
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 20 June 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.899795
This article is part of the Research Topic Co-use of medicines in surgery View all 114 articles
Purpose: To analyze the obstetric high-risk factors and serological characteristics of early-onset neonatal bacterial infections (EONBI).
Methods: 119 neonates with early-onset bacterial infection who were admitted to the neonatal ward of our hospital from October 2020 to December 2021 were recorded as the study group, and 100 neonates without bacterial infection who were admitted during the same period were used as the reference group. Comparative analysis of obstetric high-risk factors and serological characteristics of EONBI.
Results: There was no statistical difference between the two groups in terms of gender and age at admission (P > 0.05). The gestational age and birth weight of newborns in the study group were lower than those in the reference group (P < 0.001). Comparing the maternal factors of EONBI between the two groups, there was no statistical difference in age, number of obstetric inspections, whether to use antibiotics, and mode of delivery (P > 0.05). Univariate analysis showed that preterm birth, unexplained asphyxia, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h were significantly associated with EONBI (P < 0.05); while there was no significant difference between the two groups in the comparison between diabetic mother and child and maternal fever at delivery (P > 0.05). Multifactorial analysis showed that preterm birth, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h had a good multivariate dependence on EONBI (P < 0.05), while there was no significant association with unexplained asphyxia, diabetic mother and child, and maternal fever at delivery (P > 0.05). The incidence of neonatal temperature >37.9°C was higher in the study group than in the reference group (P < 0.05), and there were no statistical differences in the comparison of other clinical manifestations (P > 0.05). The CRP level of neonates in the study group (47.33 ± 4.14) mg/L was higher than that of the reference group (4.84 ± 1.03) mg/L (P < 0.001). The WBC level of neonates in the study group (5.64 ± 1.18) 109/L was higher than that of the reference group (0.28 ± 0.04) 109/L (P < 0.001). The PCT level of neonates in the study group (5.41 ± 0.85) µg/L was higher than that of the reference group (0.24 ± 0.07) µg/L (P < 0.001).
Conclusion: EONBI is closely associated with several obstetric high-risk factors, including preterm birth, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h; EONBI has no specific symptoms and signs, but serum CRP, WBC, and PCT levels are significantly higher than those of newborns without co-infection with bacteria.
Newborn infants are immunocompromised and have low resistance, so bacteria can easily invade the body from the skin mucosa, umbilical cord stump, respiratory and digestive tracts causing bacterial infections in newborns, which are common types of inflammatory infections in the first 72 h of life (1, 2). Generally, the first symptom of bacterial infection in newborns is fever (3). Coughing and coughing up sputum may occur in the case of respiratory tract infections caused by bacterial infections (4). In case of bacterial infection of the intestinal tract, abdominal pain and diarrhea may be present (5). If bacterial infection such as bacteremia and sepsis occurs, it is a more serious situation, and effective antibiotic treatment is required in time.
Currently, no global assessment data on the incidence of bacterial infections in newborns are available for each country. According to incomplete statistics at home and abroad, the incidence of early-onset neonatal bacterial infections (EONBI) can range from 1% to 10% throughout live births, and once a neonate develops a systemic infection, the chance of causing its death can be 20% to 40%, and even if the child survives, 10% to 30% of the children can still be left with varying degrees of sequelae later in life (6, 7). Alerting newborns to danger signs and early identification of bacterial infections from other pathogens in a simple bedside laboratory test is of great importance to control the severity of bacterial infections in newborns and reduce neonatal mortality.
According to previous reports, in the early stages of bacterial infection, children often present with some signs, symptoms or changes in laboratory tests of inflammatory response, and these children are often accompanied by the presence of high-risk factors predisposing the newborn to bacterial infection (8). However, the clinical manifestations after infection in children are mostly not obvious, and laboratory findings are often not specific, making early diagnosis of early-onset bacterial infections in neonates relatively difficult (9). Based on the above, it is necessary to properly assess whether bacterial infections occur in the early neonatal period and to study the relationship between the occurrence of early infections and obstetric high-risk factors. This study analyzes the obstetric high-risk factors and serological characteristics of EONBI, with a view to and provides a favorable reference for its early diagnosis and timely and effective control treatment. The details are as follows.
