
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 05 July 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.894272
Background: Clear cell renal cell carcinoma (CCRCC) is a common urological neoplasm, and even though surgical resection is effective for localized CCRCC, the prognosis of metastatic CCRCC is poor. Currently, there is a paucity of recognized effective therapeutic protocols for metastatic CCRCC.
Case presentation: A 76-year-old Asian man underwent radical left nephrectomy for CCRCC 26 years ago; this patient visited our hospital with abdominal pain due to multiple abdominal metastases 24 years after the nephrectomy. After metastasectomy, he underwent targeted therapy combined with a programmed death receptor-1 (PD-1) inhibitor, and the current imaging results indicate remarkable tumor remission.
Conclusions: Metachronous pancreatic metastasis from CCRCC after nephrectomy is rare, but clinicians and patients should not ignore this possibility. The combination of targeted therapy and immunotherapy can result in satisfactory outcomes in cases where metastatic CCRCC continues to progress despite metastasectomy and targeted therapy. The combination of local and systemic therapy can be an effective therapeutic protocol for metastatic CCRCC, but there is no consensus on suitable therapeutics.
Renal cell carcinoma (RCC) is a malignant tumor originating from the renal epithelial cells and accounts for 80%–90% of all kidney malignancies (1). Approximately 25% of all patients with RCC have distant metastases at diagnosis, and more than one-third of all patients will develop metastases after nephrectomy (2). Clear cell renal cell carcinoma (CCRCC) is the most common histopathological type of RCC, and it originates from the proximal tubular epithelial cells that can invade the renal sinus and extend to the renal vein (3). Consequently, vascular invasion by CCRCC is more common than other histopathological types of RCC. Compared to metastases to the liver, lung, adrenal gland, bone, or brain, RCC metastases to the pancreas are rare and account for 1%–4% of all malignant pancreatic tumors (4, 5). Clinically, imaging modalities that can effectively differentiate metastases from primary pancreatic tumors include enhanced computed tomography (CT), positron emission tomography (PET) associated with CT (PET/CT), magnetic resonance imaging, and endoscopic ultrasound-guided fine-needle aspiration biopsy (3). Here, we describe a case of CCRCC metastases to the pancreas and other distant organs 24 years after radical nephrectomy and review the relevant literature.
A 76-year-old Asian man underwent radical left nephrectomy due to CCRCC in 1995, but detailed medical records of the primary renal tumor were not available as the patient had undergone the procedure at another hospital. In August 2019, the patient suddenly experienced epigastric pain after eating, accompanied by abdominal distension, nausea, and vomiting. Enhanced abdominal CT revealed a space-occupying lesion in the pancreatic body and tail, multiple liver metastases, and lymph node metastases around the pancreas; however, chest CT was negative. CT imaging findings of the space-occupying lesions were similar to those of CCRCC. Whole-body fluorodeoxyglucose PET/CT scans showed pancreatic thickening along with increased fluorodeoxyglucose tracer intake, pancreatic duct dilation, and a mild increase in the metabolic activity of lymph nodes in the peripancreatic and mesenteric areas (Figure 1). Thus, as the patient’s symptoms, past history, and imaging results were indicative of malignant tumor metastasis, we did not perform a biopsy but chose to proceed with metastasectomy. The patient underwent combined exploratory laparotomy with pancreatic body and tail resection, splenectomy, and left hepatic lobectomy. In the early stage of surgery, the intraoperative frozen-section examination was performed to identify the tumor property. The dimensions of the excised pancreatic tumor were 15 × 7 × 6 cm, and the cut surface was multicolored. Metastases were visible in 1 of 3 lymph nodes around the pancreas, whereas the two liver metastases measured 2 × 1 × 3 cm and 1.5 × 1 × 2 cm, respectively. The results of histopathological and immunohistochemical analyses are provided in Figure 2. Histologically, the CCRCC metastases were found in Fuhrman grades II–III. Considering the adverse reactions and financial burden of the systemic therapy, we decided to follow up closely without the application of systemic therapies for the time being.
