- 1Department of Neurosurgery, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Department of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 3Department of Pathology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
Oncocytic carcinoma is a malignant tumor characterized by a proliferation of epithelial cells with abundant eosinophilic granular cytoplasm. In this article, we report on the first case of a 61-year-old male patient presenting with oncocytic carcinoma involving the cranio-orbital area. An oncocytic carcinoma in the patient, who reported a sudden decrease in vision in his right eye, was removed through a frontal orbital approach craniotomy. The patient's postoperative development was rapid, and he was admitted to the neurosurgery department for a combined operation after ophthalmological screening. Pathological analysis revealed the tumour cells were large, round or polygonal, and the cytoplasm was finely granular and appeared to be more pleomorphic than the eosinophilic adenoma. Oncocytic carcinoma in the cranio-orbital area is extremely rare. The most effective treatment is early resection to be performed jointly by ophthalmology and neurosurgery, and long-term follow-up and adjuvant chemoradiotherapy are beneficial.
Introduction
Oncocytic carcinoma is also known as oncocytic adenocarcinoma, malignant oncocytoma or malignant oncocytic adenoma. It is a malignant tumor characterized by a proliferation of epithelial cells with abundant eosinophilic granular cytoplasm. Oncocytic carcinoma from a pleomorphic adenoma can be primary or malignant. The majority (80%) of oncocytic carcinomas occur in the parotid gland and 10% occur in the submandibular gland. The rest occur in the small salivary glands (including in the palate, cheek, floor of the mouth, base of the tongue and the retromolar area) (1). About one-third of diagnosed patients may have local pain or numbness or facial paralysis due to oncocytic carcinoma invading the facial nerves. The tumour is hard, inactive and has no obvious boundaries with the surrounding tissue. The skin on the surface of the oncocytic carcinoma may change colour and shrink (2). Oncocytic tumours may have a history of rapid growth from recent long-standing oncocytic carcinomas (3–5). There are also reports of oncocytic carcinomas in the nasal cavity, the paranasal sinuses (i.e. the ethmoid sinus, maxillary sinus and lacrimal sac), the bronchus, thyroid, parathyroid, kidney, ovary, upper mediastinum, lung and breast (6–9). Scattered case reports show oncocytic carcinomas occurring in the orbital area are extremely rare, and there are no reports available on oncocytic carcinomas occurring in the cranio-orbital area (10–18).
Case Report
The patient was a 61-year-old Asian male. The visual acuity of his right eye gradually worsened for almost one year before surgery. There was no swelling, pain or abnormality of movement in his right eye, but there was a defect of the visual field. The patient was first diagnosed with decreased vision in an ophthalmic clinic. The orbital computed tomography (CT) results visualised the tumour in the right posterior upper orbital space as fast growing and erosive in the cranio-orbital area, eroding and destroying the bone and protruding into the brain through the supraorbital fissure (Figure 1). The orbital enhanced magnetic resonance imaging (MRI) findings detected a solid lesion outside the muscular cone of the right superior posterior orbit.
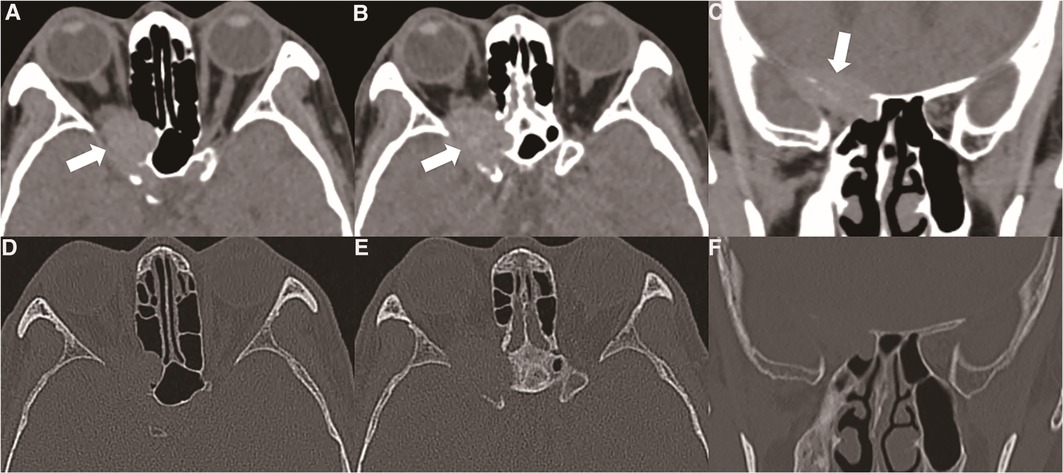
Figure 1. (A, B) orbital CT showed that the oncocytic carcinoma affects intracranial structures through the supraorbital fissure, accompanied by bone destruction (thick white arrow). (C) Oncocytic carcinoma destroyed the skull base bone and involved the dural structure (thick white arrow). (D, E, F) Orbital CT bone window showed the bone of the skull base and the outer orbital wall were absorbed or destroyed by the tumour.