The subjects of this experiment were neonates with ≥1 of the following obstetric high-risk factors who were admitted to the neonatal ward of our hospital from October 2020 to December 2021: preterm birth, unexplained asphyxia, diabetic mother and child, fecal contamination of amniotic fluid, maternal fever at delivery (≥38°C), maternal infection during pregnancy (including third trimester vaginitis, chorioamnionitis), premature rupture of membranes ≥18 h. All children were admitted to the hospital at an age of ≤3 days and had no other obstetric high-risk factors or complications outside the scope of the above study, and no congenital anomalies.
The children were divided into the study group (n = 119) with co-infection and the reference group (n = 100) without co-infection according to whether they were co-infected with bacteria or not. Inclusion criteria for the study group: ① With ≥1 obstetric high-risk factor; ② At least one of the following main conditions was met: blood culture or sputum culture or peripheral secretion culture (+); one or more clinical signs of infection (e.g., the child showed signs of systemic bacterial infection such as fever, low response, reduced milk intake); ③ If the above primary condition was (−), then at least one of the following secondary conditions was satisfied: elevated C-reactive protein (CRP) >8 mg/L; increased white blood cell (WBC) >25 × 109/L or decreased <5 × 109/L; decreased platelets <100 × 109/L. Exclusion criteria for the study group: Excluding sepsis, pneumonia, necrotizing small bowel colitis, urinary tract infection, meningitis, enteritis and impetigo and other diseases with clear etiology and site of infection, excluding infection by other pathogenic microorganisms such as viruses, mycobacteria and protozoa. Inclusion criteria for the reference group: children with obstetric high-risk factors but no clinical manifestations of infection, negative laboratory indicators, and clinical exclusion of infection.
Baseline information was collected upon admission of the child, including gender, age at admission, gestational age, and birth weight. Maternal factors that might be present in EONBI were collected, including age, number of obstetric inspections, whether to use antibiotics, and mode of delivery, etc. The obstetric high-risk factors present in the two groups of newborns were counted, and the relationship between EONBI and obstetric high-risk factors was analyzed by univariate and multiple logistic regression. Both groups of neonates were observed for clinical manifestations and examined for CRP, blood routine and blood culture before the administration of antibiotics. If there were clinical manifestations, sputum culture, umbilical or ocular secretion culture or chest X-ray were added according to the condition of the child. Comparison of CRP, WBC, and calcitoninogen (PCT) levels prior to antibiotic administration in both groups to assess their serological characteristics. The assay was performed by collecting 5 mL of fasting elbow vein blood, centrifuging it at 2,000 r/min for 10 min, and finally storing the separated plasma in a refrigerator at −20°C. 7,600 Hitachi automatic biochemical analyzer (Beijing Tailin Oriental Trading Co., Ltd.) and immunoturbidimetry were used to detect CRP and PCT levels, AU5800 automatic blood analyzer [Beckman Coulter Trading (China) Co., Ltd.] and supporting reagents were used to detect WBC levels.
The neonates in both groups were monitored continuously for 72 h, and they were given antibiotics of the third generation or more of cephalosporin immediately after infection for 1–3 weeks. Those who ruled out infection were given lower antibiotics or no antibiotics, paying attention to intensive care of local infection.
SPSS 22.0 software was applied for statistical analysis, and the measurement data were expressed as mean ± standard deviation, and paired t-test and ANOVA were performed. The statistical data were expressed as ratios, and the χ2 test was performed. In order to exclude the influence of confounding factors on the results of the study, with EONBI as the dependent variable, the relationship between EONBI and 7 obstetric high-risk factors was analyzed by multivariate Logistic regression. P < 0.05 was considered statistically significant.
There was no statistical difference between the two groups in terms of gender and age at admission (P > 0.05). The gestational age and birth weight of newborns in the study group were lower than those in the reference group (P < 0.001) (Figure 1).