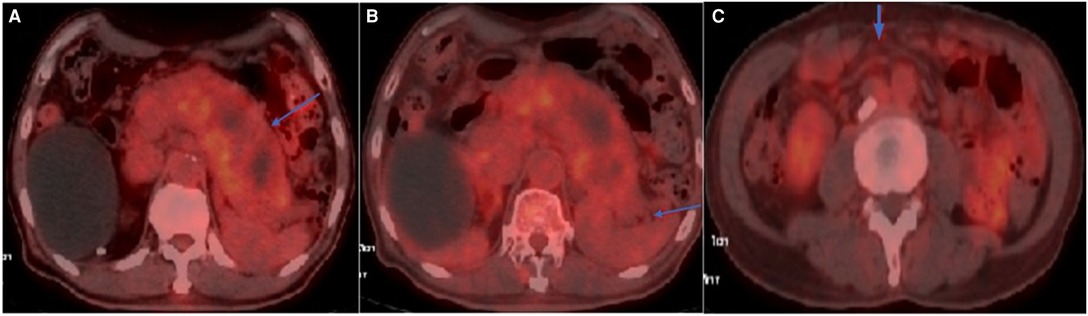
Figure 1. Axial view of FDG-PET/CT: (A) Hypermetabolic activity in the swollen pancreas with SUVmax of 3.0; (B) Mild hypermetabolic activity surrounding the pancreas; (C) Mild hypermetabolic activity in the mesenteric area with SUVmax of 1.9.
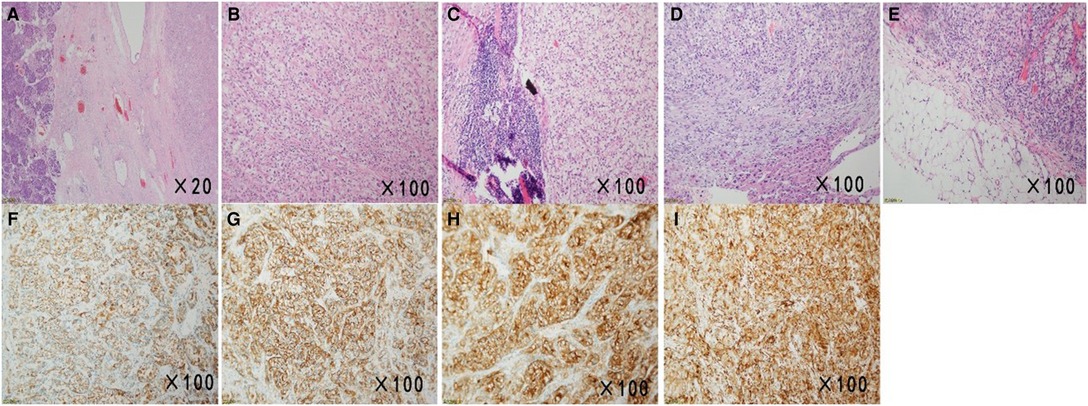
Figure 2. Micrographs of the surgical specimens and immunohistochemical examination results. (A,B) Sections of the pancreas showed atypical clear cells monomorphonuclear with bright cytoplasm (H&E, ×20, ×100). (C–E) HE staining image of the peripancreatic lymph node, sections of the liver, and the omental tissue showed histological features similar to those of the pancreatic sections (H&E, ×100, ×100, ×100). (F–I) Immunohistochemical examination of surgical specimens showed neoplastic cells with CK (+), CA9 (+), CD10(+), and Vimentin (+) (×100).
In June 2020, the high-resolution chest CT re-examination revealed new nodules in the basal segment of the right lower lobe (Figure 3). We believed that systemic therapy was necessary to prevent tumor progression and prolong survival. Then the patient received targeted therapy (Sunitinib). At this time point, the patient had a Memorial Sloan Kettering Cancer Center (MSKCC) (6) intermediate-risk feature based on clinical manifestations and laboratory examinations. In September 2020, a reexamination of the abdominal enhanced CT showed that there were multiple new-onset metastatic lesions in the left renal area, the liver, and the lymph nodes in the hilar area (Figure 4). Given the continuous progress of the tumor, we decided to use targeted therapy combined with the PD-1 inhibitor (Toripalimab). In May 2021, a chest CT scan showed no significant changes in pulmonary nodules; while an abdominal CT scan revealed that the number of liver metastases was reduced and the size was decreased. Consequently, we could conclude that the patient had a partial response after receiving targeted therapy combined with PD-1 inhibitors. Since receiving the combinatorial treatment, the patient has not suffered from any serious adverse events. Conversely, the patient’s physical and psychological conditions are gradually improving. We will continue to adopt targeted therapy combined with immunotherapy, and follow up regularly.
