
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 19 May 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.895444
This article is part of the Research TopicModern Neurosurgery and NeuroanatomyView all 18 articles
Background: Pseudomeningoceles (PMCs) as abnormal collections of cerebrospinal fluid are quite common findings on follow-up MRI after Chiari decompression surgery (CDS). However, the importance of their identification has not been truly determined, especially when PMCs are described occasionally in the process of radiological follow-up. We retrospectively analyzed surgical outcomes and imaging findings after CDS depending upon the occurrence and thickness of PMCs.
Methods: A total of 76 adult patients who underwent CDS were analyzed. The clinical and radiological outcomes of patients with a pseudomeningocele (wPMC) were evaluated and compared to those of patients without a pseudomeningocele (w/oPMC). Radiological morphometric measurements were performed and compared between groups. Comparisons of the maximal PMC thickness were made within the wPMC group.
Results: PMCs were recognized in 27 (35.5%) patients, of whom 3 (11.1%) required reoperation. Differences in satisfactory result rates regarding gestalt assessment and Chicago Chiari Outcome Scale were statistically insignificant between the w/oPMC and wPMC groups (p = 1 and p = 0.56, respectively). The postoperative syringomyelia decrease and cerebellar tonsil elevation were similar between the groups (p = 1 and p = 0.74, respectively) in the long-term follow-up. Additionally, the clinical or radiological outcomes with radiological details were not related to PMC thickness in the long-term follow-up. However, radiological details showed the cooccurrence of PMCs with a postsurgical of cerebello-tentorial distance increase (p < 0.05), basion-pontomedullary sulcus distance decrease (p < 0.05) and tonsillo-graft distance decrease (p < 0.05).
Conclusions: We found no significant relationships between PMC presence or thickness and clinical or radiological outcomes. However, postoperative changes within the posterior fossa associated with PMCs resemble brain sagging, which occurs in intracranial hypotension. Therefore, extradural cerebrospinal fluid escape may also be responsible for symptoms in some patients with PMCs after CDS.
The Chiari malformations originally described by Hans Chiari in 1891 (1) relate to a rare group of hindbrain abnormalities concerning both pediatric and adult patients. The most common, Chiari I malformation (CMI), consists of caudal herniation of elongated cerebellar tonsils through the foramen magnum, causing symptoms secondary to compression of the brain stem, dysfunction of the cerebellum and distortion of cerebrospinal fluid (CSF) flow (2). Disturbances of CSF flow are responsible for syringomyelia, which has been reported in 69% of adult patients (3), but the exact pathophysiology remains unclear (4, 5). For symptomatic cases, the treatment of choice is suboccipital decompression with duraplasty (6). However, duraplasty is related to a larger number of complications with a lower recurrence rate of symptoms than osseous decompression alone (7–9). Average complication rates have been estimated at 4.5%, and among the most common causes of CSF leaks, aseptic meningitis and pseudomeningocele have been reported (3). Pseudomeningoceles (PMCs) are defined as abnormal CSF collections visible on MRI due to leakage into the extradural space (10) and directly over the dural graft. Pseudomeningocele can reduce the volume of reconstituted cisterna magna because of compression on duraplasty. Eventually, they can become symptomatic due to compression of neural structures or the impeding of CSF flow through the foramen magnum. Nevertheless, pseudomeningocele is not a rare finding on follow-up MRI, but it is not considered a complication until it leads to recurrent symptoms or CSF fistula or causes unacceptable cosmetic effects (11).
The aim of this study was to determine the roles of co-occurring pseudomeningoceles and their sizes on long-term outcomes after decompressive surgery with duraplasty in patients with CMI.
A total of 96 adult patients who underwent posterior fossa decompression (PFD) with duraplasty for symptomatic CMI from January 2003 to December 2019 at our institution were screened. Twenty of them were excluded because their preoperative radiographic studies were not accessible. The mean Chicago Chiari Outcome Scale (CCOS) of the excluded and included patients did not significantly differ (12.85 vs. 12.4; p = 0.45). Of 76 included patients, 60 were women, and 16 were men, with an average age of 41.8 years old (range from 18 to 66 years old). The medical data were obtained from telephone questionnaires and hospital and ambulatory charts. For analysis of the long-term clinical course, gestalt assessment (improvement, unchanged or deterioration) and Chicago Chiari Outcome Scale (CCOS) were used (12–14). The mean clinical follow-up was 58 months.
All methods were carried out in accordance with relevant guidelines and regulations.
All protocols were approved by Bioethics Committee of Medical University of Warsaw (AKBE/231/2021). Informed consent was obtained from all subjects.
Preoperative and follow-up MRI was performed in every case. Follow-up MRI was scheduled 6 months after surgery, and additional studies were performed earlier or later, depending on the clinical indications. Numerous patients underwent many control MRI studies, especially those with long-term follow-up. In these cases, the last study was considered. The mean neuroimaging follow-up was 39.9 months.