A transfrontal orbital craniotomy was performed. The frontal bone flap and brow bone were removed with a milling cutter. The great wings of the sphenoid bone, the bone of the skull base and the bone of the lateral orbital wall were extensively involved, and the tumour had abundant blood supply, soft texture, no complete encapsulation and some necrosis. The bone of the lateral orbital wall was removed to reach the infraorbital fissure, the sphenoid bone was cut to reach the superior orbital fissure, the bone of the supraorbital wall was removed and the orbit was fully decompressed. The right optic canal was opened, and the sphenoid was opened inward toward the sinus. The bilateral optic canal bones were intact without tumour invasion, and the suspected eosinophilic carcinoma in the sphenoid sinus was removed (Figure 2). The dura mater of the skull base and the outer part of the orbital periosteum were also removed as they were thickened and tough. The operation exhibited difficulty in that only one fronto-orbital craniotomy method was used to deal with the tumours in the sphenoid sinus, orbit and skull base dura mater simultaneously. In reviewing the case, it was seen that the oncocytic carcinoma involved the right cranio-orbital area.
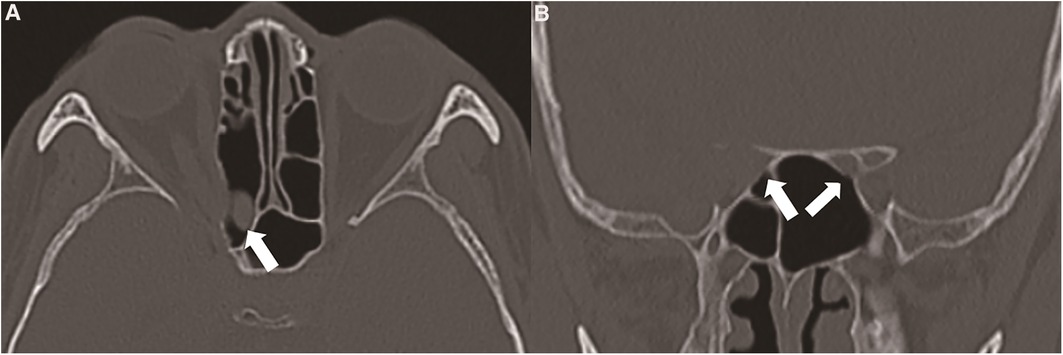
Figure 2. (A) the suspected tumour originated in the sphenoid sinus or invaded the sphenoid sinus from the cranio-orbital area (thick white arrow). (B) The tumour destroyed the right optic canal and involved the right optic nerve, and the side wall of the sphenoid sinus remained intact (thin white arrow). The left optic canal was intact (thin white arrow).
The intracranial dural structure had a natural barrier effect on this kind of tumour. According to the orbital MRI, the tumour involved the dura mater of the skull base, but it did not break through the dura mater. It only caused the enhancement of the dura mater. Note that this kind of tumour must be resected in addition to the involved bone. To avoid tumour recurrence, an extensive resection of the whole sphenoid wing and lateral orbital wall was performed (Figure 3). Through postoperative pathological analysis, the immunohistochemical staining of the tumour was found to be cytokeratin (CK) 7 (−) and tumour protein p63 (−). The CK cells were marked as epithelial cells, and p63 marked various types of cells, such as squamous epithelium and myoepithelium. To help identify some morphologically similar lesions, the negative situation raised doubts regarding the origin of the tumour. The tumour cells were large, round or polygonal, and the cytoplasm was finely granular. It appeared to be more pleomorphic than the eosinophilic adenoma. The nucleus is the centre of the cell, and nucleoli are often large and irregular (Figure 4). The patient did not undergo chemotherapy after the operation, refusing because of economic factors. After extensive resection of the tumour, there was no evidence of a recurrence at the patient's 6-month follow-up.