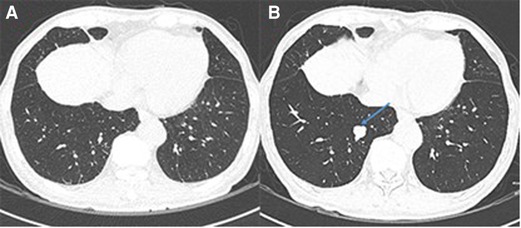
Figure 3. Newly-occurred lung metastases after metastasectomy. (A) No lung metastases before metastasectomy. (B) The metastatic lesion in the right lower lobe (arrow) 10 months after metastasectomy.
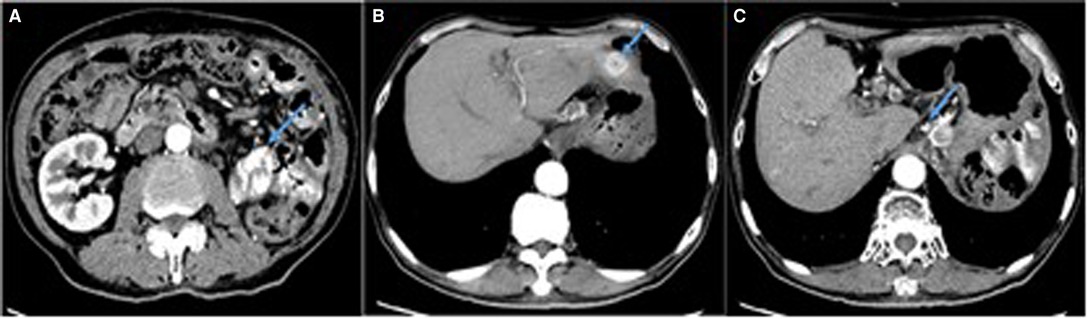
Figure 4. Enhanced CT scan showing multiple abnormal enhancement lesions (arrows). (A) The mass with heterogeneous enhancement in left kidney area. (B) A ‘target-like’ sign and heterogeneous enhancement of the nodule in liver. (C) Multiple enlarged lymph nodes in hepatic hilar region.
CCRCC is the most common and aggressive type of RCC. At the time of diagnosis, the metastasis has usually occurred due to the asymptomatic features of CCRCC, and more than 20% of CCRCC patients relapse after nephrectomy (7). CCRCC can metastasize to almost all organs or tissues. The earliest and most common metastatic site is the lung, followed by the liver, adrenal glands, bones, brain, and pancreas. Pancreatic metastases account for only 2%–5% of pancreatic malignancies (8). Among pancreatic metastases, CCRCC metastasized to the pancreas accounted for only 1.3% (9). Even though mechanisms underlying metachronous CCRCC metastases after radical nephrectomy have not been elucidated, we hypothesize that this patient must have been chronically harboring CCRCC micrometastases and immunosurveillance served to maintain a relative balance between micrometastases and the immune system. Arguably, this equilibrium lasted 24 years but was disrupted by an event/events that led to tumor progression, and we propose two potential reasons as follows. First, the tumor gradually established a stable tumor microenvironment (TME) at the metastatic site, which then promoted angiogenesis and induced immune tolerance by releasing cytokines (10). Second, physiological changes due to aging during 24 years, such as inflammaging and immunosenescence (11, 12), could have triggered progression. Inflammaging is defined as a chronic state of low-grade inflammation characterized by elevated levels of inflammatory mediators and proinflammatory cytokines and is attributed to long-term endogenous or exogenous factors. However, to avoid excessive inflammatory reactions, the immunosuppressive network is activated, which not only increases the number of immunosuppressive cells and secretion of anti-inflammatory cytokines but also decreases positive immune function(s). These long-term changes in the immune system result in immunosenescence (11). Additionally, long-term stimulation of micrometastases could have contributed to inflammaging and immunosenescence, thereby resulting in a decline in immunosurveillance, which then weakened the limitations on tumor growth and metastasis. Thus, we suggest that the formation of a conducive TME and immunosenescence together contributed to the failure of immunosurveillance.
Although the detection of metastases usually takes a long time from the diagnosis of primary renal tumors, for example, the average time of pancreatic metastasis is 7.1–10.0 years (13), metastasis occurred 24 years after radical nephrectomy in the present case. To the best of our knowledge, this situation is rare, and to date, the longest duration from diagnosis to metastasis in CCRCC is 32.7 years (13). Hence, we propose that clinicians design patient-centered follow-up plans based on the primary tumor to ensure an optimal cost-benefit ratio.
