, Harvey-Bryan KA, Sanchez-Salas R, Rozet F, Galiano M, Cathelineau X. How to diagnose and treat focal therapy failure and recurrence? Curr Opin Urol. (2014) 24:241–6. doi: 10.1097/MOU.0000000000000052
42. Sivaraman A, Marra G, Stabile A, Mombet A, Macek P, Lanz C, et al. Does mpMRI guidance improve HIFU partial gland ablation compared to conventional ultrasound guidance? Early functional outcomes and complications from a single center. Int Braz J Urol. (2020) 46:984–92. doi: 10.1590/S1677-5538.IBJU.2019.0682
43. Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol. (2021) 80:682–9. doi: 10.1016/j.eururo.2021.08.002
44. Gontero P, Marra G, Alessio P, Filippini C, Oderda M, Munoz F, et al. Salvage radical prostatectomy for recurrent prostate cancer: morbidity and functional outcomes from a large multicenter series of open versus robotic approaches. J Urol. (2019) 202:725–31. doi: 10.1097/ju.0000000000000327
45. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomized, multi-centre study. Lancet. (2020) 395:1208–16. doi: 10.1016/s0140-6736(20)30314-7
46. Marra G, Ploussard G, Futterer J, Valerio M, Ploussard G, De Visschere PJL, et al. Controversies in MR targeted biopsy: alone or combined, cognitive versus software-based fusion, transrectal versus transperineal approach? World J Urol. (2019) 37:277–87. doi: 10.1007/s00345-018-02622-5
47. Oderda M, Faletti R, Battisti G, Dalmasso E, Falcone M, Marra G, et al. Prostate cancer detection rate with koelis fusion biopsies versus cognitive biopsies: a comparative study. Urol Int. (2016) 97:230–7. doi: 10.1159/000445524
48. Shah TT, Kanthabalan A, Pavlou M, Adeleke S, Giganti F, Brew-Graves C, et al. MRI and targeted biopsies compared to transperineal mapping biopsies for targeted ablation in recurrent prostate cancer after radiotherapy: primary outcomes of the FORECAST trial. Journal of Clinical Oncology. (2021) 39(15_suppl):5009. doi: 10.1200/JCO.2021.39.15_suppl.5009
49. Marra G, Zhuang J, Beltrami M, Calleris G, Zhao X, Marquis A, et al. Transperineal freehand multiparametric MRI fusion targeted biopsies under local anaesthesia for prostate cancer diagnosis: a multicentre prospective study of 1014 cases. BJU Int. (2021) 127(1):122–30. doi: 10.1111/bju.15121
50. Marra G, Marquis A, Tappero S, D’Agate D, Oderda M, Calleris G, et al. Transperineal free-hand mpMRI fusion-targeted biopsies under local anesthesia: technique and feasibility from a single-center prospective study. Urology. (2020) 140:122–31. doi: 10.1016/j.urology.2019.11.078
51. Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol. (2021) 80(6):682–9. doi: 10.1016/j.eururo.2021.08.002
52. Sivaraman A, Barret E. Focal therapy for prostate cancer: an “à la Carte” approach. Eur Urol. (2016) 69:973–5. doi: 10.1016/j.eururo.2015.12.015
53. Linares-Espinós E, Carneiro A, Martínez-Salamanca JI, Bianco F, Castro-Alfaro A, Cathelineau X, et al. New technologies and techniques for prostate cancer focal therapy: a review of the current literature. Minerva Urol Nefrol. (2018) 46:252–63. doi: 10.23736/S0393-2249.18.03094-1
54. Ganzer R, Arthanareeswaran VKA, Ahmed HU, Cestari A, Rischmann P, Salomon G, et al. Which technology to select for primary focal treatment of prostate cancer? European Section of Urotechnology (ESUT) position statement. Prostate Cancer Prostatic Dis. (2018) 21:175–86. doi: 10.1038/s41391-018-0042-0
55. Scheltema MJ, Chang JI, van den Bos W, Gielchinsky I, Nguyen TV, de Reijke TM, et al. Impact on genitourinary function and quality of life following focal irreversible electroporation of different prostate segments. Diagnostic Interv Radiol. (2018) 24:268–75. doi: 10.5152/dir.2018.17374
56. Morozov A, Taratkin M, Barret E, Singla N, Bezrukov E, Chinenov D, et al. A systematic review of irreversible electroporation in localized prostate cancer treatment. Andrologia. (2020) 52:1–13. doi: 10.1111/and.13789
Keywords: prostate cancer, biochemical recurrence (BCR), PSMA-PET/CT, irreversible electroporation (IRE), focal treatment
Citation: Marra G, Shah TT, D’Agate D, Marquis A, Calleris G, Lunelli L, Filippini C, Oderda M, Gatti M, Valerio M, Sanchez-Salas R, Bossi A, Gomez-Rivas J, Conte F, Deandreis D, Cussenot O, Ricardi U and Gontero P (2022) The SAFE Pilot Trial—SAlvage Focal Irreversible Electroporation—For Recurrent Localized Prostate Cancer: Rationale and Study Protocol. Front. Surg. 9:900528. doi: 10.3389/fsurg.2022.900528
Received: 20 March 2022; Accepted: 4 May 2022;
Published: 7 June 2022.
Edited by:
Dmitry Enikeev, I.M. Sechenov First Moscow State Medical University, RussiaReviewed by:
Andrey O. Morozov, I.M. Sechenov First Moscow State Medical University, RussiaMatthijs Scheltema, Amsterdam University Medical Center, Netherlands
Copyright © 2022 Marra, Shah, D'Agate, Marquis, Calleris, Lunelli, Filippini, Oderda, Gatti, Valerio, Sanchez-Salas, Bossi, Gomez Rivas, Conte, Deandreis, Cussenot, Ricardi and Gontero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giancarlo Marra Z2lhbmNhcmxvLm1hcnJhQHVuaXRvLml0
Specialty section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
 Giancarlo Marra1,2*
Giancarlo Marra1,2* Taimur T. Shah
Taimur T. Shah Alessandro Marquis
Alessandro Marquis Giorgio Calleris
Giorgio Calleris Marco Oderda
Marco Oderda Marco Gatti
Marco Gatti Desiree Deandreis
Desiree Deandreis