The presence of pseudomeningocele on follow-up MRI was defined as hyperintense fluid collection above a hypointense linear dural graft on sagittal T2-weighted MRI imaging.
Because of the variable shape, precise measurement of PMC total volume is very difficult or impossible. Therefore, size was determined by the maximal thickness of the pseudomeningocele as the perpendicular distance to the graft with the best correlation clinically with complications (15) (Figure 1).
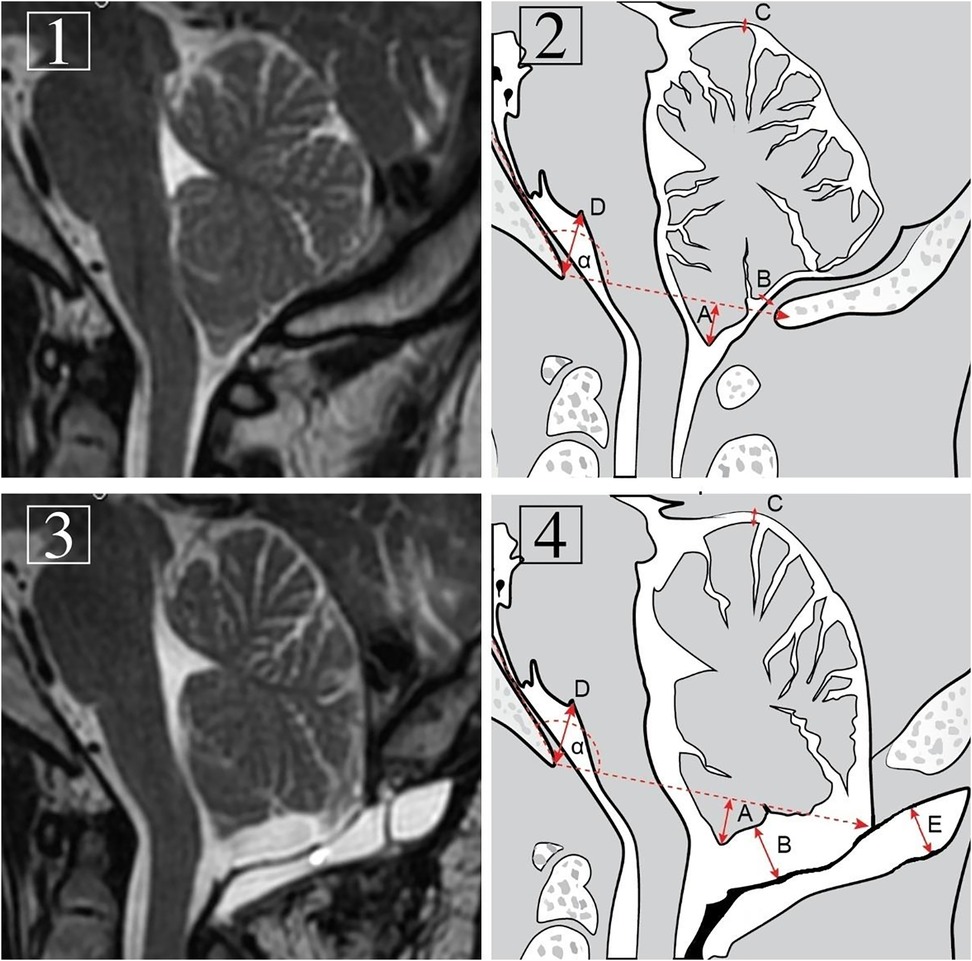
Figure 1. Preoperative (1 and 2) and follow-up (3 and 4) T1-weighted MRI images of the craniocervical junction region and diagrams presenting measurements in the same midsagittal plane. Measurements included: α: Boogaard's angle. A: max. tonsillar herniation. B: max. tonsillo-graft distance. C: max. cerebello-tentorial distance. D: max. basion-pontomedullary sulcus distance. E: pseudomeningocele thickness.
Maximal tonsillo-graft distance was also measured. Additional radiological details, such as pre- and postprocedural differences in maximal cerebello-tentorial distance and basion-pontomedullary sulcus distance, were noted. The levels of pre- and postoperative tonsillar descent were also measured and compared. For a postoperative tonsil position assessment, the level of the foramen magnum was determined by restoration of the angle between the line tangent to the clivus surface and the basion-opisthion line established on preoperative images (Boogaard’s angle; Figure 1) (16). In cases of syringomyelia in the preoperative study, their size was determined to be decreased, stable or increased. The cooccurrence of pseudomeningocele and its maximal thickness and clinical and radiological outcomes were analyzed in detail, including the poor preoperative status of 3 reoperated patients for symptomatic PMCs. Evaluation of the relationship of PMC thickness with tonsillo-graft distance and pre- and postprocedural differences in maximal cerebello-tentorial distance, basion-pontomedullary sulcus distance and tonsillar herniation was performed. In cases of pre- and postoperative distance comparisons, we received positive or negative values depending on whether a particular distance decreased or increased on follow-up MRI, respectively (Figure 1).