At present, there is still a lack of effective methods for the examination of metastatic CCRCC. Imaging examination is the main diagnostic method. However, the imaging manifestations of most metastatic tumors are similar to those of tumors originating from the metastatic site, which might lead to misdiagnosis. Notably, when the tumor metastasizes to a single organ or site. The preoperative misdiagnosis rate reaches about 70% (14). The examination measures commonly used for the metastatic tumors of the pancreas or liver include enhanced CT, enhanced MRI, PET/CT, PET/MRI, and endoscopic ultrasound-guided biopsy (3, 15, 16).
For metastatic CCRCC, there are currently two treatment options, including local therapies and systemic therapies. Local therapies include cytoreductive nephrectomy, metastasectomy, and stereotactic radiotherapy (SRT) (17–19). Systemic therapies include targeted therapy and immunotherapy (20, 21). It is generally believed that local therapies should be used in combination with systemic therapies. Yet, it has also been shown in several studies that whether combined with local therapies had little influence on the effects of systemic therapies (22). Local therapies can even increase risks, such as surgical trauma, iatrogenic metastasis, and radiotherapy toxicity.
It is believed that cytoreductive nephrectomy and metastasectomy can reduce tumor growth factors, inflammatory cytokines, and immunosuppressive factors secreted by cancer cells, thereby reducing tumor growth or improving the effects of systemic treatment (23). Furthermore, the survival and recurrence-free interval after pancreatic metastasis resection of CCRCC is significantly longer than in other tumors according to a previous meta-analysis of pancreatic metastasis of malignant tumors (24). However, the incidence of surgical complications and mortality is relatively high for metastatic RCC patients 75 years or older (25). Consequently, clinicians need to be cautious in choosing whether to perform surgery. Given the existing literature, we believe that whether to perform cytoreductive nephrectomy or metastasectomy for patients with metastatic CCRCC cannot be generalized. The final choice should be based on a comprehensive assessment of clinical manifestations, degree of frailty, pathological type, metastatic location and burden, drug treatment response, and life expectancy.
SRT is an emerging treatment method for metastatic CCRCC. Although CCRCC was considered to be radiation resistant in the past, SRT can provide very high local doses to kill tumor cells (22). More importantly, several prospective trials have exhibited that radiotherapy might induce PD-1 ligand 1(PD-L1) expression in tumor tissues, thereby increasing the anti-tumor ability of immune checkpoint inhibitors (ICIs) (26). A retrospective study by Kroeze et al. (27) showed that targeted therapy or immunotherapy combined with SRT could significantly improve the progression-free survival and overall survival of metastatic RCC patients without increasing toxic effects. As far as we know, SRT has been applied to metastases of RCC to lungs, bones, lymph nodes, liver, intracranial, and spinal (22).
Recently, the systemic therapies of metastatic CCRCC have undergone tremendous changes. The use of vascular endothelial growth factor (VEGF) inhibitors alone is no longer recommended. The combination of PD-L1 inhibitors and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors or PD-L1 inhibitors and VEGF inhibitors is currently recommended (28). ICIs and VEGF inhibitors are both first-line standard treatments for metastatic CCRCC (7). The VEGF targeted therapy can enhance the infiltration capacity of T cells, which provides a strong theoretical basis for the combination of VEGF targeted therapy and ICIs therapy in metastatic CCRCC (29). Additionally, Li et al. confirmed that Sunitinib could inhibit the PD-L1 expression in tumor tissues (30), which furnishes another supporting evidence for Sunitinib combined with ICIs. The combination of immunotherapy and targeted therapies has shown potent metastases suppressing effects in both first-line and second-line treatment, and prolonged the overall survival (29).
At present, there is still controversy about the specific regimen of the combination of systemic therapies and local therapies. Most researchers consider that local therapies should be used after systemic therapies (31, 32). On the one hand, great progress has been made in systemic therapies. Some metastatic CCRCC patients can achieve desired outcomes with systemic therapies, and there is no significant difference in whether combined with surgical treatment (33). In the case that systemic therapies effectively control disease progression, patients can avoid trauma or related complications caused by local therapies. On the other hand, local therapies are able to address the puzzle of drug resistance in tumors after systemic therapies. However, some researchers believe that local therapies will provide a certain drug-free interval, thereby reducing adverse events caused by systemic therapies and maintaining the quality of life (34, 35). Therefore, local therapies should precede system therapies.
