
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg. , 04 May 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.889999
This article is part of the Research Topic The Impact of COVID-19 on Immune System-Related Complications in Surgical Patients View all 5 articles
 Connor M. Bunch1
Connor M. Bunch1 Ernest E. Moore2
Ernest E. Moore2 Hunter B. Moore2
Hunter B. Moore2 Matthew D. Neal3
Matthew D. Neal3 Anthony V. Thomas4
Anthony V. Thomas4 Nuha Zackariya4
Nuha Zackariya4 Jonathan Zhao5
Jonathan Zhao5 Sufyan Zackariya5
Sufyan Zackariya5 Toby J. Brenner5
Toby J. Brenner5 Margaret Berquist5
Margaret Berquist5 Hallie Buckner5
Hallie Buckner5 Grant Wiarda5
Grant Wiarda5 Daniel Fulkerson4,6
Daniel Fulkerson4,6 Wei Huff4,6
Wei Huff4,6 Hau C. Kwaan7
Hau C. Kwaan7 Genevieve Lankowicz5
Genevieve Lankowicz5 Gert J. Laubscher8
Gert J. Laubscher8 Petrus J. Lourens8
Petrus J. Lourens8 Etheresia Pretorius9,13
Etheresia Pretorius9,13 Maritha J. Kotze10
Maritha J. Kotze10 Muhammad S. Moolla11
Muhammad S. Moolla11 Sithembiso Sithole11
Sithembiso Sithole11 Tongai G. Maponga12
Tongai G. Maponga12 Douglas B. Kell9,13,14
Douglas B. Kell9,13,14 Mark D. Fox4
Mark D. Fox4 Laura Gillespie15
Laura Gillespie15 Rashid Z. Khan16
Rashid Z. Khan16 Christiaan N. Mamczak4,17
Christiaan N. Mamczak4,17 Robert March18
Robert March18 Rachel Macias4,19
Rachel Macias4,19 Brian S. Bull20†
Brian S. Bull20† Mark M. Walsh4,5*
Mark M. Walsh4,5*
Early in the coronavirus disease 2019 (COVID-19) pandemic, global governing bodies prioritized transmissibility-based precautions and hospital capacity as the foundation for delay of elective procedures. As elective surgical volumes increased, convalescent COVID-19 patients faced increased postoperative morbidity and mortality and clinicians had limited evidence for stratifying individual risk in this population. Clear evidence now demonstrates that those recovering from COVID-19 have increased postoperative morbidity and mortality. These data—in conjunction with the recent American Society of Anesthesiologists guidelines—offer the evidence necessary to expand the early pandemic guidelines and guide the surgeon’s preoperative risk assessment. Here, we argue elective surgeries should still be delayed on a personalized basis to maximize postoperative outcomes. We outline a framework for stratifying the individual COVID-19 patient’s fitness for surgery based on the symptoms and severity of acute or convalescent COVID-19 illness, coagulopathy assessment, and acuity of the surgical procedure. Although the most common manifestation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is COVID-19 pneumonitis, every system in the body is potentially afflicted by an endotheliitis. This endothelial derangement most often manifests as a hypercoagulable state on admission with associated occult and symptomatic venous and arterial thromboembolisms. The delicate balance between hyper and hypocoagulable states is defined by the local immune-thrombotic crosstalk that results commonly in a hemostatic derangement known as fibrinolytic shutdown. In tandem, the hemostatic derangements that occur during acute COVID-19 infection affect not only the timing of surgical procedures, but also the incidence of postoperative hemostatic complications related to COVID-19-associated coagulopathy (CAC). Traditional methods of thromboprophylaxis and treatment of thromboses after surgery require a tailored approach guided by an understanding of the pathophysiologic underpinnings of the COVID-19 patient. Likewise, a prolonged period of risk for developing hemostatic complications following hospitalization due to COVID-19 has resulted in guidelines from differing societies that recommend varying periods of delay following SARS-CoV-2 infection. In conclusion, we propose the perioperative, personalized assessment of COVID-19 patients’ CAC using viscoelastic hemostatic assays and fluorescent microclot analysis.
The global impact of the coronavirus disease 2019 (COVID-19) pandemic on surgical procedures was unprecedented (1, 2). After the induction of the national state of emergency in the United States in March 2020, the Centers for Medicare and Medicaid Services (CMS), the American College of Surgeons (ACS), and other international surgical societies recommended postponing, minimizing, and cancelling elective surgical procedures based largely on SARS-CoV-2 transmissibility precautions (3–5). During this time, there was a 50% reduction in the number of surgical procedures performed relative to baseline (1). Five weeks later, a joint recommendation was published that advised the resumption of elective surgical procedures (6). As the demand for elective procedures increased, clinicians were faced with a growing number of patients still recovering from COVID-19, and surgeons had little available evidence for the optimal timing of surgery and postoperative prognosis for those recovering from acute and/or convalescent COVID-19 illness (7).
As the pandemic continued and data accrued, postoperative morbidity and mortality were confirmed to be significantly higher for patients with perioperative COVID-19 infection (8). Perioperative COVID-19 infection correlated with higher rates of postoperative pulmonary complications and venous thromboemboli (VTE) (e.g., pulmonary emboli [PE] and deep venous thromboses [DVT]) (9). Notably, those with ongoing symptoms at the time of operation—regardless of length from diagnosis/onset of COVID-19 symptoms—have a significantly greater risk of VTE and associated mortality. VTE has been independently associated with 30-day postoperative mortality (odds ratio 5.4 [95%CI 4.3–6.7]) (9).
One prospective cohort study by the COVIDSurg Collaborative evaluated >140,000 surgical patients from 116 countries who underwent elective or emergency surgery during October 2020 (10). Surgical patients with preoperative SARS-CoV-2 infection were compared to those without previous SARS-CoV-2 infection. There was a significant increase in postoperative pulmonary complications and mortality in patients with a recent diagnosis of SARS-CoV-2 infection (10). The calculated 30-day mortality rates were stratified by time between diagnosis of SARS-CoV-2 infection and surgery (groups included those diagnosed 0–2, 3–4, 5–6, and ≥7 weeks prior to surgery). For those patients without SARS-CoV-2 infection, the adjusted 30-day mortality rate was 1.5%. For those patients diagnosed with SARS-CoV-2 infection, 30-day postoperative mortality rates were 9.1% for 0–2 weeks; 6.9% for 3–4 weeks; 5.5% for 5–6 weeks; and 2.0% at ≥7 weeks between diagnosis and surgery. Surgery performed ≥7 weeks after SARS-CoV-2 diagnosis was associated with a similar mortality risk to baseline for controls (odds ratio 1.5 [95%CI 0.9–2.1]). Further stratifying this analysis to those patients with ongoing symptoms at time of surgery, it was noted that even after a ≥7 week delay from SARS-CoV-2 infection diagnosis, these symptomatic convalescent patients had a higher mortality than patients whose symptoms had resolved or who had been asymptomatic at operation (6.0% for ongoing symptoms, vs. 2.4% for resolved symptoms, vs. 1.3% for controls).
Consequently, the authors recommended that surgery be delayed for at least seven weeks following SARS-CoV-2 infection, and concluded that patients with ongoing symptoms at seven weeks may benefit from even longer delay (10). A recent multidisciplinary consensus statement aligned with these study conclusions, stating that elective surgery should be scheduled seven or more weeks after the diagnosis of COVID-19 for patients with asymptomatic or transient SARS-CoV-2 infection, while patients with persistent symptoms (i.e., “Long COVID”) remain at elevated risk for postoperative morbidity and mortality even after seven weeks. It is therefore recommended to stratify patient risk in delaying surgery beyond seven weeks on a personalized basis (11). In one systematic review including >250,000 patients, more than half had post-acute sequelae of COVID-19 at six months follow-up (12). Clearly, there is a significant need to risk stratify this patient population on an individualized basis.
The most recent American Society of Anesthesiologists (ASA) and Anesthesia Patient Safety Foundation (APSF) joint recommendation for the delay of surgery have been divided into four blocks of time based upon the symptoms and severity of acute COVID-19 illness:
“Four weeks for an asymptomatic patient or recovery from only mild, non-respiratory symptoms
Six weeks for a symptomatic patient (e.g., cough, dyspnea) who did not require hospitalization
Eight to 10 weeks for a symptomatic patient who is diabetic, immunocompromised, or hospitalized
Twelve weeks for a patient who was admitted to an intensive care unit due to COVID-19 infection”
The ASA and APSF joint recommendation further states: “These timelines should not be considered definitive; each patient’s preoperative risk assessment should be individualized, factoring in surgical intensity, patient co-morbidities, and the benefit/risk ratio of further delaying surgery” (13). Specifically, these recommendations have not considered the differing degrees of coagulopathy associated with COVID-19 variants. Additionally, there is little guidance on elective surgery for patients afflicted with Long COVID, and there are few proposals for preoperative laboratory measurements (e.g., D-dimer, fibrinogen, ferritin, lactate dehydrogenase (LDH), and other clinical and laboratory markers of severity of disease) to guide surgeons and anesthesiologists for the appropriate timing of surgery (14, 15).
Likewise, sparse guidance exists for the postoperative thromboprophylaxis of this patient group. In a large study of 11,249 patients from the first COVID-19 surge in the Spring of 2020 in New York City, 1.7% of all patients discharged from the hospital had VTE or arterial thromboembolism (16). This study also reported that prophylactic anticoagulation with either enoxaparin or rivaroxaban engenders a 46% reduction in clotting disorders (16). However, the unique pathophysiological aspect of COVID-19 pneumonitis is that these patients may bleed and clot at the same time when anticoagulated (17). It was reported that 1.73% of discharged patients suffered major bleeding within 90 days of discharge while only 13.2% of the entire cohort was administered thromboprophylaxis (16).