Suboccipital craniectomy with C1 posterior arch removal and sometimes with partial (No. 24) or whole (No. 6) C2 laminectomy was performed with Y-shaped dural incision and with subsequent duraplasty in all cases. Depending on surgeon preference, two types of graft material were used for dural closure: nonautologous grafts or autologous grafts. The former, represented by synthetic collagen matrices, was used in 48 (63.2%) cases. Autologous grafts in this series, including previously harvested pericranium or fascia lata, were used in 28 (36.8%) cases. The arachnoid layer, 4th ventricle and tonsils were left intact, and no local or lumbar drains after surgery were used (17, 18).
The Shapiro-Wilk test was used to test the assumption of normality, and Levene’s test was used to examine the assumption of homogeneity. Mean values and standard deviations (SDs) are reported. Fisher's exact test was used to examine the relationship between PMC presence and changes in symptom severity expressed in a binary manner, while the chi-square test was used in the case of symptom severity changes in three categories (improvement vs. unchanged vs. deterioration). The Mann-Whitney U test was used to compare values of continuous and ordinal variables between independent groups. The significance level was set at alpha = 0.05.
Twenty-seven (35.5%) patients demonstrated PMC on posttreatment MRI. Only 3 (11.1%) of 27 patients required revision surgery, representing 3.9% of the overall study cohort. Satisfactory clinical results according to gestalt assessment and CCOS were obtained in 75.5% and 81.6% of patients without pseudomeningocele (w/oPMC) and 66.7% and 70.4% of patients with pseudomeningocele (wPMC), respectively (p = 0.43, p = 0.27). For this outcome analysis, the prereoperative clinical condition of 3 reoperated patients was considered to capture the worst condition potentially related to PMC. Long-term follow-up showed even more comparable satisfactory results: 75.5% vs. 77.7% (p = 1) in gestalt and 81.6% vs. 77.7% (p = 0.56) in CCOS score for w/oPMC and wPMC patients, respectively (Table 1). Analysis of individual signs and symptoms showed no significant correlations between the wPMC and w/oPMC groups postoperatively (Supplementary Table S1).
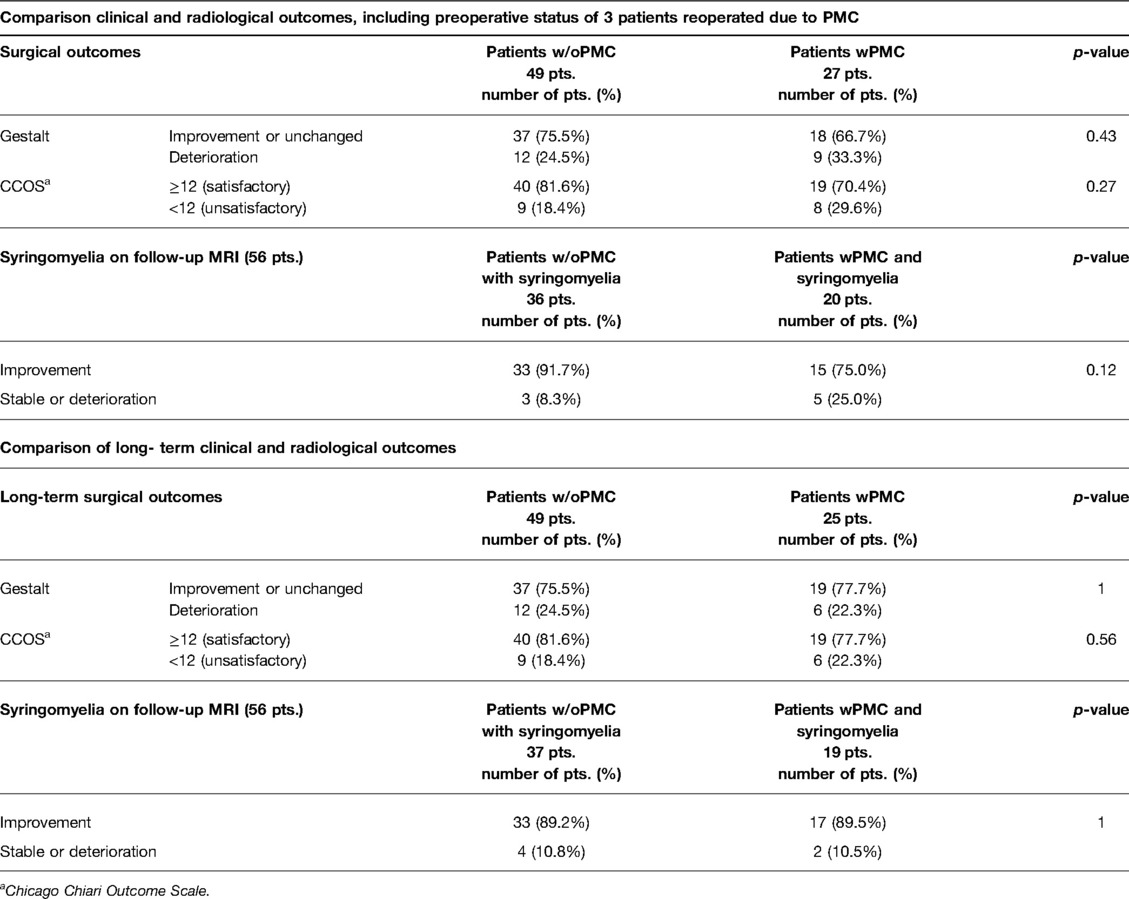
Table 1. Comparison of clinical and radiological outcomes between patients without pseudomeningocele (w/oPMC) and with pseudomeningocele (wPMC) after decompression surgery in patients with Chiari I malformation, including preoperative status of 3 patients reoperated due to PMC and over long-term follow-up.