This patient had a partial response after receiving targeted therapy combined with PD-1 inhibitors. Thus, the inhibitory effects of Sunitinib and Toripalimab on metastatic CCRCC are obvious and encouraging. During the combinatorial treatment, this patient did not experience serious adverse reactions or complications. However, Liu et al. found that ICIs combined with radiotherapy promoted the development of lung metastases from CCRCC (36). This might be because ICIs significantly activated and increased tumor-infiltrating PD-1+ Treg cells and led to suppression of anti-tumor immunity (37). Therefore, we should review the changes in patients with metastatic CCRCC regularly when applying ICIs, such as PD-1 inhibitors.
In the case of multiple organ metastases from CCRCC, the European strategy is more inclined to initiate a systemic treatment first. Next, whether a local treatment will be proposed depends on the response to systemic treatment (38). Nevertheless, our study suggests that systemic therapy following metastasectomy may be also safe and effective. Certainly, the final clinical decision should be made after a comprehensive analysis of the patients’ situation, which is in the best interest of the patients.
Although metachronous pancreatic metastasis of CCRCC after radical nephrectomy is rare, clinicians and patients should not ignore this possibility. The combination of targeted therapy and immunotherapy can result in satisfactory outcomes in cases where metastatic CCRCC continues to progress despite metastasectomy and targeted therapy. However, the effectiveness and safety of such a treatment scheme require large-scale research to verify in the future. The combination of local and systemic therapy can be an effective therapeutic protocol for metastatic CCRCC, but there is no consensus on suitable therapeutics.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HC conceived and designed the manuscript. JW and CH provided language help. ZS and WW reviewed the manuscript. All authors contributed to the article and approved the submitted version.
The authors thank the Department of Urology of Affiliated Beijing Chaoyang Hospital of Capital Medical University for technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chow W, Devesa S, Warren J, Fraumeni J. Rising incidence of renal cell cancer in the United States. JAMA. (1999) 281(17):1628–31. doi: 10.1001/jama.281.17.1628
2. Janzen N, Kim H, Figlin R, Belldegrun A. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. (2003) 30(4):843–52. doi: 10.1016/s0094-0143(03)00056-9
3. Zianne M, Takahashi N, Tsujibata A, Miwa K, Goto Y, Matano Y. Asymptomatic Pancreatic Metastasis from Renal Cell Carcinoma Diagnosed 21 Years after Nephrectomy. Case Rep Gastrointest Med. (2017) 2017:8765264. doi: 10.1155/2017/8765264
4. Bianchi M, Sun M, Jeldres C, Shariat S, Trinh Q, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. (2012) 23(4):973–80. doi: 10.1093/annonc/mdr362
5. Rupert K, Kural T, Skalický T, Zeithaml J, Hess O, Třeška V. Clear cell renal carcinoma metastases to the pancreas. Rozhl Chir. (2020) 99(7):311–5. doi: 10.33699/PIS.2020.99.7.311-315
6. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. (1999) 17(8):2530–40. doi: 10.1200/JCO.1999.17.8.2530
7. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
8. Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce A, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. (2008) 15(4):1161–8. doi: 10.1245/s10434-007-9782-0
9. Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. (2006) 13(1):75–85. doi: 10.1245/aso.2006.03.064
10. Mennitto A, Huber V, Ratta R, Sepe P, de Braud F, Procopio G, et al. Angiogenesis and immunity in renal carcinoma: can we turn an unhappy relationship into a happy marriage? J Clin Med. (2020) 9(4):930. doi: 10.3390/jcm9040930