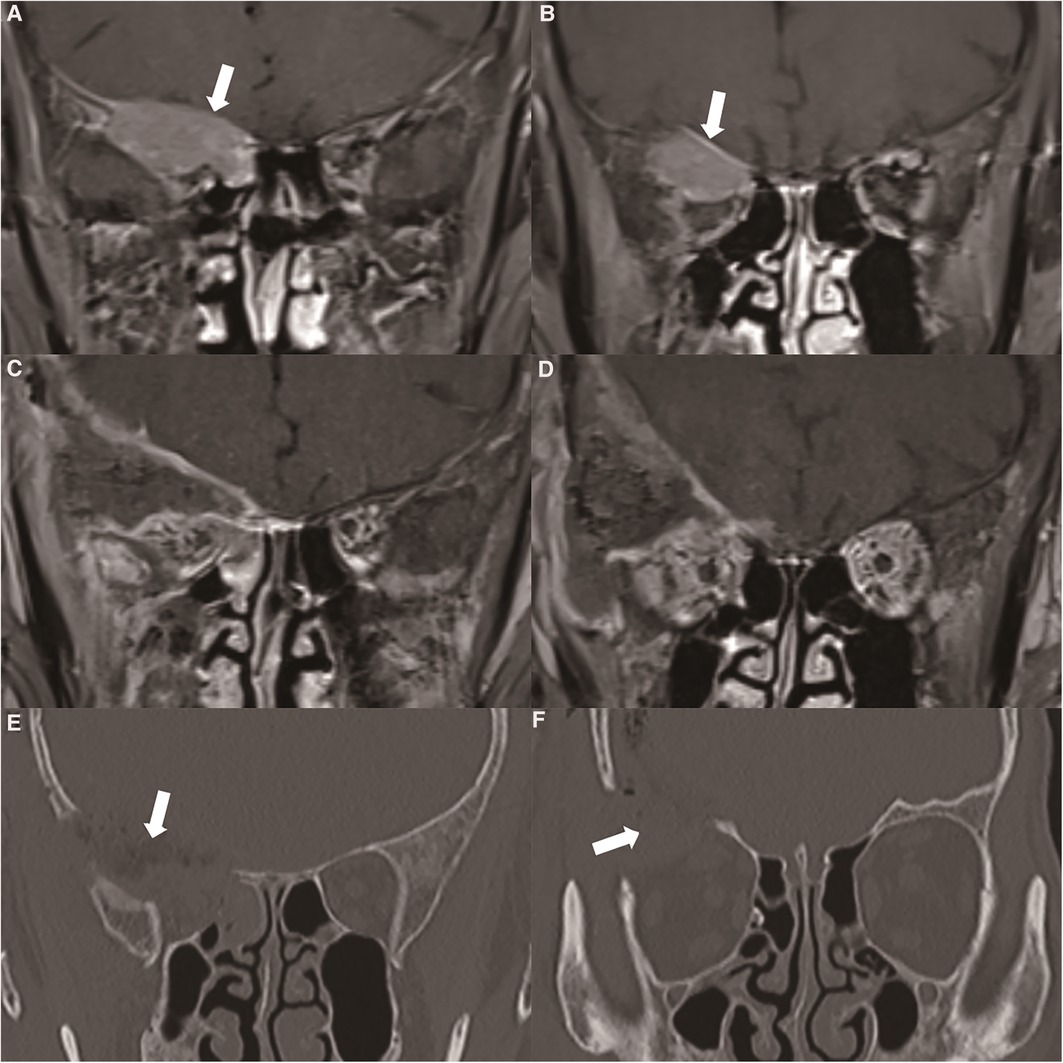
Figure 3. (A) orbital MRI showed that the tumour broke through the skull base and involved the intracranial structure (thick white arrow). (B) Enhanced MRI showed enhanced performance of the dura mater located at the base of the skull (thick white arrow). (C, D) Postoperative enhanced MRI showed complete resection of the tumour. (E, F) We performed a frontal orbital craniotomy to remove the tumours in the cranio-orbital area, and simultaneously performed bone removal of the skull base and the lateral wall of the orbit. Postoperative CT showed that the bone involved in the tumour was completely removed (thick white arrow).