Syringomyelia was present in 73.7% of patients preoperatively. A reduction in syringomyelia size was obtained in 91.7% of cases in the w/oPMC group and 75.0% of cases in the wPMC groups (p = 0.12). Two patients who underwent reoperation due to PMC had syringomyelia that remained unchanged after the first operation. In the long-term follow-up, including the results of 3 revision surgeries, the decrease in syrinx was similar: 89.2% vs. 89.5% for the w/oPMC and wPMC groups, respectively (p = 1; Table 2).
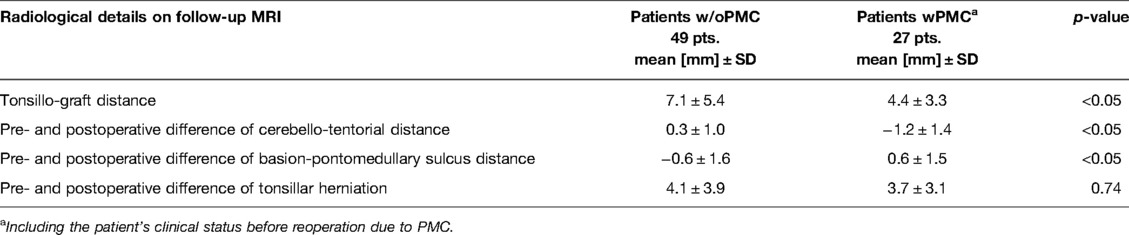
Table 2. Comparison of long-term radiological details on follow-up MRI between patients without pseudomeningocele (w/oPMC) and with pseudomeningocele (wPMC) after decompression surgery in patients with Chiari I malformation.
Pseudomeningocele was associated with a reduction in the average maximal tonsillo-graft distance on follow-up MRI (4.4 mm vs. 7.1 mm; p < 0.05). The cerebello-tentorial distance increased postsurgically in the wPMC group by an average of 1.2 mm, in contrast to the patients with w/oPMC, in whom it slightly decreased by an average of 0.3 mm (p < 0.05). Additionally, the existence of PMC was associated with a decrease in basion-pontomedullary sulcus distance by an average of 0.6 mm compared to the w/oPMC group, in which it was increased by an average of 0.6 mm (p < 0.05). However, we did not observe a substantial difference in tonsil elevation after surgery between the w/oPMC and wPMC groups on follow-up MRI (4.1 mm vs. 3.7 mm; p = 0.74; Table 3).