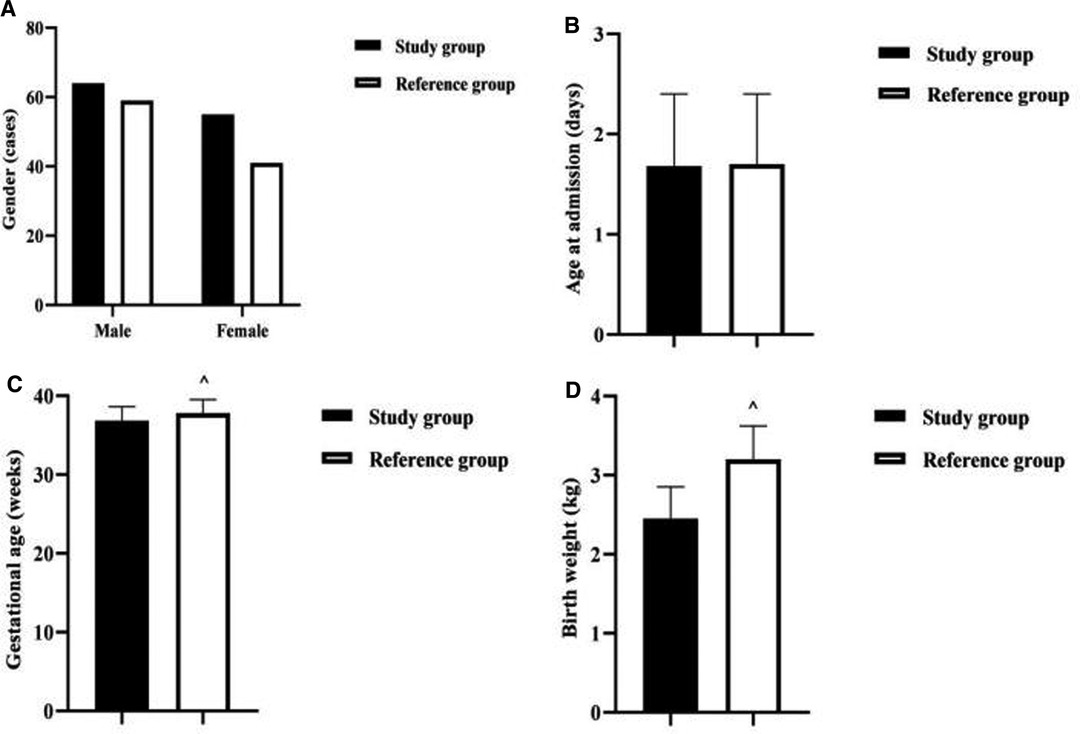
Figure 1. Comparison of baseline information between the two groups of newborns. Note: (A) Comparison of neonatal gender between the two groups. (B) Comparison of neonatal age at admission between the two groups. (C) Comparison of neonatal gestational age between the two groups. (D) Comparison of neonatal birth weight between the two groups. Comparison with the study group ^P < 0.001.
Comparing the maternal factors of EONBI between the two groups, there was no statistical difference in age, number of obstetric inspections, whether to use antibiotics, and mode of delivery (P > 0.05) (Figure 2).