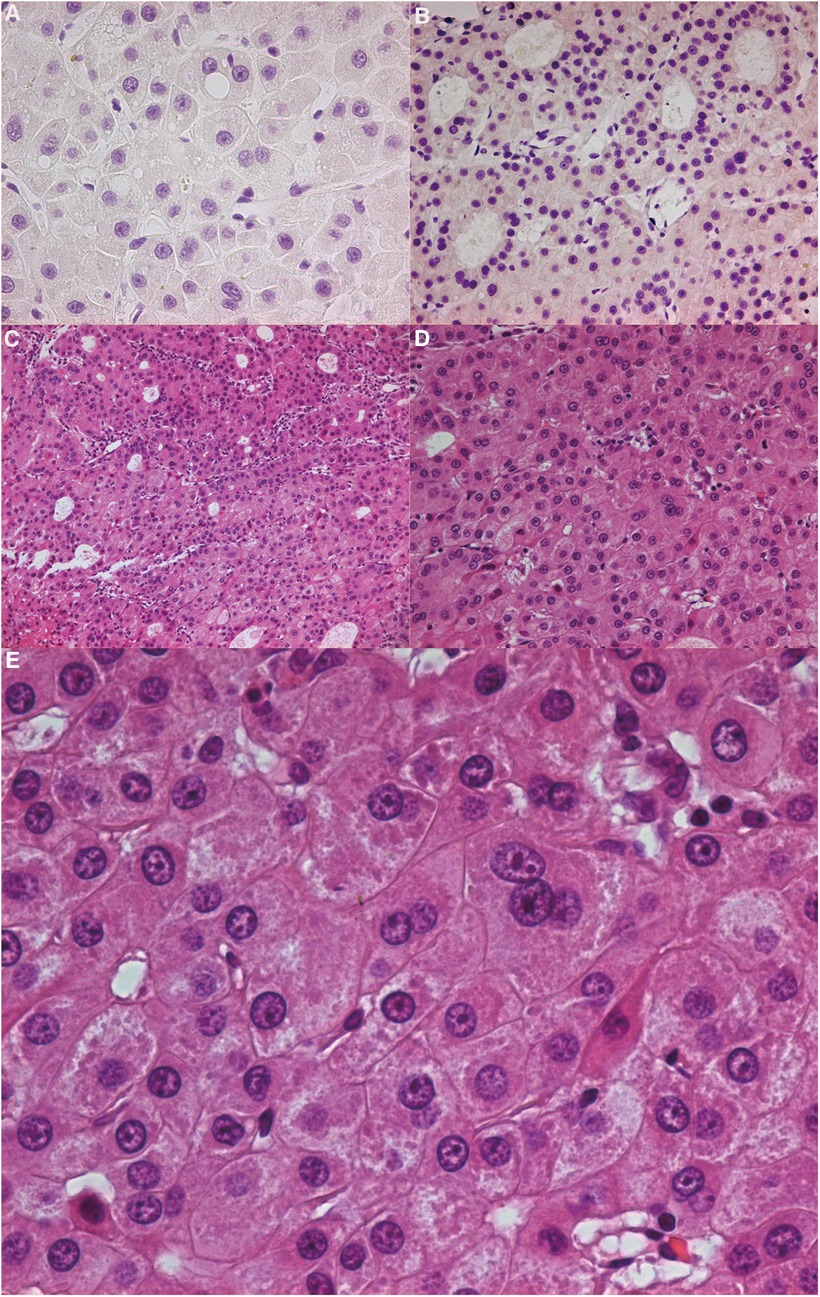
Figure 4. (A) immunohistochemistry CK7 (−). (B) Immunohistochemistry P63 (−). (C) (HE 100×): Tumour cells were usually arranged in slices, beams or alveolar structures. Occasionally, there was extensive duct differentiation in tumours. The tumour had grown invasively, and perineural and vascular invasion were common. (D) (HE 200×), (E) (HE 400×): It could be seen that the tumour cells of eosinophilic carcinoma were large, round or polygonal, and the cytoplasm was finely granular. It was more pleomorphic than an eosinophilic adenoma. The nucleus was located in the centre of the cell and often had large and irregular nucleoli. HE: Hematoxylin-Eosin staining.