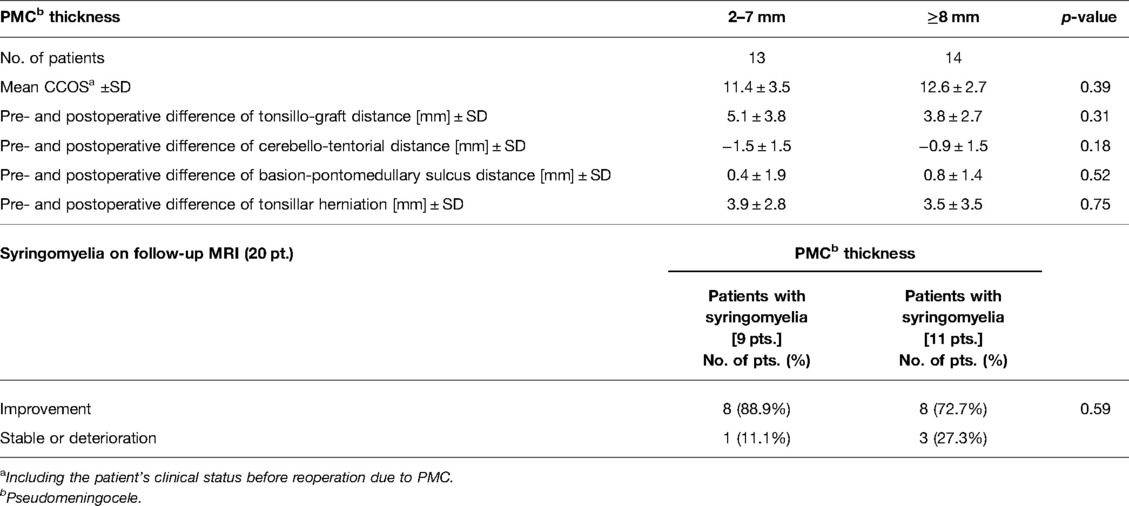
Table 3. Correlation between pseudomeningocele thickness on follow-up MRI related and clinical outcome in CCOS (Chicago Chiari Outcome Scale) and radiological details.
The average thickness of PMC was 8.7 mm ± 4.5 (SD) (range 2.0–21.0 mm). Relationships of PMC thickness with CCOS, individual signs and symptoms were not statistically significant (Supplementary Table S1). Additionally, we did not find an association between PMC thickness and changes in syrinx size on follow-up (p = 0.59) or other radiological details (Table 3).
A distinct group consisted of 3 reoperated patients due to symptomatic PMCs. Significant cerebellar subsidence coexisted in 2 patients. The average time between the operation and the onset of new symptoms was 7.7 days (range: 3–13). The predominant symptoms were severe headache, nausea, and vomiting with depressed levels of consciousness. Reduraplasty was performed in all cases, with optimization of craniectomy size in 2. The clinical condition assessed with the CCOS in these cases before reoperation was significantly worse than that of the rest of the wPMC group (7.3 vs. 12.3; p = 0.02; Table 4). The mean CCOS of the reoperated patients improved in the long-term follow-up to 13.7 (range: 12–15), although persistent PMC was noted in 1 case on follow-up MRI. Comparison of radiological details of the reoperated PMCs to the remaining PMCs in the PMC group showed an insignificantly larger mean PMC thickness (p = 0.37) and a smaller reduction in tonsillo-graft distance (p = 0.73) in the reoperated patients. Prepostoperative differences in tonsillar herniation (p = 0.27), cerebello-tentorial distance (p = 0.20), and basion-pontomedullary sulcus distance (p = 0.73) were insignificantly less favorable in the reoperated patients than in nonreoperated patients with PMC (Table 4). We have not observed external CSF leaks in our series.
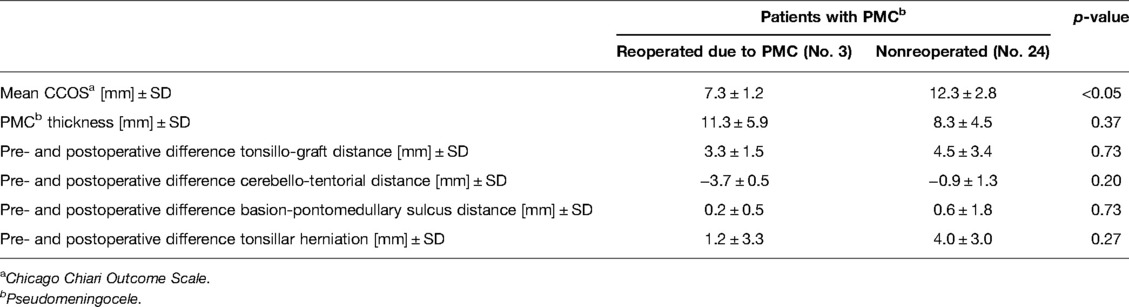
Table 4. Comparison of clinical outcomes and radiological details on follow-up MRI between reoperated and nonreoperated patients for pseudomeningocele (wPMC) after decompression surgery in patients with Chiari I malformation.
Surgical treatment for CMI has a particular predisposition to the development of CSF-related complications, including PMCs. Among predisposing factors worth mentioning are duraplasty, craniectomy, midline approach, and surgery concerning the posterior fossa (10, 15, 19). Thus, PMCs are among the most common complications after Chiari decompression surgery (20). Smith et al. noted that CM was the second most common cause of PMC after surgery for posterior fossa extraaxial tumors (10). PMC rates reported in the literature range from 2.5 to 24% (15, 21, 22). We observed PMCs in 35.5% of cases. However, we assessed even barely visible PMCs on follow-up MRI to define their exact significance. This wide disparity presumably resulted from some authors having considered PMCs recognized only on imaging studies as asymptomatic or incidental findings and not, therefore, reporting them as complications (10, 21).