Together these findings offer surgeons the opportunity to familiarize themselves with the unique hemostatic derangement of CAC affecting postoperative outcomes worldwide. A basic flow chart for triaging acute and convalescent COVID-19 patients based upon recent ASA and surgical guidelines for the timing of surgery and postoperative VTE prophylaxis is presented in Figure 1. Clear data demonstrates unvaccinated individuals with COVID infection procure a higher risk of thrombosis and more severe illness. Breakthrough infections in vaccinated individuals have less thrombotic risk and lower risk of severe illness (27). Following the ASA guidelines, patients’ length of delay for surgery should be based on the severity of illness regardless of vaccination status.
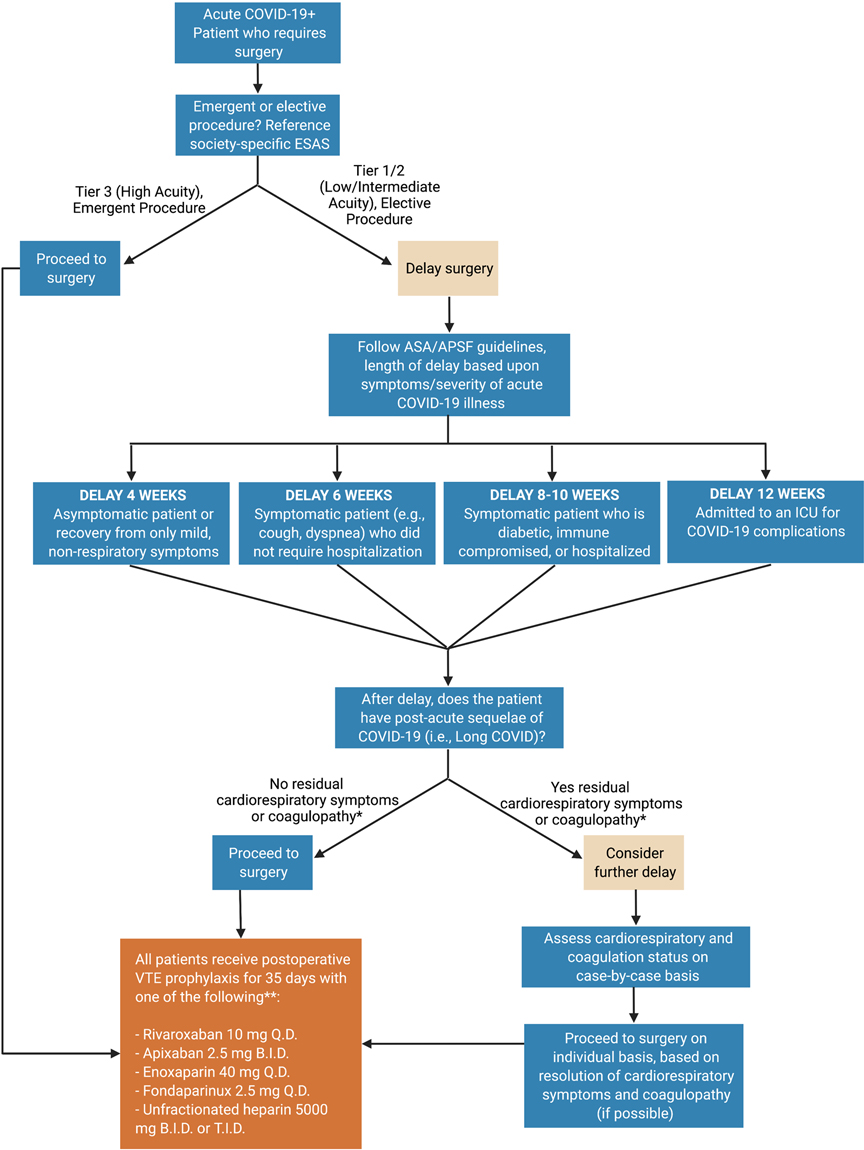
Figure 1. COVID-19 patient surgical fitness flow chart. The surgeon assesses the acuity of the procedure using the specialty-specific Elective Surgery Acuity Scale (ESAS). If the procedure is of low/intermediate acuity and can be delayed, the length of delay may follow American Society of Anesthesiologists’ recommendations. After the delay, timing of surgery is a function of both the necessity of surgery and the degree of recovery of respiratory and hemostatic derangement post-COVID-19. Post-operatively, due to a high degree of hypercoagulability in many convalescent COVID-19 patients, standard pharmacologic VTE prophylaxis may be considered in all these patients. Created using BioRender.com. *Cardiorespiratory symptoms such as shortness of breath, chest pain, and fatigue are common in patients with convalescent COVID-19 (12, 13, 18, 19). Coagulopathy, including both hyper and hypocoagulable states, should be assessed pre- and post-operatively using fibrinogen levels, platelet count, and viscoelastic hemostatic assays (e.g., thromboelastography (TEG) and rotational thromboelastometry (ROTEM)). The definition of hyper and hypocoagulability are a function of institutional preference. **This is not an exhaustive list of anticoagulant agents, nor a comprehensive dosing regimen. For inpatients acutely ill with COVID-19, those with VTE, those who undergo emergent surgery, or those at risk for hemorrhage, personalized titration of anticoagulation with adjunctive viscoelastic hemostatic assays is recommended (17, 20–23). VTE prophylaxis for postoperative patients with acute or convalescent COVID-19 is not specifically addressed in recent expert consensus statements or society recommendations (24–26). As such, this recommendation is the opinion of the authors and should be judiciously applied taking into account each individual patient’s risk and benefit to anticoagulant therapy.
The impact of COVID-19 on immune system-related complications in surgical patients is a crosstalk between immunology and coagulation at the level of the endothelium. This is more than a theoretical concept; rather, it is an important pathophysiologic understanding of the unique challenges of operating on patients who have either recent or remote infections with the SARS-CoV-2 virus. Few guidelines exist concerning the timing and nature of this dual threat of bleeding and clotting that occur in a surgical patient with COVID-19 (28, 29). This review will address the pathophysiologic underpinnings of COVID-19 as it relates to surgical timing and thromboprophylaxis, followed by a specific application of these immune-thrombotic changes to perioperative and postoperative management of patients.
Recent consensus and guidelines exist, but they lack granularity to assist the surgeon for optimizing the timing of elective surgery and guiding postoperative thromboprophylaxis for their individual convalescent COVID-19 patients. The long-term effects of COVID-19 infection are still under investigation. Hence, there are limited studies or specific guidelines aiding the surgeon in weighing risks and benefits of elective surgery for patients post-recovery from COVID-19. Persistent complications of COVID-19 infection after recovery are a product of the vast endothelial damage induced by SARS-CoV-2 (29). It is pertinent for the surgeon and anesthesiologist to understand the mechanisms of this endotheliitis to individualize care for their convalescent COVID-19 patients in this time of limited evidence-based guidance.
Immuno-thrombosis is often described as the dysregulated interaction between innate immunity and coagulation (30–32). A reported 30-80% of COVID-19 ICU patients endure thrombotic complications during their clinical course (33–36). The most common thrombotic complication in COVID-19 patients is VTE, which occurs in 15-85% of patients (37). Although the exact mechanism behind CAC is poorly understood, endotheliitis likely incites the hypercoagulable state (Figure 2) (38–42). The SARS-CoV-2 virus directly invades endothelial cells through the angiotensin converting enzyme 2 (ACE2) receptor. This leads to localization of viral inclusion bodies in the lungs, liver, small intestines, and kidneys, shedding of endothelial glycocalyx proteins, and decreases in the level of heparanase-2. This results in a progression from normal hemostasis to a relatively antifibrinolytic state (31, 38, 43–45). The SARS-CoV-2 viral saturation of ACE2 increases circulating levels of angiotensin II, leading to increased production of the important antifibrinolytic mediator, plasminogen activator inhibitor 1 (PAI-1) (46–48). Elevated PAI-1 and thrombin-activatable fibrinolysis inhibitor (TAFI) levels in the alveolar space have been identified in COVID-19 patients (46, 49–51). Elevated tissue plasminogen activator (tPa) levels have also been noted in COVID-19 patients. This tPa release may be due to malperfusion secondary to microvascular fibrin deposition. Despite this increase in tPa, pro-thrombosis persists in these patients, suggesting that elevated PAI-1 and TAFI overwhelm the action of tPa, resulting in microvascular deposition of fibrin (52). These factors contribute to the disruption of the tightly regulated balance between thrombosis and fibrinolysis in COVID-19 patients, leading to what is known as “fibrinolytic shutdown” (17, 46, 50, 52–58). When combined with elevated thrombin generation, it leads to adverse complications such as a type of adult respiratory distress syndrome (ARDS), multisystem organ failure, and mortality (29, 59–62). This unique type of ARDS is often associated with a localized hypercoagulability of the pulmonary vasculature due to abnormal deposition of fibrin and development of microthrombosis (63–66). Postmortem analyses confirm a similar presentation in patients with COVID-19-induced ARDS (67–70). This localized hypercoagulability is partially induced by inflammation of tissue, leading to elevated tissue factor (TF) production by epithelial cells and alveolar macrophages which results in the deposition of fibrin and the development of microthrombosis (46, 71). Thromboses are additionally potentiated by a deficient fibrinolytic response primarily induced by the overexpression of PAI-1 from activated platelets and endothelial cells (31, 72, 73).