Discussion
Oncocytomas are common in the kidneys, parotid glands and other organs, and have corresponding diagnosis and treatment characteristics (19, 20). The case presented here was an oncocytic carcinoma located in the cranio-orbital area, which has not been found in the literature previously. Although there have been 15 reported cases of oncocytic carcinoma in the orbital area, there have been no reports of oncocytic carcinoma involving the brain (21). Oncocytic carcinomas in the orbital area generally originate from the lacrimal tissue, and most of these were located on the outside the orbit. Patients usually develop exophthalmos, making clinical discovery and early diagnosis straightforward. Like oncocytic carcinomas, patients with ocular lymphoma also commonly present with exophthalmos. Sang et al. described a case of a 6-year-old male who presented with bilateral rapid orbital swelling for 10 days, and the final diagnosis was bilateral orbital secondary non-Hodgkin's lymphoma (22). Ngoc et al. reported a case of a 74-year-old man with non-Hodgkin's lymphoma who also presented with bilateral eye proptosis (23). The final judgement of tumour type must be determined by means of biopsy.
In this case, the oncocytic carcinoma involved the deep part of the orbit, and the main body was located above the orbit. The main clinical manifestation was decreased vision with an insidious onset. The oncocytic carcinoma involved the intracranial structure through the supraorbital fissure. These situations are difficult for ophthalmologists to deal with through the transorbital surgical approach. Neurosurgeons are required to perform a craniotomy through the frontal orbital approach, removing the frontal and brow bones to completely expose the surgical area. In these cases, the oncocytic carcinoma involves the contents of the orbit, the optic nerve, the total tendon ring tissue and the dura mater of the frontal lobe. It destroys the bone of the optic canal and supraorbital fissure, the sphenoid bone and its great wings and the bone in the lateral orbital wall. This is obviously characteristic of malignant eosinophilic carcinomas. Gray et al. described the diagnostic criteria for oncocytic carcinoma as: (1) distant metastasis; (2) regional lymph node metastasis; (3) perineural, intravascular or intralymphatic invasion; and (4) common mitosis and cellular pleomorphism accompanied by extensive invasion and destruction of adjacent tissues (24).
The origin of this case of oncocytic carcinoma is worth exploring because of three aspects: (a) The patient had bone involvement in the sphenoid sinus. Part of the main body of the oncocytic carcinoma protruded into the sphenoid sinus (Figure 2). This kind of oncocytic carcinoma is known to occur in the paranasal sinus. Oncocytic carcinoma originates from the gland mucosa in the paranasal sinus and invades the orbital structure. It next invades the intracranial dural structure through the supraorbital fissure. However, in this case, the sphenoid sinus bone was basically intact, albeit thin; (b) The distant metastasis of this atypical cranio-orbital oncocytic carcinoma could not be ruled out. This kind of oncocytic carcinoma has a high degree of malignancy and is prone to distant metastasis through the lymphatic and blood systems. However, the patient was examined with positron emission CT, and no original lesions in the related organs were found; (c) The lacrimal gland structure of the patient was intact, and the main body of the oncocytic carcinoma was in the orbital apex region. This kind of oncocytic carcinoma did not originate from the lacrimal gland structure, but a primary oncocytic carcinoma in the intracranial and cranio-orbital area had not been reported. The origin of oncocytic carcinomas in the cranio-orbital area is worth discussing. The possibility of (a) and (b) still existed. In this case, postoperative enhanced MRI confirmed that the oncocytic carcinoma had been completely removed surgically. During the operation, the resection of the bones involved was extended, including the great wings of the sphenoid bone, the lateral wall outside the orbit and the lateral wall of the sphenoid sinus. The visual acuity of the patient recovered from 0.1 to 0.3, and there was no eye movement disorder. At the patient's 3-month follow-up, no recurrence of the oncocytic carcinoma was found.