According to the current state of knowledge, the appearance of PMC at the operation site might have no effect on duraplasty, or it can lead to slight reduction in recreated cisterna magna or obstruction of CSF flow, potentially leading to hydrocephalus (11). Further PMC enlargement can lead to compression of the posterior fossa neural structures. The patient either remains stable, or new symptoms can appear. PMC manifestation evolves from local pain in distended tissue or simple headache to posterior fossa syndrome or even impaired consciousness. Moreover, PMC can lead to CSF fistula and meningitis in cases of skin rupture (19).
The pathophysiology of PMC formation is unclear, but it seems that PMCs are initially formed as a result of suture CSF leakage between the dura and dural grafts or a tear in one of them. Leakage can occur immediately after an operation due to poor dural closure or could be caused by a progressive increase in intracranial CSF pressure in the course of hydrocephalus (23). However, knowing the role of CSF pressure, Valsalva maneuvers (e.g., coughing, sneezing, or defecating) likely cause a sudden increase in pressure, acting as a trigger factor in leakage (20). Symptomatic PMCs are most often observed shortly after surgery, which would suggest their onset until strong scarring among the graft, dura and neck muscles is created (15, 24).
Comparison of postoperative radiographic images of patients without and with PMC showed significant differences. A tendency to decrease the brainstem with the cerebellum in the wPMC group, defined as increased cerebello-tentorial and reduced basion-pontomedullary sulcus distances, was noted. This tendency was the opposite to that observed in the w/oPMC group, in which these structures had ascended (Table 2).
Considering our findings, we propose that the clinical consequences of some PMCs could develop via a similar mechanism to that of spontaneous intracranial hypotension (SIH). Spontaneous CSF fistula manifests as a small CSF reservoir (meningeal diverticula), usually associated with nerve root sleeves. CSF leakage is self-limiting, or CSF constantly leaks out in intracranial hypotension, e.g., after lumbar puncture (25–27). Similarly, postoperative leakage usually does not result in a constant increase in PMC volume. Similar to brain sagging in SIH, we noted slight hindbrain subsidence in the wPMC group, expressed as a postoperative increase in the supracerebellar space and a decrease in the basion-pontomedullary sulcus distance (Figure 1). The cooccurrence of cerebello-tentorial distance increases and basion-pontomedullary sulcus distance decreases in patients with PMCs might derive from the pressure gradient between the posterior fossa and PMC spaces, with a subsequent downward shift of all posterior fossa neural structures. Hypotension in the posterior fossa corresponds to partial hypotension syndrome but is not as severe as SIH in causing sagging of the whole brain (25, 28). This difference might indicate that postoperative cerebellar subsidence could be related not only to oversized occipital bony decompression but also to CSF leakage.
Raising and changing the shape of primarily herniated tonsils after decompression surgery from thin and extended to rounder and shorter are well known (16). Interestingly, despite sagging of the superior surface of the cerebellum in patients wPMC, we did not observe a significant difference in postsurgical tonsil tip ascent between the two groups, suggesting that favorable and unfavorable displacements in the posterior fossa might coexist after decompression.
Although the recreated cisterna magna was significantly smaller in patients with PMC, and the worst “prerevision” condition related to PMC was used for analysis, the comparison of the w/oPMC and wPMC groups showed no significant differences in clinical outcomes. Furthermore, the long-term observation of 25 patients with persistent PMC demonstrated that they achieved very similar clinical and radiological outcomes as their counterparts without PMC. It appears that the relative reduction in the recreated intradural space was not sufficient to block CSF flow in nonreoperated patients with PMC (29).
Pare and Batzdorf reported three cases in which PMCs were a reason for persistent syringomyelia in long-term follow-up (30). In our series, in which it was never a main cause of reoperation for PMC, all 3 revisions were performed too early after the first surgery to expect a decrease in the syrinx. We did not find any impact of the presence or size of PMCs on syringomyelia evolution.
Thus far, based on our analysis, neither the presence nor any size of PMC can be identified as a risk factor for worse outcomes. All PMCs requiring revision manifested shortly after surgery, long before scheduled follow-up MRI. Therefore, early clinical postsurgical deterioration is much more suggestive of PMC importance than any long-term radiological parameter. However, the differences in hindbrain rearrangement after decompression between patients with and without PMC shed new light on the potential mechanism increasing symptoms from some PMCs.