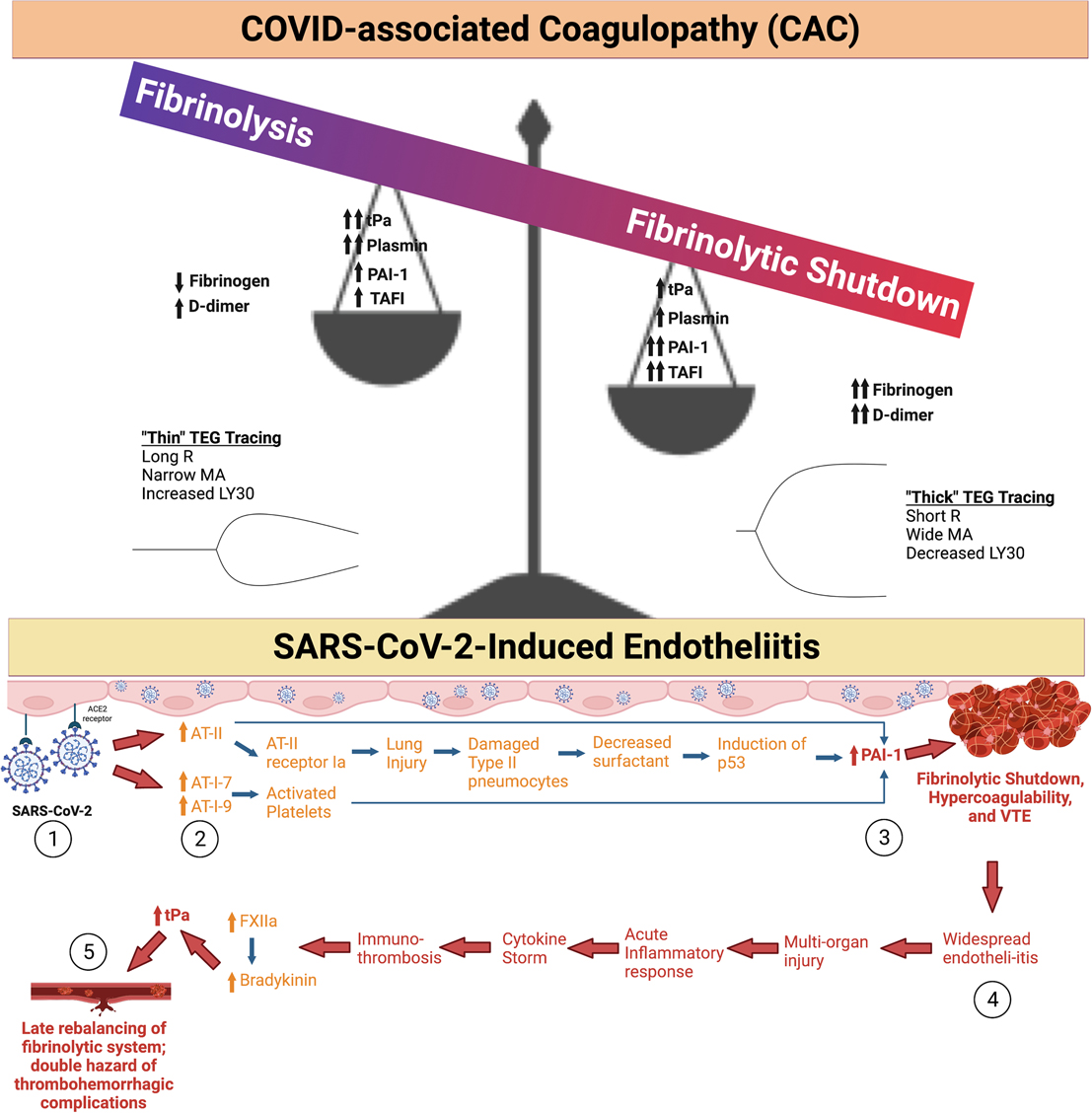
Figure 2. COVID-19-associated coagulopathy (CAC) and Fibrinolytic Shutdown. (1) The SARS-CoV-2 Spike protein binds with angiotensin converting enzyme 2 (ACE2), which causes internalization of the receptor and virus into the host cell. (2) In turn, ACE2 is not available for breakdown of angiotensin II (AT-II) leading to its build-up. AT-II causes subsequent lung injury and increases in plasminogen activated inhibitor 1 (PAI-1). Angiotensin I-7 (AT-I-7) and angiotensin I-9 (AT-I-9) are increased by other pathways involving activated platelets, but also lead to increased levels of PAI-1. (3) Increased circulating PAI-1 leads to fibrinolytic shutdown, a hypercoagulable state characterized by increased fibrin deposition and thrombus formation. (4) Among other mechanisms, widespread endotheliitis and microthrombosis can cause multi-organ injury in patients with severe COVID-19 illness. This exacerbates the acute inflammatory response, often causing a cytokine storm and worsening immuno-thrombosis. Inflammatory cytokines activate Factor XII (FXII), which subsequently increases bradykinin levels. Bradykinin increases tissue plasminogen activator (tPa) levels. (5) This subacute rebalancing of the fibrinolytic system predisposes the COVID-19 patient to both thrombosis and hemorrhage as a function of local endothelial derangement. This is particularly the case for hospitalized COVID-19 patients who are anticoagulated; these patients’ anticoagulant regimen must be judiciously titrated on a personalized basis (17, 29, 31). The spectrum of fibrinolysis is depicted as a balance whereby the predominate hypercoagulable fibrinolytic shutdown phenotype is portrayed as a balance with high levels of PAI-1, thrombin activatable fibrinolysis inhibitor (TAFI), fibrinogen, D-dimer, and lower levels of tPa and plasmin. The opposite occurs at the opposite end of the fibrinolysis spectrum when fibrinolysis predominates, which occurs much less frequently in CAC. Created using BioRender.com
Primary hemostasis, which is accomplished via activated platelets at the site of endothelial injury, contributes to the hypercoagulable state of the COVID-19 patient. Overwhelming platelet activation is driven by many stimuli, and significantly facilitated by increased levels of von Willebrand Factor (VWF), as well as activation of the enzymatic (intrinsic) clotting pathway. COVID-19 patients have increased activation of circulating platelets as demonstrated by elevated soluble P-selectin as well as markers for endothelial activation (74). A direct stimulatory role of SARS-CoV-2 Spike protein on the COVID-19 patient’s platelets has also been described (75). Severe COVID-19 illness correlates not just with platelet activation but is also associated with enhanced platelet-monocyte aggregation. Finally, ICU admitted COVID-19 patients’ platelets induce P-selectin and αIIb/β3-dependent monocyte TF expression which may augment inflammation and hypercoagulability (76).
Together, these aberrancies in hemostasis mediated by platelets and reduced fibrinolysis (documented by whole blood viscoelastic hemostatic assays [VHAs], thromboelastography [TEG] and rotational thromboelastometry [ROTEM]) inevitably lead to uncontrollable hypercoagulopathy (76–78). The TEG/ROTEM evaluates the patient’s position along the hemostatic spectrum from hypo to hypercoagulable states. The tracings resemble a shovel, whereby the length of the handle reflects coagulation factor competence, the slope of the blade reflects fibrinogen concentration, the thickness of the blade reflects clot contraction mediated by fibrin-platelet interaction, and the tapering end of the blade represents fibrinolysis. Therefore, a characteristic early COVID-19 patient with a hypercoagulable state presents with a short handle, a steep slope, and a thick blade with no tapering of the blade, which represents enhanced effect of coagulation factors, increased fibrinogen concentration, increased fibrin/platelet function, and fibrinolytic shutdown. At the other end of the spectrum lies the hypocoagulable state as manifested by a long handle, flat slope, thin blade, with significant tapering. In Figure 3, examples of this paradigm are represented along various stages of this spectrum of coagulopathies associated with COVID-19.