Conclusions
Oncocytic carcinomas in the cranio-orbital area are extremely rare. Because surgery involves ophthalmological and neurosurgical areas, it is difficult to completely resect the oncocytic carcinoma in a single department. Early resection is the most effective treatment for this kind of oncocytic carcinoma, and long-term follow-up and adjuvant chemoradiotherapy are beneficial. As this oncocytic carcinoma has an uncertain origin, continual systemic observation and local re-examination are needed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Tongren Hospital of China Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YP and JLB conceived of the study, and LHG and ZJL participated in its design and coordination and KJ helped to draft the manuscript. All authors contributed to the article and approved the submitted version.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang M-W, Zhang J-G, Zhang J, Liu S-M, Zheng L, Shi Y, et al. Oncocytic carcinoma of the parotid gland. Laryngoscope. (2013) 123(2):381–5. doi: 10.1002/lary.23696.23404488
2. Wen Y, Lv P, Wang F, Shi M, Zhang X, Feng B. A case of oncocytic carcinoma at the base of the tongue with cervical lymph node metastasis and literature review. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2021) 35(8):748–51. doi: 10.13201/j.issn.2096-7993.2021.08.016.34304539
3. Wu C, Yu Y, Qiao C, Song L, Liu Q. Oncocytic carcinoma in the retromolar trigone: a case report. Ear Nose Throat J. (2022):1455613221080074. doi: 10.1177/01455613221080074. [Epub ahead of print]35192390
4. Pivovarcikova K, Alaghehbandan R, Vanecek T, Ohashi R, Pitra T, Hes O. Tsc/mtor pathway mutation associated eosinophilic/oncocytic renal neoplasms: a heterogeneous group of tumors with distinct morphology, immunohistochemical profile, and similar genetic background. Biomedicines. (2022) 10(2):322. doi: 10.3390/biomedicines10020322.35203531
5. Westergaard-Nielsen M, Godballe C, Andersen LJ, Primdahl H, Kristensen CA, Andersen E, et al. Oncocytic carcinoma of the salivary glands: a danish national study. Auris Nasus Larynx. (2018) 45(4):825–30. doi: 10.1016/j.anl.2017.10.001.29100750
6. Hu Y-W, Lin C-Z, Li W-Y, Chang C-P, Wang L-W. Locally advanced oncocytic carcinoma of the nasal cavity treated with surgery and intensity-modulated radiotherapy. J Chin Med Assoc. (2010) 73(3):166–72. doi: 10.1016/S1726-4901(10)70033-2.20231003
7. Mohanty SK, Satapathy A, Aggarwal A, Mishra SK, Sampat NY, Sharma S, et al. Oncocytic renal neoplasms with diffuse keratin 7 immunohistochemistry harbor frequent alterations in the mammalian target of rapamycin pathway. Mod Pathol. (2022) 35(3):361–75. doi: 10.1038/s41379-021-00969-6.34802045
8. Hodzic Z, Rowan NR, Kashiwazaki R, Willson TJ, Wang EW, Lee SE. A systematic review of sinonasal oncocytomas and oncocytic carcinomas: diagnosis, management, and technical considerations. Int Forum Allergy Rhinol. (2017) 7(5):514–24. doi: 10.1002/alr.21902.28092140
9. Villaret AB, Lombardi D, Schreiber A, Farina D, Nicolai P. Oncocytic carcinoma of the nasolacrimal duct treated by transnasal endoscopic resection. Head Neck. (2013) 35(1):E24–E7. doi: 10.1002/hed.21803.21717525
10. Liu R, Ge X, Ma JM, Luan FX. A case of communicating oncocytic carcinoma of the naso-orbit. Zhonghua Yan Ke Za Zhi. (2022) 58(1):49–50. doi: 10.3760/cma.j.cn112142-20210510-00222.34979794
11. Østergaard J, Prause JU, Heegaard S. Oncocytic lesions of the ophthalmic region: a clinicopathological study with emphasis on cytokeratin expression. Acta Ophthalmol. (2011) 89(3):263–7. doi: 10.1111/j.1755-3768.2009.01677.x.