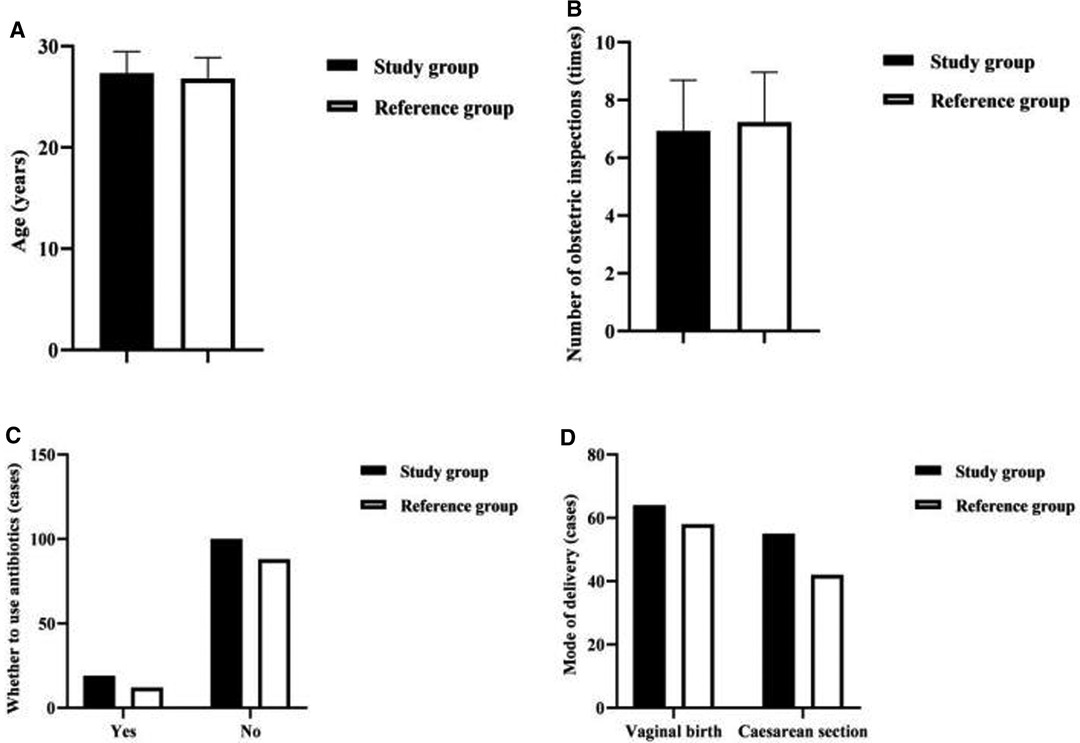
Figure 2. Analysis of the maternal factors of EONBI. Note: (A) Comparison of maternal age in both groups (years). (B) Comparison of the number of obstetric inspections in both groups (times). (C) Comparison of whether to use antibiotics in both groups (cases). (D) Comparison of the mode of delivery in both groups (cases).
Univariate analysis showed that preterm birth, unexplained asphyxia, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h were significantly associated with EONBI (P < 0.05); while there was no significant difference between the two groups in the comparison between diabetic mother and child and maternal fever at delivery (P > 0.05) (Table 1).
Multifactorial analysis showed that preterm birth, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h had a good multivariate dependence on EONBI (P < 0.05), while there was no significant association with unexplained asphyxia, diabetic mother and child, and maternal fever at delivery (P > 0.05) (Table 2).
The incidence of neonatal temperature >37.9°C was higher in the study group than in the reference group (P < 0.05), and there were no statistical differences in the comparison of other clinical manifestations (P > 0.05) (Table 3).
The CRP level of neonates in the study group (47.33 ± 4.14) mg/L was higher than that of the reference group (4.84 ± 1.03) mg/L (P < 0.001). The WBC level of neonates in the study group (5.64 ± 1.18) 109/L was higher than that of the reference group (0.28 ± 0.04) 109/L (P < 0.001). The PCT level of neonates in the study group (5.41 ± 0.85) µg/L was higher than that of the reference group (0.24 ± 0.07) µg/L (P < 0.001) (Figure 3).