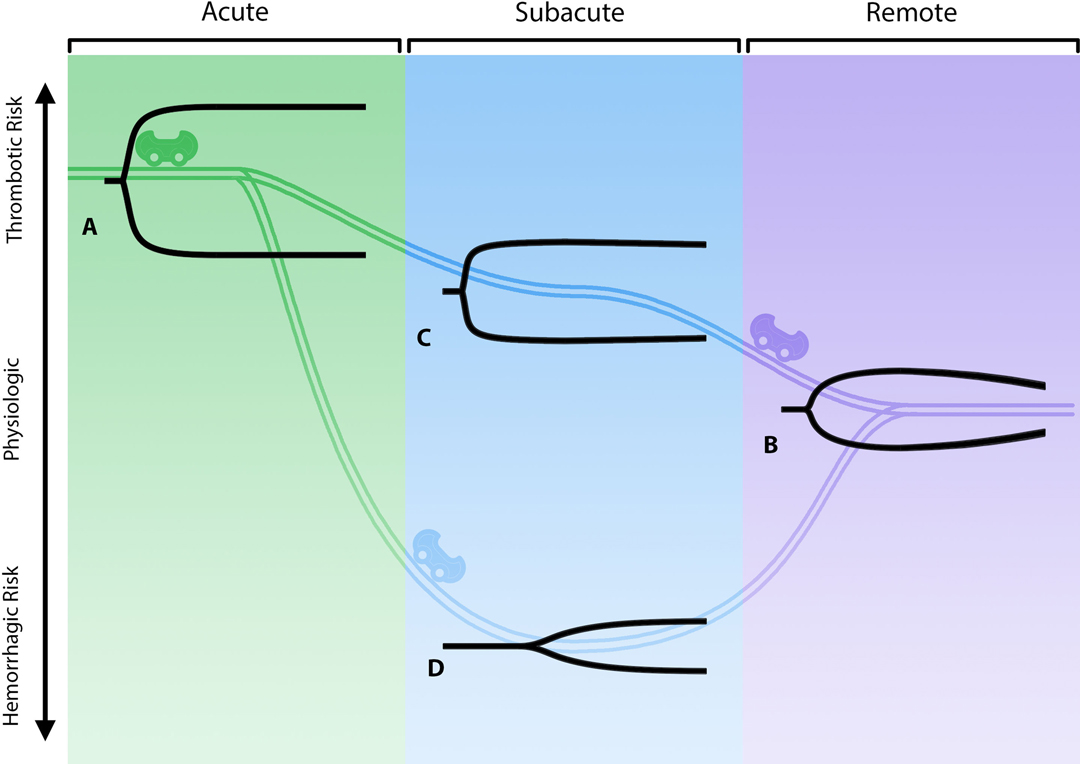
Figure 3. The “rollercoaster” phenomenon of COVID-19-associated coagulopathy. The three stages of COVID-19-associated coagulopathy and their relationship to the immuno-thrombotic derangement caused by the cytokine storm are represented above by viscoelastic hemostatic assays (e.g., thromboelastography [TEG]). (A) represents the acute fibrinolytic shutdown phenotype (short R, steep α, thick MA, no lysis). (B) represents the eventual evolution to a normal physiologic fibrinolytic phenotype (normal R, α, MA, and LY30). (C) represents the TEG in evolution without anticoagulation and the subacute recovery stage where the patient is less hypercoagulable (parameters intermediate between acute and remote). (D) represents the patient with or without anticoagulant with a hypocoagulopathic phenotype due to hypofibrinogenemia and/or a thrombocytopenia in spite of persistent fibrinolytic shutdown (long R, flat α, narrow MA, no lysis) (17, 20, 77, 79–81). Created using Adobe Illustrator.
The hypercoagulability of COVID-19 patients is clearly complex and multifactorial, but understanding these complex mechanisms allows for the pursuit of specific treatment strategies to improve patient outcomes. Management is complicated because of fibrinolytic shutdown due to the reduction of natural fibrinolysis at the level of the endothelium. While a multitude of studies have shown the detrimental effect of fibrinolytic shutdown on COVID-19 patient outcomes, bleeding complications such as gastrointestinal bleeding and hemorrhagic stroke are also common, especially in patients on therapeutic anticoagulation (17, 79, 80, 82–86). These patients tend to clot and bleed at the same time, a function of the “rollercoaster” phenomenon (77).
Even for those patients without comorbidities or illness requiring hospitalization, a large number of convalescent COVID-19 patients at four months post-infection are reported to have one or more residual organ system impairments as defined by quantitative magnetic resonance imaging (MRI) T1 mapping (12, 87). This phenomenon is termed Long COVID (or post-acute sequelae of COVID-19 [PASC]) and can involve any organ system (88). Hypercoagulability and VTE have been described as potential complications in Long COVID patients (88). In the convalescent COVID-19 patient, it is important to understand that the timing of surgery is a function of where the patient lies within the context of their individual immuno-thrombotic state (77).
Further research is necessary for determining potential biomarkers which may allow surgeons to weigh the risks and benefits of elective surgery. Multiple studies have shown persistently elevated D-dimer, fibrinogen, C-reactive protein (CRP), interleukin (IL)-4, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ-induced protein 10, factor VIII, VWF, and plasma soluble thrombomodulin in patients up to six months post-recovery from COVID-19 (81, 89–94), indicating that these may serve as potential biomarkers for assessing the risk of postoperative complications. Hypercoagulability markers are often associated with postoperative complications in non-COVID-19 perioperative settings (e.g., PAI-1, TAFI, increased fibrinogen and platelet counts) (7, 95–97). Research on these and other biomarkers in assessing postoperative morbidity and mortality in convalescent COVID-19 patients undergoing elective surgery, however, is lacking. Future studies are required in order to determine the most prognostic biomarkers for evaluating this patient population (88).
COVID-19 and the subsequent damage to endothelial cells result in a progression from hemostasis to a relatively prothrombotic/ antifibrinolytic state (31, 38, 43–45). Clinical data demonstrate significant thrombophilia with fibrinolysis shutdown being a primary component, regulated by higher circulating levels of antifibrinolytic factors, namely PAI-1 and TAFI. Yet, coagulation is a rigorously regulated process: fibrinolytic and thrombotic pathways are continuously competing against each other such that neither hemorrhage nor thrombosis is favored under normal physiological conditions (98). Therapeutic strategies for COVID-19 patients that utilize these findings, including the use of fibrinolytics, have been under examination and show potential for improving patient outcomes. For patients being treated with COVID-19, therapeutic anticoagulation is the standard regimen for patients with macrothrombosis. Furthermore, the use of anticoagulant heparinoids is advised for all COVID-19 patients (99). The use of tPa has been suggested for patients with severe ARDS caused by COVID-19 (100). In these studies, the use has been late in the disease course. Theoretically, if tPa is used earlier during the hypercoagulable stages of the disease, the fibrinolytic and therapeutic effect may be greater than those reported in the recent literature. Also, when administered later in the course of the disease—as the cytokine storm recedes—the patients may be prone to hemorrhage similar to anticoagulated COVID-19 patients (17, 101).
Therapeutic anticoagulation and thromboprophylaxis for the hospitalized COVID-19 surgical patient vary substantially. Recommendations have been made from adherence to standard VTE prophylactic guidance to intermediate or therapeutic dosing for moderately ill COVID-19 patients (85, 86). The preferred agents have been enoxaparin with anti-Xa monitoring or unfractionated heparin with either activated partial thromboplastin time (aPTT) or anti-Xa guidance. This heterogeneity is based on large multiplatform randomized controlled trials (RCTs) as well as society consensus statements. Recent guidelines and consensus statements have considered conditional support for therapeutic and/or intermediate heparinoid thromboprophylaxis for moderately ill COVID-19 patients not requiring high-flow oxygen (Table 1) (24–26, 85, 86, 99, 102–104). The importance of this foundational literature has been highlighted by observations that clinicians find it difficult to practice medicine during the COVID-19 pandemic bereft of RCTs to guide treatment (105). Therefore, reliance on categorization of hemostatic phenotype by VHAs fulfills the criteria of personalized-based medicine as a guidance for anticoagulation and thromboprophylaxis for the COVID-19 surgical patient.
For elective cases, a high incidence of patients discharged from the hospital have VTEs within 90 days of discharge from COVID-19 (16). In addition, a subsection of the population who have COVID-19 and have undergone surgery have a markedly elevated rate of VTEs (9). Because of the absence of specific RCTs recommending postoperative DVT prophylaxis in this group of patients, use of Elective Surgery Acuity Scale (ESAS) guidelines as well as co-morbidities and adjunctive VHA analysis would allow for postoperative DVT prophylaxis similar to that which has been recommended for the post-COVID discharged patient. Specifically, the recommendations vary from 10 mg rivaroxaban for 35 days, 2.5 mg apixaban b.i.d. for 35 days, or for patients with normal renal function, 40 mg enoxaparin for 35 days (102, 106, 107). Specific co-morbid analysis using conventional coagulation tests with adjunctive TEG-guided anticoagulation for COVID-19 patients with and without macrothrombosis has demonstrated the ability of the TEG to assist in the prediction of bleeding and clotting in this group of patients (17, 108). Similarly, the large RCTs which have demonstrated therapeutic benefit of heparinoids in moderately ill COVID-19 patients would be refined by the addition of VHAs to guide anticoagulant therapy (17, 85, 86, 109).
As has been shown, so-called “intermediate” and therapeutic anticoagulation has been found to protect patients with moderately severe COVID-19 pneumonitis (86). Surgeons will find themselves during the acute and subacute period considering emergent and semi-emergent procedures for patients on anticoagulation not just for known macrothromboses but also for the specific antiviral and antiinflammatory functions of heparinoids in these patients (85, 86, 109). In addition to conventional coagulation tests, anticoagulation for these patients is facilitated by adjunctive hemostatic monitoring with VHAs (17, 20–22).
The sequelae of Long COVID may be a function of residual endothelial damage from the SARS-CoV-2 endothelial cell invasion (88, 110). As such, thromboprophylaxis for postoperative convalescent COVID-19 patients should draw from strategies to manage acute COVID-19 patients along with the strategies for non-COVID-19 patients undergoing elective surgery.
Surgeons—because of their more than half century experience with TEG/ROTEM to diagnose and treat coagulopathies in liver transplantation, cardiac surgery, and trauma—have been at the forefront of pathophysiologic investigations of CAC (111). Because of surgeons’ historical experience on the intricacies of hemostasis, surgeons have employed TEG/ROTEM to guide and personalize anticoagulation during the COVID-19 pandemic (20–22, 28, 29, 101, 112). TEG/ROTEM have also been used to profile postoperative patients at risk for VTE (23, 113–116). This background in using TEG/ROTEM to predict a patient’s fibrinolytic phenotype prepares the surgeon to manage CAC patients. Given the significant variation in risk of the development of either a hypocoagulopathic- or hypercoagulopathic-related complication in the perioperative period as a function of the severity of COVID-19 illness, it is difficult to rely on arbitrary standards regarding the timing of surgery and the nature of anticoagulation and thromboprophylaxis in this group of patients (17, 28, 29, 31, 108).