11. Salminen A. Activation of immunosuppressive network in the aging process. Ageing Res Rev. (2020) 57:100998. doi: 10.1016/j.arr.2019.100998
12. Pang W, Schrier S, Weissman I. Age-associated changes in human hematopoietic stem cells. Semin Hematol. (2017) 54:39–42. doi: 10.1053/j.seminhematol.2016.10.004
13. Hoshino Y, Shinozaki H, Kimura Y, Masugi Y, Ito H, Terauchi T, et al. Pancreatic metastases from renal cell carcinoma: a case report and literature review of the clinical and radiological characteristics. World J Surg Oncol. (2013) 11:289. doi: 10.1186/1477-7819-11-289
14. Ma Y, Yang J, Qin K, Zhou Y, Ying X, Yuan F, et al. Resection of pancreatic metastatic renal cell carcinoma: experience and long-term survival outcome from a large center in China. Int J Clin Oncol. (2019) 24(6):686–93. doi: 10.1007/s10147-019-01399-w
15. Li J, Xue F, Xu X, Wang Q, Zhang X. Dynamic contrast-enhanced MRI differentiates hepatocellular carcinoma from hepatic metastasis of rectal cancer by extracting pharmacokinetic parameters and radiomic features. Exp Ther Med. (2020) 20(4):3643–52. doi: 10.3892/etm.2020.9115
16. Zhang C, O’Shea A, Parente C, Amorim B, Caravan P, Ferrone C, et al. Evaluation of the Diagnostic Performance of Positron Emission Tomography/Magnetic Resonance for the Diagnosis of Liver Metastases. Invest Radiol. (2021) 56(10):621–8. doi: 10.1097/rli.0000000000000782
17. Laru L, Ronkainen H, Ohtonen P, Vaarala M. Nephrectomy improves the survival of metastatic renal cell cancer patients with moderate to good performance status-results from a Finnish nation-wide population-based study from 2005 to 2010. World J Surg Oncol. (2021) 19(1):190. doi: 10.1186/s12957-021-02308-0
18. Hsieh P, Hung S, Li J, Wang S, Yang C, Chen C, et al. The effect of metastasectomy on overall survival in metastatic renal cell carcinoma: A systematic review and meta-analysis. Urol Oncol. (2021) 39(7):422–30. doi: 10.1016/j.urolonc.2021.02.026
19. Klausner G, Troussier I, Biau J, Jacob J, Schernberg A, Canova C, et al. Stereotactic Radiation Therapy for Renal Cell Carcinoma Brain Metastases in the Tyrosine Kinase Inhibitors Era: Outcomes of 120 Patients. Clin Genitourin Cancer. (2019) 17(3):191–200. doi: 10.1016/j.clgc.2019.02.007
20. Bracarda S, Iacovelli R, Boni L, Rizzo M, Derosa L, Rossi M, et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol. (2016) 27(2):366. doi: 10.1093/annonc/mdv589
21. McDermott D, Sosman J, Sznol M, Massard C, Gordon M, Hamid O, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol. (2016) 34(8):833–42. doi: 10.1200/jco.2015.63.7421
22. Christensen B, Hajja Y, Koshkin V, Barata P. Update on First-Line Combination Treatment Approaches in Metastatic Clear-Cell Renal Cell Carcinoma. Curr Treat Options Oncol. (2021) 22(2):15. doi: 10.1007/s11864-020-00814-z
23. Alzahrani A, Aggamy M, Joudeh A, Alabdullatif H, Alzahrani H, Gomha M. Metastasectomy of Sequential Asynchronous Metastatic Renal Cell Carcinoma to the Pancreas, Thyroid, Skin, Contralateral Kidney, and Lung with Cumulative Survival Beyond 10 Years: A Case Report and Clinicopathologic Review. Am J Case Rep. (2021) 22:e931696. doi: 10.12659/ajcr.931696
24. Sperti C, Pozza G, Brazzale A, Buratin A, Moletta L, Beltrame V, et al. Metastatic tumors to the pancreas: a systematic review and meta-analysis. Minerva Chir. (2016) 71(5):337–44.PMID: 27412234
25. van den Brom R, van Es S, Leliveld A, Gietema J, Hospers G, de Jong I, et al. Balancing treatment efficacy, toxicity and complication risk in elderly patients with metastatic renal cell carcinoma. Cancer Treat Rev. (2016) 46:63–72. doi: 10.1016/j.ctrv.2016.04.002
26. Buttigliero C, Allis S, Tucci M, Zichi C, Leone G, Di Stefano R, et al. Role of radiotherapy in improving activity of immune-modulating drugs in advanced renal cancer: Biological rationale and clinical evidences. Cancer Treat Rev. (2018) 69:215–23. doi: 10.1016/j.ctrv.2018.07.010