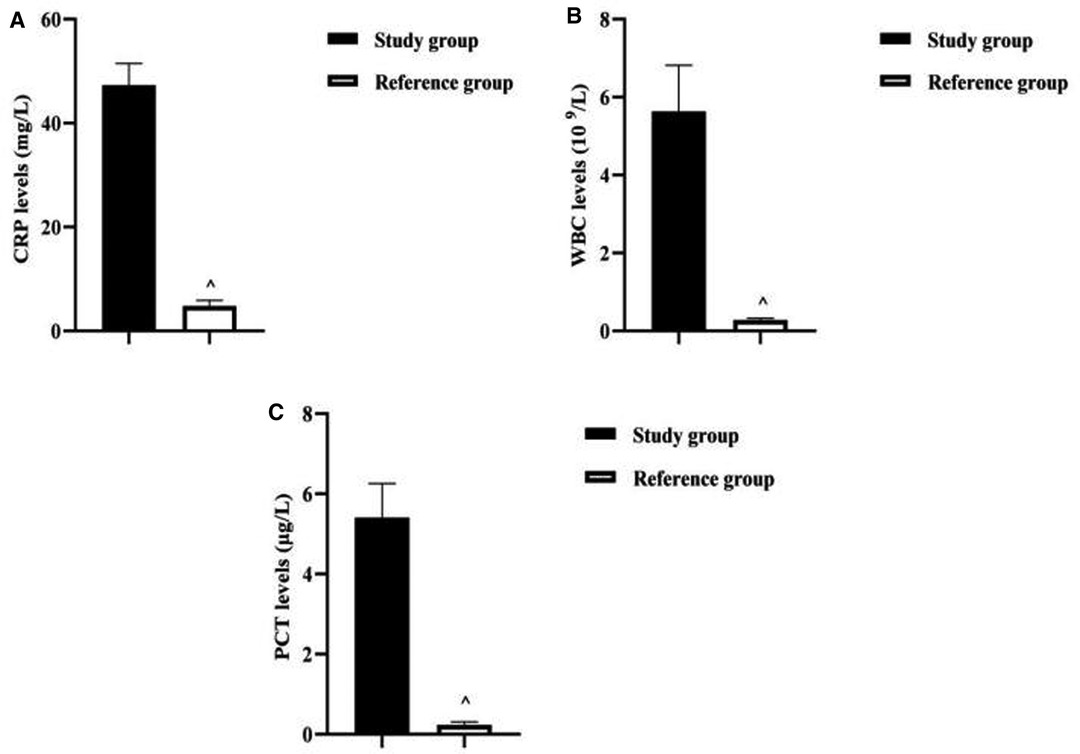
Figure 3. Analysis of serological characteristics of two groups of newborns. Note: (A) Comparison of CRP levels between two groups of neonates. (B) Comparison of WBC levels between two groups of neonates. (C) Comparison of PCT levels between two groups of neonates. Comparison with the study group ^P < 0.001.
The clinical symptoms of early neonatal bacterial infections are atypical. At present, the diagnostic criteria for neonatal bacterial infection at home and abroad are mostly based on the clinical manifestations of severe infection or even sepsis. There is a lack of scientificity and consistency in the early judgment of infection, and there are often two drawbacks: One is the failure to detect bacterial infections in newborns in a timely manner, leading to the spread of infection; the other is the use of antibiotic treatment for all newborns with obstetric high-risk factors, leading to the misuse of antibiotics (10). As seen above, it is extremely important to clarify the obstetric high-risk factors of EONBI and its infection status for the smooth implementation of the next treatment.
The results of this paper showed that preterm birth, unexplained asphyxia, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h were significantly associated with EONBI. Preterm delivery has been reported as a high risk factor significantly associated with EONBI and 64% of fecal contamination of amniotic fluid is associated with chorioamnionitis (11). The present study confirmed this finding, analyzing the reasons for this, premature infants have immature immune systems and all systems, and are more likely to acquire infections via the above-mentioned routes or through the skin and respiratory tract. In neonatal asphyxia, humoral and cellular immune functions are impaired, and the number of invasive operations during treatment predisposes to bacterial invasion, resulting in early bacterial infections (12). And EONBI mostly originates from intrauterine and is closely related to fecal contamination of amniotic fluid, maternal infection during pregnancy, premature rupture of membranes, etc. The infection routes include maternal hematogenous spread, ascending infection, retrograde fallopian tube implantation, invasive operation, etc. (13, 14). The most common of these are episodic infections, such as those caused by the upward movement of bacteria in the birth canal after premature rupture of membranes, and when bacteria spread to the amniotic cavity and grow in the amniotic fluid, the fetus can also become ill by inhaling contaminated amniotic fluid before and during delivery (15, 16).