The recent evolution of the less virulent but more infectious variants, such as the omicron (B.1.1.529), may render guidelines regarding the timing and nature of therapeutic anticoagulation and thromboprophylaxis for the surgical patient less applicable. Although there is no data directly demonstrating lower risk of thrombosis in patients infected with omicron versus other more virulent variants, thrombosis risk in COVID-19 patients is in large part a function of illness severity (117, 118). By logical extension of this evidence, it is likely omicron infection carries less thrombotic risk compared to more virulent variants such as alpha or delta. Age, co-morbidities, and overall clinical picture are paramount to consider when deciding to what degree the individual patient should be anticoagulated. There are no plasma-based laboratory tests which allow for prediction of the likelihood of perioperative complications regarding coagulation for the surgical patient with either acute or remote COVID-19 infection. Historically, VHAs have been used to predict both bleeding and clotting in patients undergoing liver transplantation, cardiac surgery, trauma surgery, and most recently, bleeding and clotting obstetric patients. Most recently, adjunctive TEG has demonstrated prediction of VTE in critically ill COVID-19 patients (20–23). However, categorizing patients’ fibrinolytic and coagulopathic phenotype with TEG/ROTEM, as has been done by surgeons for decades, may allow the surgeon to better predict the development of perioperative clot and to manage pre- and post-surgical clots more effectively in the COVID-19 patient. In addition, platelet activation can be graded with plasma microclot density analysis, which has demonstrated that Long COVID patients have a persistent coagulopathy and increased microclot formation (119). Together, TEG/ROTEM with microclot analysis may predict the surgical patient’s position in the hemostatic and fibrinolysis spectrum in the absence of evidenced biomarkers. Figure 3 illustrates the ‘rollercoaster’ phenomenon of hospitalized COVID-19 patients as characterized by TEG/ROTEM in the acute and subacute periods, as well as the remote period after discharge (77). Figure 4 provides a prototype spectrum of CAC wherein the patient’s individual coagulopathy may be modified by the COVID-19 variant and is characterized by TEG/ROTEM and plasma microclot analysis. Studies are in progress regarding microclot analysis as an enhanced method of hemostatic grading (78).
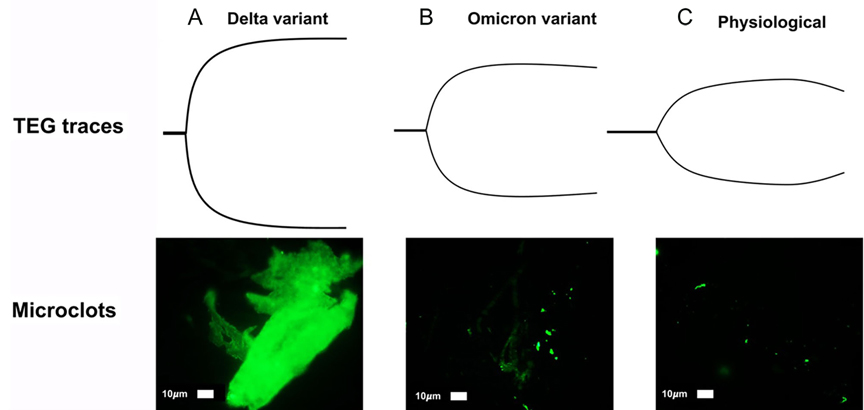
Figure 4. Prototype spectrum of COVID-19-associated coagulopathy used to assess the patient’s coagulopathy with thromboelastography (TEG) and plasma microclot analysis. Shown above is an example of correlation between the most hypercoagulable delta (B.1.617.1) variant, intermediate hypercoagulable omicron (B.1.1.529) variant, and physiologic TEGs with corresponding plasma microclot analyses. Using Thioflavin-T as a fluorescent marker specifically for microclot staining, fluorescence microscopy demonstrates microclots in plasma with representative examples of different degrees of microclot formation as related to delta (A), omicron (B), and physiologic (C) TEGs. Previously described, Stage 1 to 4 is a qualitative numerical scoring system where a score of 4 is given for significant and widespread microclot formation and a score of 1 is given for minimal microclot formation (78, 119). In this figure, delta variant (A) equals stage 4, omicron (non-hospitalized patient) (B) is stage 3, normal physiologic non-infected individual (C) is stage 2. A hypocoagulable state either from anticoagulation of the COVID-19 patient or due to naturally acquired disease from COVID-19-associated coagulopathy is stage 1 (not pictured). Patients with Long COVID also demonstrate microclot formation, which may be used to assess platelet dysfunction and surgical candidacy in this group of patients (77, 78, 119). The omicron variant (BA.1 sub-lineage) was detected by polymerase chain reaction (PCR) based on S-gene target failure (SGTF) as a proxy for variant status (https://www.science.org/doi/10.1126/science.abn4543) (120). The TaqPath COVID-19 Combo Kit® (Thermo Fisher Scientific) identifies SARS-CoV-2 infections by detecting 3 viral gene regions, E, RdRP, and N gene. The laboratory reported positive cycle threshold of 32 cycles for both the E gene, 35 for RdRP and 34 for the N gene. Positive N gene with negative S gene (SGTF) is an acceptable proxy of the BA.1 variant when the gold-standard whole genome sequencing is not done. The PCR result combined with reported case counts and known genomic epidemiology of the 4th wave in South Africa has been used to track changes in transmission over time for the BA.1 variant (121, 122).
Emergency surgery in the acute period of COVID-19 infection requiring hospitalization is associated with high mortality (8, 123). These increases in mortality have been observed across various surgical specialties (123). As a result, there are surgical specialty-specific recommendations regarding the timing of surgery for patients with symptomatic and asymptomatic SARS-CoV-2 infection. These recommendations are varied and a function of institutional preference and expert consensus. Table 2 and the text below summarizes the literature and recommendations of the leading surgical and obstetric organizations regarding the timing and administration of thromboprophylaxis for patients with acute and remote SARS-CoV-2 infection.
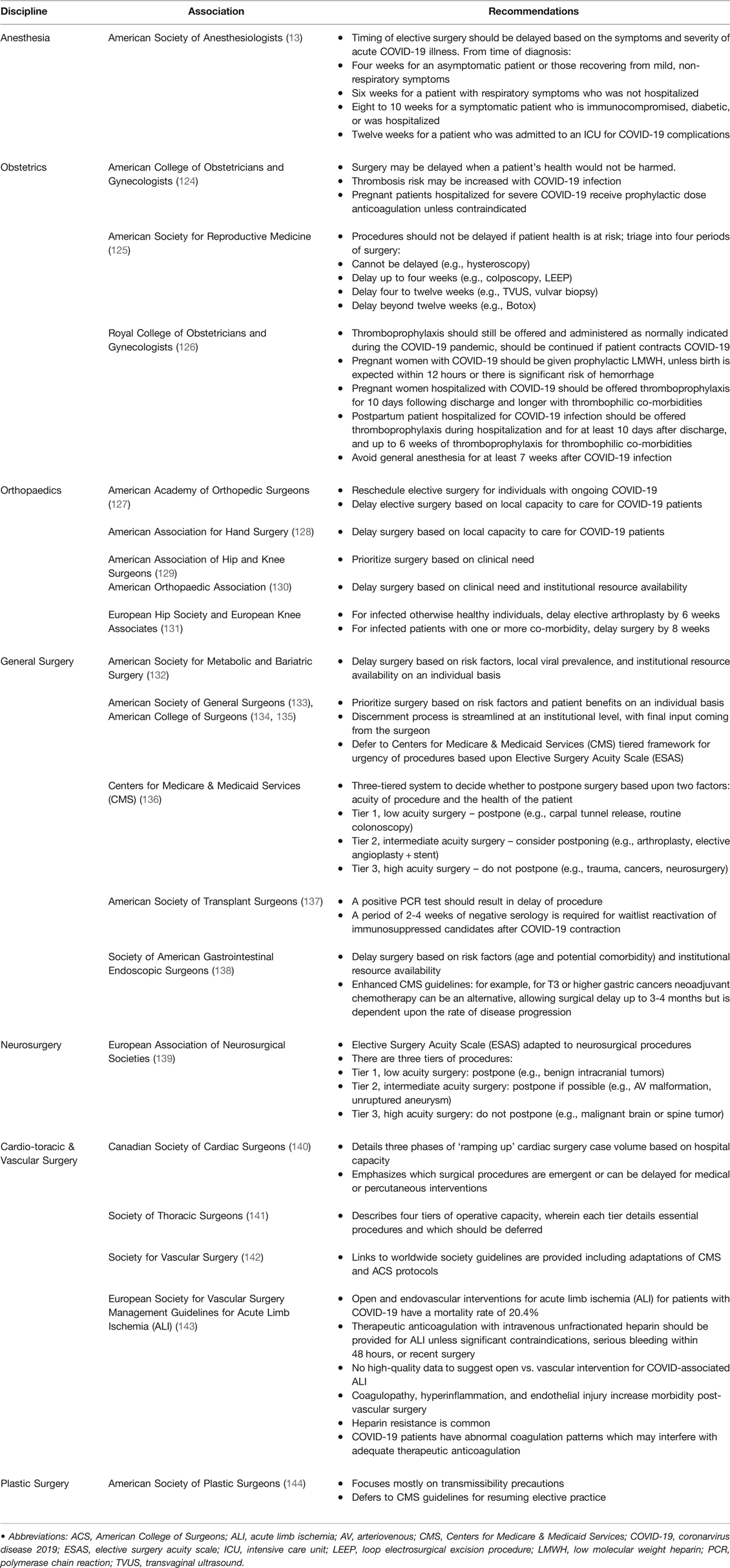
Table 2. Society recommendations on timing of surgery and thromboprophylaxis in acute and convalescent COVID-19 patients. Note this table is not exhaustive, but reflects the diverse and heterogeneous recommendations regarding the timing of surgery and anticoagulation for this patient population.