27. Kroeze S, Fritz C, Schaule J, Siva S, Kahl K, Sundahl N, et al. Stereotactic radiotherapy combined with immunotherapy or targeted therapy for metastatic renal cell carcinoma. BJU Int. (2021) 127(6):703–11. doi: 10.1111/bju.15284
28. Escudier B, Motzer R, Tannir N, Porta C, Tomita Y, Maurer M, et al. Efficacy of Nivolumab plus Ipilimumab According to Number of IMDC Risk Factors in CheckMate 214. Eur Urol. (2020) 77(4):449–53. doi: 10.1016/j.eururo.2019.10.025
29. Powles T, Atkins M, Escudier B, Motzer R, Rini B, Fong L, et al. Efficacy and Safety of Atezolizumab Plus Bevacizumab Following Disease Progression on Atezolizumab or Sunitinib Monotherapy in Patients with Metastatic Renal Cell Carcinoma in IMmotion150: A Randomized Phase 2 Clinical Trial. Eur Urol. (2021) 79(5):665–73. doi: 10.1016/j.eururo.2021.01.003
30. Li H, Kuang X, Liang L, Ye Y, Zhang Y, Li J, et al. The Beneficial Role of Sunitinib in Tumor Immune Surveillance by Regulating Tumor PD-L1. Adv Sci. (2021) 8(2):2001596. doi: 10.1002/advs.202001596
31. Hsiang W, Kenney P, Leapman M. Redefining the Role of Surgical Management of Metastatic Renal Cell Carcinoma. Curr Oncol Rep. (2020) 22(4):35. doi: 10.1007/s11912-020-0895-y
32. Bhindi B, Abel E, Albiges L, Bensalah K, Boorjian S, Daneshmand S, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur Urol. (2019) 75(1):111–28. doi: 10.1016/j.eururo.2018.09.016
33. Ishihara H, Takagi T, Kondo T, Fukuda H, Tachibana H, Yoshida K, et al. Prognostic impact of metastasectomy in renal cell carcinoma in the postcytokine therapy era. Urol Oncol. (2021) 39(1):77.e17–77.e25. doi: 10.1016/j.urolonc.2020.08.011
34. Johns A, Wei L, Grogan M, Hoyd R, Bridges J, Patel S, et al. Checkpoint inhibitor immunotherapy toxicity and overall survival among older adults with advanced cancer. J Geriatr Oncol. (2021) 12(5):813–9. doi: 10.1016/j.jgo.2021.02.002
35. Cheung P, Patel S, North S, Sahgal A, Chu W, Soliman H, et al. Stereotactic Radiotherapy for Oligoprogression in Metastatic Renal Cell Cancer Patients Receiving Tyrosine Kinase Inhibitor Therapy: A Phase 2 Prospective Multicenter Study. Eur Urol. (2021) 80(6):693–700. doi: 10.1016/j.eururo.2021.07.026
36. Liu C, Piao J, Shang Z. Hyperprogressive disease after radiotherapy combined with anti-PD-1 therapy in renal cell carcinoma: a case report and review of the literature. BMC Urol. (2021) 21(1):42. doi: 10.1186/s12894-021-00813-8
37. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1 regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. (2019) 116(20):9999–10008. doi: 10.1073/pnas.1822001116
Keywords: Clear cell renal cell carcinoma, pancreatic metastasis, metastasectomy, systemic therapy, combinatorial treatment
Citation: Cao H, Sun Z, Wu J, Hao C and Wang W (2022) Metastatic Clear Cell Renal Cell Carcinoma to Pancreas and Distant Organs 24 Years After Radical Nephrectomy: A Case Report and Literature Review. Front. Surg. 9:894272. doi: 10.3389/fsurg.2022.894272
Received: 11 March 2022; Accepted: 20 June 2022;
Published: 5 July 2022.
Edited by:
Qiang Hu, Tongde Hospital of Zhejiang Province, ChinaReviewed by:
Chunhua Lin, Yantai Yuhuangding Hospital, ChinaCopyright © 2022 Cao, Sun, Wu, Hao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang d2Vpd2FuZzA5MjBAMTYzLmNvbQ==
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Abbreviations: CCRCC, Clear cell renal cell carcinoma; PD-1, Programmed death receptor-1; PD-L1, PD-1 ligand 1; RCC, Renal cell carcinoma; CT, Computed tomography; PET, Positron emission tomography; MSKCC, Memorial Sloan Kettering Cancer Center; MRI, Magnetic resonance imaging; TME, Tumor microenvironment; SRT, Stereotactic radiotherapy; ICIs, Immune checkpoint inhibitors; VEGF, Vascular endothelial growth factor; CTLA-4, Cytotoxic T-lymphocyte-associated antigen 4.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.