The multifactorial analysis in this paper shows that preterm birth, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h had a good multivariate dependence on EONBI, and the most significant risk factor for EONBI is maternal infection during pregnancy. This further confirms that genital tract infection during maternal pregnancy is an important factor in triggering intrauterine infection (17). During intrauterine infection, bacteria and their products stimulate the mRNA expression of cytokines such as IL-1β, IL-6, IL-8, and TNF-α in the amniotic membrane and trophoblast cells, which induce preterm labor and lead to EONBI (18). In this study, the OR value of maternal infection during pregnancy was 71.165, which suggested that the risk of EONBI in the presence of this high-risk factor was 71.165 times higher than that in the absence of this high-risk factor. Unexplained asphyxia was not significantly associated with EONBI after controlling for confounders. This may be because the rapid development of aseptic technique and medical devices in recent years has led to a decrease in infections acquired through resuscitation.
In recent years, there has been a significant increase in the prevalence of gestational diabetes, which has been reported to be as high as 5.8% to 25.1% (19). As the placenta is rich in blood supply, the number of villi increases, the villi gap narrows accordingly, and cells proliferate, making the circulatory barrier between mother and fetus widen and the small metaplastic arteries narrow, while the potential microangiopathy in pregnant women with diabetes can aggravate the obstruction of small arteries on the fetal side of the villi stem, leading to chronic intrauterine hypoxia, acidosis, and secondary infection in the fetus (20). However, the present results after controlling for confounders showed no significant association between diabetic mother and child and EONBI. This may be related to the popularization of gestational diabetes screening in my country and the vigorous intervention of gestational diabetes in recent years. The present results also showed that maternal fever at delivery was also not significantly associated with EONBI. This may be related to the higher priority given to it by obstetricians and therefore earlier intervention. It is also possible that the sample size in this study was limited and further studies with larger sample sizes are needed to understand more precisely the relationship between the two. In this result, the gestational age and birth weight of newborns in the study group were lower than those in the reference group. This may be due to the fact that 71.43% of the newborns in the study group were preterm, much higher than the 31.00% in the reference group, so the children were under gestational age and most of them had a birth weight of no more than 2.5 kg. In this paper, the incidence of neonatal temperature >37.9°C was higher in the study group than in the reference group, and there were no statistical differences in the comparison of other clinical manifestations. This further confirms that the clinical signs and symptoms of EONBI are atypical and that the first symptom of bacterial infection in newborns is fever.
Previously, the clinical diagnosis of neonatal infectious diseases was based on the detection of peripheral blood WBC counts, and the results of WBC detection were affected by various factors, and some neonates had poor WBC count bases, and the detected WBC levels were still in the normal range even though bacterial infections had occurred in the body, so WBC could only be used as a routine reference index for the diagnosis of infectious diseases (21, 22). CRP, synthesized by inflammatory factors stimulating hepatocytes, is an acute phase response protein that triggers immunomodulation, phagocytosis, formation of immune complexes, and activation of the complement system, and has been clinically used as a nonspecific marker of the systemic inflammatory response (23). When inflammatory reaction or tissue damage occurs in the organism, CRP levels can rise abruptly within hours or 1–2 days, and its level is positively correlated with the degree of infection in the organism, so it is often used as an early diagnostic indicator of bacterial infection (24). Besides, in recent years, PCT has been considered as an ideal serological marker for the diagnosis of bacteriological infections, which is considered to have not only high specificity and sensitivity, but also can compensate for the lack of lag in bacterial culture, thus facilitating the accurate and rapid diagnosis and treatment of diseases (25). The levels of CRP, WBC, and PCT in the newborns of this result study group were higher than those of the reference group. This suggests that health care providers can use this serological characteristics as an important reference indicator to identify the presence of bacterial infection in newborns.