Obstetricians have long experience with surgical interventions on patients who have peripartum coagulopathies wherein the patient may switch between hemorrhagic and thrombotic phenotypes rapidly. Obstetricians have similar experience as CAC with pathologies such as preeclampsia, Hemolysis, Elevated Liver enzymes, and Low Platelets (HELLP) syndrome, morbidly adherent placenta (MAP) and other significant diseases associated with the peripartum state (145–156). This spectrum of disorders in the peripartum patient allow pathophysiological comparison to the hemostatic derangement of CAC in the nonpregnant surgical patient (29, 157). In each disease entity, there is endotheliitis that results in an immuno-thrombotic crosstalk resulting in local thrombosis and/or hemorrhage at the level of the inflamed endothelium (17, 157). For example, prepartum patients with preeclampsia must be given thromboprophylaxis to prevent clotting, but during parturition, they are at higher risk for bleeding, and again return to a hypercoagulable state postpartum (157). In tandem, postpartum patients with preeclampsia or other underlying risk factors for a hypercoagulable state should be treated for at least six weeks with thromboprophylaxis postpartum (146). Hence, discussion of the obstetrical approaches to providing thromboprophylaxis serves as an ideal foundation for describing the other surgical disciplines with more straightforward pathophysiological proclivities for the formation of perioperative clots. International guidelines for thromboprophylaxis for women who have undergone a C-section are heterogeneous in their recommendations regarding the timing and duration of thromboprophylaxis (146, 158).
In the United States and other countries, thromboprophylaxis for women undergoing C-sections has been described by the American College of Obstetricians & Gynaecologists guidelines that recommend thromboprophylaxis for all patients with C-sections who have at least one major indication or two minor indications for anticoagulation (159). The three major indications are previous VTE, body mass index (BMI) > 35, and thrombophilia. The minor indications are preeclampsia, hypertension, diabetes, peripartum infection, and other comorbidities (145, 159–163). Since guidelines are dynamic, the inclusion of COVID-19 as a minor indication seems reasonable. However, it would also be judicious to refer to the spectrum of diseases whereby women with severe COVID-19 are included in the group of patients with major indications for anticoagulation such that they would receive six weeks thromboprophylaxis. This paradigm has been useful in the most recent literature and serves as a template for applications to the other disciplines.
Since there is sparse literature regarding the guidance of thromboprophylaxis and treatment of the peripartum COVID-19 patient, surgeons must rely on consensus recommendations from interim guidelines. These recommendations for peripartum anticoagulation and thromboprophylaxis have been extrapolated from the COVID-19 literature for non-pregnant patients. Interim guidelines have been published by many organizations with specific references to pregnant women (164–166).
Similar to obstetric patients, there is a spectrum of risk for developing postoperative VTE as a function of COVID-19 illness severity in patients undergoing orthopaedic surgery. Significant literature has demonstrated the benefit of serial VHAs to predict postoperative VTE (167–169). Therefore, for a disease which, by its very nature presents with a hypercoagulopathic state manifested clinically by increased VTEs and arterial thromboses, the use of VHAs in this COVID-19 population is a logical extension. While consensus recommendations have been proposed to optimize safety for patients undergoing elective surgery after previously being diagnosed with COVID-19, adjunctive VHAs may enhance these guidelines to better predict VTE and guide anticoagulant therapy postoperatively (131, 170–172).
Early intervention tends to be optimal for fracture fixation, but timing of surgery is historically complicated in patients with hemostatic derangement such as that which is induced in the post-COVID-19 patient. Decreased time between hip fracture and surgery correlates with decreased morbidity and mortality. However, correct timing of surgery in patients diagnosed with COVID-19 is difficult to assess (173). As an example of the heterogeneity of recommendations, it has been noted that a delay in fracture fixation of up to three months postinjury is acceptable, allowing for the risks of delayed surgery to be weighed against the risks of early fracture fixation performed during early post-COVID-19 prognosis (170).
Therefore, it is generally recommended that clinicians address each post-COVID-19 case individually and tailor a unique treatment plan for each patient based on their severity of injury and COVID-19 prognosis (172, 173). Patients waiting for elective orthopaedic surgery need to be reassessed in orthopaedic pathology, COVID-19 prognosis, surgical fitness, and desire to undergo the procedure with regards to evolving risks and benefits. Robust appraisal during the preoperative period to evaluate fitness for surgery is necessary to mitigate the risk of postoperative complications and decrease strain on postoperative intensive care units (170).
Specific orthopaedic society recommendations are outlined in Table 2. The American Association for Hand Surgery suggests delaying surgery based on the capacity to care for COVID-19 patients rather than postoperative prognosis (128). The American Orthopaedic Association offers broad suggestions regarding delay of surgery based on clinical need and institutional availability (130). Most of these orthopedic organizations do not have specific guidelines for delaying surgery to reduce the incidence of VTEs. However, the European Hip Society and European Knee Associates recommend delaying arthroplasty for six weeks following infection for otherwise healthy individuals, and for infected individuals with one or more comorbidities delaying the surgery for at least 8 weeks (131). When compared to anesthesia and non-orthopaedic society recommendations, the recommendations from European societies seem the most judicious provided the well-recognized increases in mortality and VTE complications associated with post-COVID-19 elective surgeries (13, 134). An example ESAS for delaying procedures is provided in Table 3.
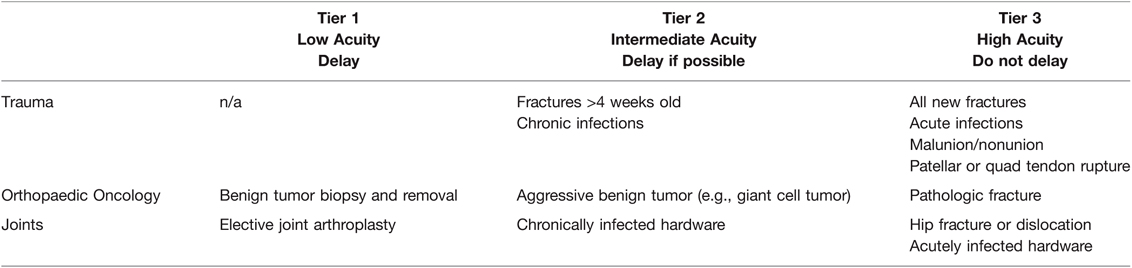
Table 3. Orthopaedics Elective Surgery Acuity Scale (ESAS). All candidates for surgery should be assessed for surgical fitness on an individual basis. This is an example ESAS and not comprehensive. Procedure acuity for the individual patient may shift among tiers based upon acuity of illness, severity of COVID-19 infection and coagulopathy, and hospital surgical capacity (135). For specific length of operative delay, please see the text and Association of Anesthesiologists guidelines (13).
The COVID-19 pandemic resulted in a significant reduction of general surgical procedures, including procedures for both benign and malignant diseases. While elective surgery for benign disease was intentionally halted during the pandemic, the reduction in procedures for malignancies was likely an unintentional consequence of reduced patient access to clinical examinations and diagnostic testing as a result of limited capacity of healthcare systems (174).
At the beginning of the pandemic, it was recommended that emergent cases be individualized based on patient risk, urgent cases be delayed at least until COVID-19 infection clears, and elective cases not be performed and potentially delayed for months (175). Emergency surgery was performed on patients regardless of their COVID-19 infection status.
The ACS and the American Society of General Surgeons initially published guidelines based on risk factors and patient benefits related to SARS-CoV-2 transmissibility during acute infection. These guidelines are heterogeneous and do not specifically address hemostatic complications related to elective and acute surgery during acute and convalescent COVID-19 (133, 134). These two associations adopted the CMS 3-tiered framework for urgency of procedures based on the ESAS (134, 136). This three-tiered system is defined by Tier 1, elective surgery which is to be postponed; Tier 2, intermediate acuity surgery postpone if possible; and Tier 3, indications for acute surgery where postponement is not an option (136). Table 4 specifically defines example surgical procedures based on this three-tiered system.