Our study is limited by several factors, the most significant of which is the retrospective nature and single-center design of the research. Moreover, the cohort was represented by only 76 patients, with a significant effect on the statistical analysis of certain differences between groups. Additionally, the radiological data were based on MRI studies performed at various times after surgery and sometimes with different resolution of studies, which might have had an impact on the measured details. Standard follow-up MRI was performed without contrast, which could have shown other intracranial hypotension features. However, we obtained a relatively long-term follow-up by choosing to measure the last available MRI study. Future prospective research with a larger cohort is needed, especially to confirm our observations contained in the conclusion.
We did not find any significant relationships of pseudomeningocele presence or pseudomeningocele thickness with clinico-radiological outcomes after decompressive surgery. In rare cases, PMCs might be a cause of clinical deterioration over short postoperative periods. However, the symptoms could be secondary to hindbrain lowering caused by posterior fossa hypotension, resulting from extradural CSF leakage, rather than from narrowing of the intradural space at the foramen magnum level.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Bioethics Committee of Medical University of Warsaw (AKBE/231/2021). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conceptualization: AB, PK. Methodology: AB, PK. Formal analysis and investigation: AB, MB. Writing–original draft preparation: AB. Writing–review and editing: PK. Mathematical analysis: SK. Supervison: AM. All authors contributed to the article and approved the submitted version.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.895444/full#supplementary-material.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chiari H. Über veränderungen des kleinhirns infolge von hydrocephalie des grosshirns. Dtsch Med Wochenschr (1891) 17(42):1172–75. doi: 10.1055/s-0029-1206803
2. Steinbok P. Clinical features of Chiari I malformations. Childs Nerv Syst. (2004) 20(5):329–331. doi: 10.1007/s00381-003-0879-x.
3. Arnautovic A, Splavski B, Boop FA, Arnautovic KI. Pediatric and adult Chiari malformation Type I surgical series 1965–2013: a review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr. (2015) 15(2):161–77. doi: 10.3171/2014.10.PEDS14295.
4. Holly L, Batzdorf U. Chiari malformation and syringomyelia. J Neurosurg Spine. (2019) 31:619–28. doi: 10.3171/2019.7.SPINE181139.
5. Klekamp J. The pathophysiology of syringomyelia - historical overview and current concept. Acta Neurochir (Wien). (2002) 144(7):649–64. doi: 10.1007/s00701-002-0944-3.
6. Zhao JL, Li MH, Wang CL, Meng W. A Systematic Review of Chiari I Malformation: Techniques and Outcomes. World Neurosurg. (2016) 88:7–14. doi: 10.1016/j.wneu.2015.11.087.
7. Hankinson T, Tubbs RS, Wellons JC. Duraplasty or not? An evidence-based review of the pediatric Chiari I malformation. Childs Nerv Syst. (2011) 27(1):35–40. doi: 10.1007/s00381-010-1295-7.
8. Klekamp J, Batzdorf U, Samii M, Bothe HW. The surgical treatment of Chiari I malformation. Acta Neurochir (Wien). (1996) 138(7):788–801. doi: 10.1007/BF01411256.
9. Del Gaudio N, Vaz G, Duprez T, Raftopoulos C. Comparison of dural peeling versus duraplasty for surgical treatment of Chiari Type I Malformation: results and complications in a monocentric patients’ cohort. World Neurosurg. (2018) 117:e595–e602. doi: 10.1016/j.wneu.2018.06.093.
10. Smith GA, Strohl MP, Manjila S, Miller JP. Incidence, management, and outcome of symptomatic postoperative posterior fossa pseudomeningocele: a Retrospective single-institution experience. Oper Neurosurg (Hagerstown). (2016) 12(3):298–304. doi: 10.1227/NEU.0000000000001329.
11. Mazzola CA, Fried AH. Revision surgery for Chiari malformation decompression. Neurosurg Focus. (2003) 15(3):E3. doi: 10.3171/foc.2003.15.3.3.
12. Aliaga L, Hekman KE, Yassari R, Straus D, Luther G, Chen J, et al. A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery. (2012) 70(3):656–64; discussion 664–55. doi: 10.1227/NEU.0b013e31823200a6.
13. Greenberg JK, Milner E, Yarbrough CK, Lipsey K, Piccirillo JF, Smyth MD, et al. Outcome methods used in clinical studies of Chiari malformation Type I: a systematic review. J Neurosurg. (2015) 122(2):262–72. doi: 10.3171/2014.9.JNS14406.
14. Bidzinski J. Late results of the surgical treatment of syringomyelia. Acta Neurochir Suppl (Wien). (1988) 43:29–31. doi: 10.1007/978-3-7091-8978-8_7.
15. Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. MRI study of the natural history and risk factors for pseudomeningocoele formation following postfossa surgery in children. Br J Neurosurg. (2003) 17(6):530–36. doi: 10.1080/02688690310001627777.