EONBI is closely associated with several obstetric high-risk factors, including preterm birth, fecal contamination of amniotic fluid, maternal infection during pregnancy, and premature rupture of membranes ≥18 h; EONBI has no specific symptoms and signs, but serum CRP, WBC, and PCT levels are significantly higher than those of newborns without co-infection with bacteria.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by This study was approved by the ethics committee of our hospital (21459-0-01). The patients/participants provided their written informed consent to participate in this study.
The first author is YW, she is the executor and writer of the paper. The second author is QC, he is responsible for searching data and data analysis. The third author is SX, she is responsible for research design. SC is the corresponding author, she is ensuring that the descriptions are accurate and agreed by all authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Johansson Gudjónsdóttir M, Elfvin A, Hentz E, Adlerberth I, Tessin I, Trollfors B. Changes in incidence and etiology of early-onset neonatal infections 1997–2017 - a retrospective cohort study in western Sweden. BMC Pediatr. (2019) 19:490. doi: 10.1186/s12887-019-1866-z
2. Kaftan H, Kinney JS. Early onset neonatal bacterial infections. Semin Perinatol. (1998) 22:15–24. doi: 10.1016/s0146-0005(98)80004-9
3. Brown JC, Burns JL, Cummings P. Ampicillin use in infant fever: a systematic review. Arch Pediatr Adolesc Med. (2002) 156:27–32. doi: 10.1001/archpedi.156.1.27
4. Wiegers HMG, van Nijen L, van Woensel JBM, Bem RA, de Jong MD, Calis JCJ. Bacterial co-infection of the respiratory tract in ventilated children with bronchiolitis; a retrospective cohort study. BMC Infect Dis. (2019) 19:938. doi: 10.1186/s12879-019-4468-3
5. Fasano A. Toxins and the gut: role in human disease. Gut. (2002) 50(Suppl 3):III9–14. doi: 10.1136/gut.50.suppl_3.iii9
6. Ranosiarisoa ZN, El Harrif S, Andrianirina AZ, Duron S, Simon-Ghediri MJ, Ramparany L, et al. Epidemiology of Early-onset Bacterial Neonatal Infections in Madagascar. Pediatr Infect Dis J. (2019) 38:76–81. doi: 10.1097/INF.0000000000001993
7. Chacko B, Sohi I. Early onset neonatal sepsis. Indian J Pediatr. (2005) 72:23–6. doi: 10.1007/BF02760574
8. Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. (2014) 68(Suppl 1):S24–32. doi: 10.1016/j.jinf.2013.09.011
9. Cottineau M, Launay E, Branger B, Caillon J, Muller JB, Boscher C, et al. Valeur diagnostique des critères de suspicion d’infection néonatale précoce : bilan dix ans après les recommandations de l’Anaes [Diagnostic value of suspicion criteria for early-onset neonatal bacterial infection: report ten years after the Anaes recommendations]. Arch Pediatr. (2014) 21:187–93. doi: 10.1016/j.arcped.2013.11.011
10. Caffrey Osvald E, Prentice P. NICE clinical guideline: antibiotics for the prevention and treatment of early-onset neonatal infection. Arch Dis Child Educ Pract Ed. (2014) 99:98–100. doi: 10.1136/archdischild-2013-304629
11. Neunhoeffer F, Dabek MT, Renk H, Rimmele P, Poets C, Goelz R, et al. Diagnostic value of immature myeloid information in early-onset bacterial infection in term and preterm neonates. Klin Padiatr. (2015) 227:66–71. doi: 10.1055/s-0034-1395552
12. Mussi-Pinhata MM, Nobre RA, Martinez FE, Jorge SM, Ferlin ML, Gonçalves AL. Early-onset bacterial infection in Brazilian neonates with respiratory distress: a hospital-based study. J Trop Pediatr. (2004) 50:6–11. doi: 10.1093/tropej/50.1.6