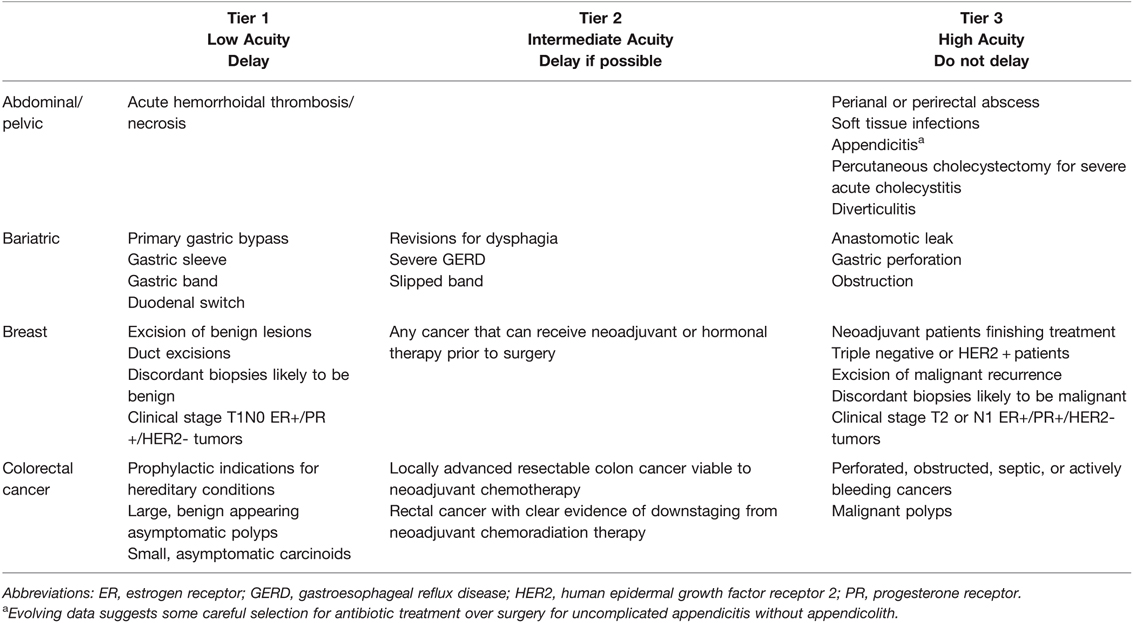
Table 4. General Surgery Elective Surgery Acuity Scale (ESAS). All candidates for surgery should be assessed for surgical fitness on an individual basis. This is an example ESAS and not comprehensive. Procedure acuity for the individual patient may shift among tiers based upon acuity of illness, severity of COVID-19 infection and coagulopathy, and hospital surgical capacity (134–136). For specific length of operative delay, please see the text and Association of Anesthesiologists guidelines (13).
With regards to transplant surgery, COVID-19 infection following organ implantation is associated with increased mortality (176, 177). Pre-vaccination prior to transplant has been suggested to reduce the risk of mortality (178). In the context of receiving an organ from a COVID-19 positive donor, there is growing evidence that viral transmission from abdominal organs is a nearly negligible risk (179). This has led to a call for increased use of COVID-19 positive donors without evidence of systemic disease, as the low risk of transmission does not appear to outweigh the potential lifesaving benefit from organ transplantation (180). However, a positive PCR test in a recipient should delay the procedure, and a delay of at least two to four weeks of negative serologies is required for waitlist reactivation of immunosuppressed candidates after COVID-19 contraction.
The Society of American Gastrointestinal Endoscopic Surgeons has provided specific guidelines for gastrointestinal cancers similar to the ESAS (138). A large study of over four million cancer patients found that most oncological procedures can be delayed by four weeks without a significant impact on patient morbidity or mortality (181, 182). However, a patient’s age and comorbidities are critical for weighing the relative benefit and risk of potentially exposing the patient to COVID-19 as opposed to choosing alternative options. The resources available to the surgeon must also be considered, since the pandemic has caused large fluctuations in patient acuity, volume, and hospital resources (183). In COVID-19 positive patients with primary or secondary cancer, elective liver or adrenal resection should be delayed until patients fully recover from acute COVID-19. In cases of jaundice or infection, percutaneous transhepatic biliary drainage (PTBD) or endoscopic retrograde cholangiopancreatography (ERCP) should be first implemented as a bridge therapy (184).
The literature concerning timing of neurosurgical procedures has mostly considered SARS-CoV-2 transmissibility and healthcare worker safety (185–188). For example, it has been demonstrated that tracheostomies may be safely delayed two weeks, whereas craniectomy and tumor resections, as well as evacuation of subdural hematomas, need to be evaluated on a case-by-case basis (188, 189). Surgical treatment of chronic subdural hematoma, a usually successful intervention with a reported mortality rate of 3.7%, has a markedly increased mortality risk in COVID-19 positive surgical patients (190). Further investigation attributes multiple factors to this mortality rate, including thrombocytopenia, immune system impairment, and interstitial pneumonia progression post-surgery (191).
Like other surgical societies, there is heavy reliance on the modified ESAS for determining the timing of neurosurgical procedures for COVID (139). A similar 3-tiered system has been proposed regarding low, intermediate, and high acuity indications for neurosurgical intervention. Examples of surgeries that are divided into these three tiers are Tier 1, benign intracranial tumors which are low acuity; Tier 2, unruptured cerebral aneurysms and AV malformations as intermediate acuity; and Tier 3, malignant brain and spinal tumors for high acuity surgery where postponement is not an option (139). Table 2 and 5 summarizes current neurosurgical guidelines regarding the timing of surgery for COVID-19 patients.
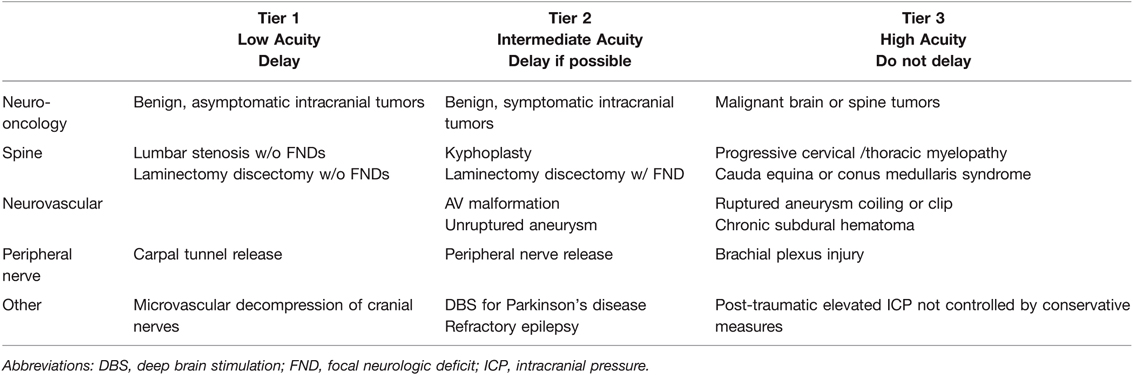
Table 5. Neurosurgery Elective Surgery Acuity Scale (ESAS). All candidates for surgery should be assessed for surgical fitness on an individual basis. This is an example ESAS and not comprehensive. Procedure acuity for the individual patient may shift among tiers based upon acuity of illness, severity of COVID-19 infection and coagulopathy, and hospital surgical capacity (135, 139). For specific length of operative delay, please see the text and Association of Anesthesiologists guidelines (13).
Cardiothoracic and vascular surgery society guidelines have likewise adapted the CMS recommendations as well as ESAS guidelines for determining the timing of surgery during the COVID-19 pandemic (140, 141). The cardiothoracic guidelines have also benefited from the cardiology literature, which recommends adherence to standard anticoagulation procedures for patients with post-acute sequelae of COVID-19 who are undergoing percutaneous coronary intervention with or without angioplasty for ST segment elevation myocardial infarcts (192). During the acute period of COVID-19 infection, adherence to standard heparin boluses, anti-platelet, and intravenous and oral anti-factor agents is recommended without any change (193). For those patients whose intervention is complicated by a hypercoagulable state, treatment should be titrated with a personalized medicine approach and the use of VHAs to guide anticoagulation has been suggested (194–196). Acutely ill patients with severe coronary artery disease and valvular pathology not amenable to non-surgical interventions can either be operated immediately or temporized with a left ventricular assist device, intra-aortic balloon pump, or extracorporeal membrane oxygenation while waiting for the cytokine storm to abate if clinically possible (197). These guidelines are heterogeneous and likewise founded on the ASA and ACS three-tiered system of classifying patients with COVID-19 who require surgery (13, 134).
Provided the protracted nature of this pandemic, cardiac surgery programs must continue to proactively manage every patient on their waitlist with re-expansion of case volumes. Aspects of this management have been divided into three separate tiers of so-called ‘ramp up’ levels which determine the timing of surgery based on a combination of return to surgical case volume and acuity of indication for cardiothoracic surgery (141, 198).
The Canadian Cardiothoracic Society has described the following criteria: For ramp up phase one where the surgical volume is increased 0-25%, the patients who should avoid surgery when possible include frail elderly patients (clinical frailty score > 4) and vulnerable co-morbid patients including those with renal insufficiency, poor ejection fractions, and advanced congestive heart failure. Overall, in a phase one scenario, the cardiothoracic surgeon should attempt to avoid complex procedures including re-operative surgery while prioritizing CABG, isolated valve procedures, and less complex procedures to maximize patient flow. There should be an emphasis on patients with aortic stenosis and coronary artery disease with prognostic benefit, such as critical aortic stenosis and left main coronary disease. In the ramp up phase two, where surgical volume is increased 25-50%, inpatient urgent and emergency surgeries should be continued with broadening inclusion of appropriately prioritized outpatients. Ramp up phase three occurs with 50-100% increase in capacity with a return to normal outpatient services while continuing to prioritize those as greatest risk such as asymptomatic and severe mitral regurgitation, atrial septal defect or patent foramen ovale surgery, and asymptomatic aneurysm with demonstrated stable size.
In the thoracic surgery literature, a four-tier system of patient triage has been recommended by The Society of Thoracic Surgeons COVID-19 Task Force and the Workforce for Adult Cardiac and Vascular Surgery. For consistency here, we provide a three-tiered ESAS in Table 6.