16. Heiss JD, Suffredini G, Bakhtian KD, Sarntinoranont M, Oldfield EH. Normalization of hindbrain morphology after decompression of Chiari malformation Type I. J Neurosurg. (2012) 117(5):942–46. doi: 10.3171/2012.8.JNS111476.
17. Sindou M, Chavez-Machuca J, Hashish H. Cranio-cervical decompression for Chiari type I-malformation, adding extreme lateral foramen magnum opening and expansile duroplasty with arachnoid preservation. Technique and long-term functional results in 44 consecutive adult cases – comparison with literature data. Acta Neurochir (Wien). (2002) 144(10):1005–19. doi: 10.1007/s00701-002-1004-8.
18. Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. implications for diagnosis and treatment. J Neurosurg. (1994) 80(1):3–15. doi: 10.3171/jns.1994.80.1.0003.
19. Steinbok P, Singhal A, Mills J, Cochrane DD, Price AV. Cerebrospinal fluid (CSF) leak and pseudomeningocele formation after posterior fossa tumor resection in children: a retrospective analysis. Childs Nerv Syst. (2007) 23(2):171–74; discussion 175. doi: 10.1007/s00381-006-0234-0.
20. Parker SL, Godil SS, Zuckerman SL, Mendenhall SK, Tulipan NB, McGirt MJ. Effect of symptomatic pseudomeningocele on improvement in pain, disability, and quality of life following suboccipital decompression for adult Chiari malformation type I. J Neurosurg. (2013) 119(5):1159–65. doi: 10.3171/2013.8.JNS122106.
21. Foreman P, Safavi-Abbasi S, Talley MC, Boeckman L, Mapstone TB. Perioperative outcomes and complications associated with allogeneic duraplasty for the management of Chiari malformations Type I in 48 pediatric patients. J Neurosurg Pediatr. (2012) 10(2):142–49. doi: 10.3171/2012.5.PEDS11406.
22. Hoffman CE, Souweidane MM. Cerebrospinal fluid-related complications with autologous duraplasty and arachnoid sparing in type I Chiari malformation. Neurosurg. (2008) 62(3 Suppl 1):156–60; discussion 160–151. doi: 10.1227/01.neu.0000317387.76185.ac
23. Pirouzmand F, Tator CH, Rutka J. Management of hydrocephalus associated with vestibular schwannoma and other cerebellopontine angle tumors. Neurosurgery. (2001) 48(6):1246–53; discussion 1253–44. doi: 10.1097/00006123-200106000-00010.
24. Mehendale NH, Samy RN, Roland PS. Management of pseudomeningocele following neurotologic procedures. Otolaryngol Head Neck Surg. (2004) 131(3):253–62. doi: 10.1016/j.otohns.2004.01.018.
25. Ferrante E, Trimboli M, Rubino F. Spontaneous intracranial hypotension: review and expert opinion. Acta Neurol Belg. (2020) 120(1):9–18. doi: 10.1007/s13760-019-01166-8.
26. Schievink WI, Maya MM, Moser FG. Digital subtraction myelography in the investigation of post-dural puncture headache in 27 patients: technical note. J Neurosurg Spine. (2017) 26(6):760–64. doi: 10.3171/2016.11.SPINE16968.
27. Paldino M, Mogilner AY, Tenner MS. Intracranial hypotension syndrome: a comprehensive review. Neurosurg Focus. (2003) 15(6):ECP2. doi: 10.3171/foc.2003.15.6.8
28. Kranz PG, Gray L, Malinzak MD, Amrhein TJ. Spontaneous Intracranial Hypotension: Pathogenesis, Diagnosis, and Treatment. Neuroimaging Clin N Am. (2019) 29(4):581–94. doi: 10.1016/j.nic.2019.07.006.
29. De Tommasi C, Bond AE. Complicated Pseudomeningocele Repair After Chiari Decompression: Case Report and Review of the Literature. World Neurosurg. (2016) 88(688):e681–87. doi: 10.1016/j.wneu.2015.11.056.
Keywords: Chiari I malformation, pseudomeningocele, surgical and radiological outcomes, decompressive surgery, complications
Citation: Balasa A, Kunert P, Bielecki M, Kujawski S and Marchel A (2022) Significance of Pseudomeningocele After Decompressive Surgery for Chiari I Malformation. Front. Surg. 9:895444. doi: 10.3389/fsurg.2022.895444
Received: 13 March 2022; Accepted: 12 April 2022;
Published: 19 May 2022.
Edited by:
Albert Sufianov, Federal Center of Neurosurgery, RussiaReviewed by:
Hidehito Kimura, Graduate School of Medicine, Kobe University, Kobe, JapanCopyright © 2022 Balasa, Kunert, Bielecki, Kujawski and Marchel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Przemysław Kunert cHJ6ZW15c2xhdy5rdW5lcnRAd3VtLmVkdS5wbA==
Specialty section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.