13. Chan GJ, Lee AC, Baqui AH, Tan J, Black RE. Risk of early-onset neonatal infection with maternal infection or colonization: a global systematic review and meta-analysis. PLoS Med. (2013) 10:e1001502. doi: 10.1371/journal.pmed.1001502
14. Korbage de Araujo MC, Schultz R, do Rosário Dias de Oliveira L, Ramos JL, Vaz FA. A risk factor for early-onset infection in premature newborns: invasion of chorioamniotic tissues by leukocytes. Early Hum Dev. (1999) 56:1–15. doi: 10.1016/s0378-3782(99)00027-4
15. Gérardin P, Fianu A, Choker G, Carbonnier M, Jamal-Bey K, Heisert M, et al. Infection bactérienne néonatale précoce dans le sud de la Réunion: incidence et application des critères de risque Anaes 2002 [Early onset neonatal infections in the South of the Reunion Island: incidence and use of 2002 ANAES risk criteria]. Med Mal Infect. (2008) 38:192–9. doi: 10.1016/j.medmal.2008.01.008
16. Liu G, He S, Zhu X, Li Z. Early onset neonatal bacterial meningitis in term infants: the clinical features, perinatal conditions, and in-hospital outcomes: a single center retrospective analysis. Medicine (Baltimore). (2020) 99:e22748. doi: 10.1097/MD.0000000000022748
17. Chan GJ, Lee AC, Baqui AH, Tan J, Black RE. Prevalence of early-onset neonatal infection among newborns of mothers with bacterial infection or colonization: a systematic review and meta-analysis. BMC Infect Dis. (2015) 15:118. doi: 10.1186/s12879-015-0813-3
18. Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis. (1999) 180:1987–93. doi: 10.1086/315135
19. Alvarez-Bulnes O, Monés-Llivina A, Cavero-Roig L, Ventura PS, Llagostera-Benedico J, Valls-Sirera C, et al. Ophthalmic pathology in the offspring of pregnant women with gestational diabetes mellitus. Matern Child Health J. (2020) 24:524–9. doi: 10.1007/s10995-020-02887-6
20. Dincer UD. Fetal exposure to a diabetic intrauterine environment resulted in a failure of cord blood endothelial progenitor cell adaptation against chronic hypoxia. Stem Cells Cloning. (2014) 8:1–14. doi: 10.2147/SCCAA.S73658
21. Go H, Nagano N, Katayama D, Akimoto T, Imaizumi T, Aoki R, et al. Diagnostic accuracy of biomarkers for early-onset neonatal bacterial infections: evaluation of serum procalcitonin reference curves. Diagnostics (Basel). (2020) 10:839. doi: 10.3390/diagnostics10100839
22. Bressan S, Andreola B, Cattelan F, Zangardi T, Perilongo G, Da Dalt L. Predicting severe bacterial infections in well-appearing febrile neonates: laboratory markers accuracy and duration of fever. Pediatr Infect Dis J. (2010) 29:227–32. doi: 10.1097/INF.0b013e3181b9a086
23. Volante E, Moretti S, Pisani F, Bevilacqua G. Early diagnosis of bacterial infection in the neonate. J Matern Fetal Neonatal Med. (2004) 2:13–6. doi: 10.1080/14767050410001727116
24. Naramura T, Imamura H, Yoshimatsu H, Hirashima K, Irie S, Inoue T, et al. The predictive value of procalcitonin and high-sensitivity C-reactive protein for early bacterial infections in preterm neonates. Neonatology. (2021) 118:28–36. doi: 10.1159/000512523
Keywords: neonatal, early-onset bacterial infections, obstetrics, high-risk factors, serological features
Citation: Wang Y, Chen Q, Xu S and Chao S (2022) Obstetric Risk Factors and Serological Characteristics of Early-Onset Neonates Bacterial Infections. Front. Surg. 9:899795. doi: 10.3389/fsurg.2022.899795
Received: 19 March 2022; Accepted: 31 May 2022;
Published: 20 June 2022.
Edited by:
Songwen Tan, Central South University, ChinaReviewed by:
Fang Wei, Second Xiangya Hospital, Central South University, ChinaCopyright © 2022 Wang, Chen, Xu and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Chao cWhjZ3l5ODhAMTI2LmNvbQ==
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.