12. Shin M, Kondo K, Hanakita S, Suzukawa K, Kin T, Shojima M, et al. Endoscopic transnasal approach for resection of locally aggressive tumors in the orbit. J Neurosurg. (2015) 123(3):748–59. doi: 10.3171/2014.11.JNS141921.26052982
13. Fenelon EM, Balby IT, Neves N, Figueiredo F, Duarte ECB, Tzelikis PF. Oncocytoma of the lacrimal gland: a case report. Arq Bras Oftalmol. (2017) 80(2):128–30. doi: 10.5935/0004-2749.20170031.28591289
14. Harris GJ, Paul S, Hunt BC. Oncocytic adenocarcinoma of the orbit. Ophthalmic Plast Reconstr Surg. (2017) 33(1):e18–20. doi: 10.1097/IOP.0000000000000419.25719368
15. Jittapiromsak N, Hou P, Williams MD, Chi TL. Orbital oncocytoma: evaluation with dynamic contrast-enhanced magnetic resonance imaging using a time-signal intensity curve and positive enhancement integral images. Clin Imaging. (2017) 42:161–4. doi: 10.1016/j.clinimag.2016.11.020.28063355
16. Kim J-Y, Park H-Y, Paik J-S, Kim D-C, Yang S-W. Oncocytoma of the lacrimal gland: an Asian case. Jpn J Ophthalmol. (2010) 54(3):239–41. doi: 10.1007/s10384-009-0796-y.20577859
17. Timoney PJ, Bradley M, Cowen DE. A rare case of progressive ptosis caused by lacrimal gland oncocytoma. Ophthalmic Plast Reconstr Surg. (2011) 27(4):e85–e7. doi: 10.1097/IOP.0b013e3181ef7275.21283034
18. Oestreicher J, Dookeran R, Nijhawan N, Kolin A. Necrobiotic Xanthogranuloma with predominant periorbital involvement. Ophthalmic Plast Reconstr Surg. (2010) 26(6):473–5. doi: 10.1097/IOP.0b013e3181d92955.21099384
19. Warren H, Neves JB, Tran MGB. Renal oncocytoma: landscape of diagnosis and management. BJU Int. (2021) 128(6):685–7. doi: 10.1111/bju.15496.34036709
20. Lv K, Cao X, Geng D, Zhang J. Imaging features of parotid gland oncocytoma: a case series study. Gland Surg. (2021) 10(3):870–6. doi: 10.21037/gs-20-790.33842232
21. Say EAT, Shields CL, Bianciotto C, Eagle RC, Shields JA. Oncocytic lesions (oncocytoma) of the ocular Adnexa: report of 15 cases and review of literature. Ophthalmic Plast Reconstr Surg. (2012) 28(1):14–21. doi: 10.1097/IOP.0b013e31822dd236.21956543
22. Sang N-V, Duc NM, My T-TT, Trung H-V, Quyen H-TD, Lenh B-V. A case report of secondary bilateral orbital lymphoma in a child. Radiol Case Rep. (2021) 16(7):1669–71. doi: 10.1016/j.radcr.2021.03.069.34007380
23. Ngoc L-TB, Doan N-V, Ngan VK, Phan N-V, Giang NQ, Quyen H-TD, et al. Bilateral lacrimal gland lymphoma: case report and literature review. Radiol Case Rep. (2022) 17(1):240–4. doi: 10.1016/j.radcr.2021.10.046.34840638
Keywords: oncocytic carcinoma, craniotomy, cranio-orbital, paranasal sinus, orbital
Citation: Yang P, Jiang L, Liu H, Zhang J and Kang J (2022) Oncocytic Carcinoma of the Cranio-Orbital Area: A Case Report. Front. Surg. 9:897319. doi: 10.3389/fsurg.2022.897319
Received: 16 March 2022; Accepted: 14 June 2022;
Published: 1 July 2022.
Edited by:
Rocco Cappellesso, University Hospital of Padua, ItalyReviewed by:
Nguyen Minh Duc, Pham Ngoc Thach University of Medicine, VietnamIlker Sengul, Faculty of Medicine, Giresun University, Turkey
Copyright © 2022 Yang, Jiang, Liu, Zhang and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jialiang Zhang amlhbGlhbmdfemgyMDAwQHNpbmEuY29t Jun Kang anVua2FuZzIwMTVAMTYzLmNvbQ==
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Abbreviations CT, Computed Tomography; MRI, Magnetic Resonance Imaging; CK, Cytokeratin; PET, Positron Emission Computed Tomography; HE, Hematoxylin-Eosin staining.
 Peng Yang1
Peng Yang1 Jun Kang
Jun Kang