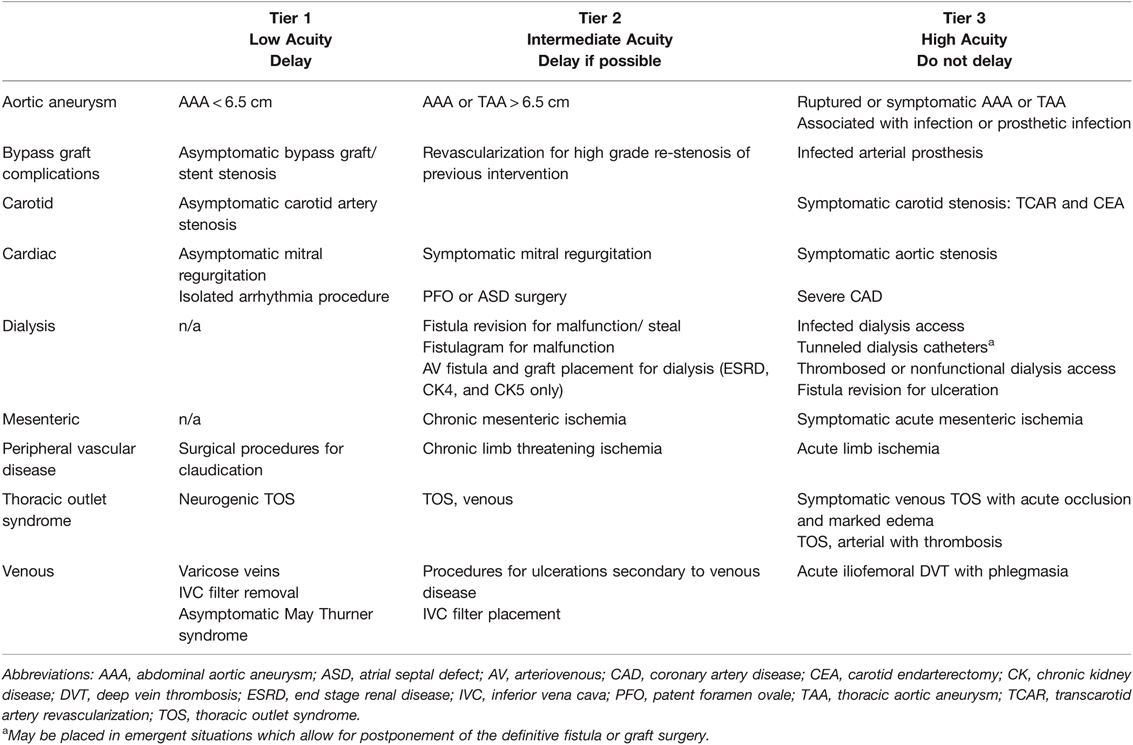
Table 6. Cardiothoracic & Vascular Elective Surgery Acuity Scale (ESAS). All candidates for surgery should be assessed for surgical fitness on an individual basis. This is an example ESAS and not comprehensive. Procedure acuity for the individual patient may shift among tiers based upon acuity of illness, severity of COVID-19 infection and coagulopathy, and hospital surgical capacity (135, 140, 141). For specific length of operative delay, please see the text and Association of Anesthesiologists guidelines (13).
Systemic anticoagulation with intravenous unfractionated heparin should be provided for all patients with acute limb ischemia (ALI). The performance of endovascular or open procedures for ALI is a function of the acuity of the limb ischemia and its relation to the hemostatic derangement present in the COVID-19 patient. There is no high-quality data to suggest that either open or vascular intervention is the preferred treatment ALI. It has been noted that a heparin resistance is in acute COVID-19 illness is common, while at the same time, the capricious nature of the cytokine storm causes the COVID-19 patient to have abnormal coagulation patterns. This interference with therapeutic anticoagulation requires frequent monitoring of not only aPTT and anti-Xa levels, but also with VHAs (17, 20–23, 108).
Similar guidelines based on the CMS and ESAS criteria have been adapted to the oculo/plastic and reconstructive procedures (199, 200). These guidelines are based on the three-tiered system which also incorporates the supply and demand for elective and acute surgeries and the acuity of the surgical intervention required. Of all specialties, the oculoplastic international societies have the most exhaustive list of procedures incorporated within the context of the three-tiered system since most of their surgeries are elective (201). These are summarized in Table 7 below.
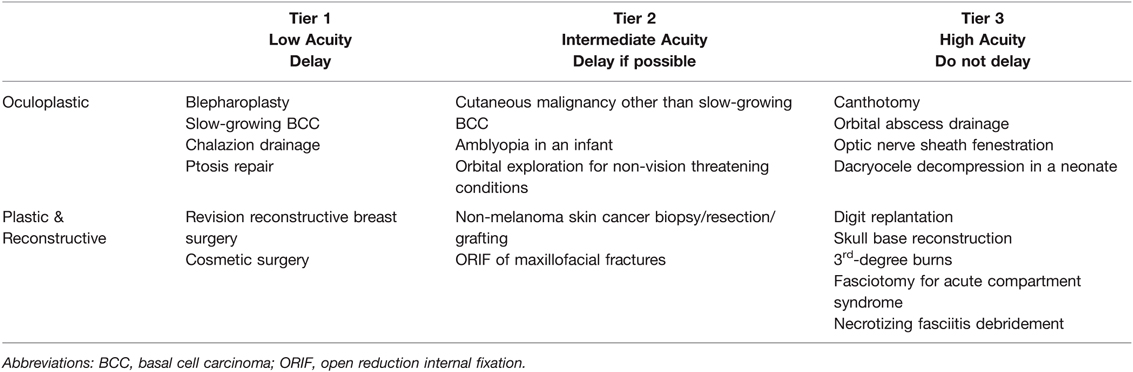
Table 7. Plastic, Oculoplastic, and Reconstructive Elective Surgery Acuity Scale (ESAS). All candidates for surgery should be assessed for surgical fitness on an individual basis. This is an example ESAS and not comprehensive. Procedure acuity for the individual patient may shift among tiers based upon acuity of illness, severity of COVID-19 infection and coagulopathy, and hospital surgical capacity (199–201). For specific length of operative delay, please see the text and Association of Anesthesiologists guidelines (13).
Of particular importance for this group of patients is the viability of microvascular flaps which, because of the hypercoagulability mediated by the immuno-thrombotic derangements that are part of CAC, require evaluation by the surgeon for the timing of surgery and the risk of ischemic flap loss. For example, criteria for the administration of continuous unfractionated heparin have been suggested for microvascular free flaps after head and neck cancer excision (202, 203). Recent publications have demonstrated the use of VHAs in guiding anticoagulation therapy to maintain vascular patency of microvascular vessels in flap surgery and reconstruction (204).
The pathophysiological derangements of patients infected with SARS-CoV-2 require an understanding of the fundamental endotheliitis and immunothrombotic crosstalk causing fibrinolytic shutdown. For the surgeon who must confront an acutely ill patient with COVID-19, important decisions must be made regarding not just the timing of surgery, but also the nature of anticipating the thrombohemorrhagic complications of CAC and whether the patients are fully or prophylactically anticoagulated (205). For those patients who require elective surgery, a large study suggests a delay of up to seven weeks for elective surgery following SARS-CoV-2 infection, a guideline which has recently been adopted by specialty-specific societies (10). Yet, other studies have found abnormal hypercoagulability biomarkers up to six months after COVID-19 recovery, indicating potentially increased risk for some patients past seven weeks (81, 89–93). Refined use of these biomarkers for risk-benefit analysis can help to determine the most favorable time for surgery (7). In regard to the administration of thromboprophylaxis and full anticoagulation in the convalescent COVID-19 postoperative patient, certain biomarkers can potentially be used in a personalized-based medicine approach (77). More studies are needed to analyze biomarkers and physiologic derangements in COVID-19 recovery patients focusing on how these profiles forecast postoperative outcomes. VHAs have historically been used to profile postoperative patients at risk for VTE, and may help guide surgeons with timing of elective surgery, thromboprophylaxis, and full anticoagulation in this patient population (113–116). As the pandemic evolves, one can anticipate changing guidelines that may vary based on the COVID variant with accruing data on managing elective and acute surgery in this unique group of patients.
Ethical clearance for microclot analysis was obtained from the Health Research Ethics Committee (HREC) of Stellenbosch University (South Africa): N19/03/043, project ID 9521. Confirmation of omicron variant: N20/04/008_COVID-19. Ethics approval for the inclusion of the microclot micrographs in this review paper was received from the Ethics committee of Stellenbosch University (HREC2-2022-24525).
Conceptualization, CMB, EEM, HBM, AVT, NZ, DF, WH, RM, BSB, and MMW; Writing – Original Draft Preparation, CMB, AVT, NZ, JZ, SZ, TJB, MB, HB, RM, BSB, and MMW; Writing – Review & Editing, CMB, EEM, HBM, MDN, AVT, NZ, JZ, SZ, TJB, MB, HB, GW, DF, WH, HCK, GL, GJL, PJL, EP, MJK, MSM, SS, TGM, DBK, MDF, LG, RZK, CNM, RM, RM, BSB, and MMW; Visualization, AVT, MMW; Supervision, AVT, MMW; Project Administration, CMB, AVT, NZ, and MMW; Funding Acquisition, MMW, EP. Patient recruitment, fluorescent microscopic and viral sequencing analysis, GJL, PJL, EP, MJK, MSM, SS, TGM, DBK. All authors contributed to the article and approved the submitted version.
DBK: Novo Nordisk Foundation for support (grant NNF20CC0035580) (Project Code 96825). MJK and TGM: Research reported this article was supported by the South African Medical Research Council with funds received from the Department of Science and Innovation (Project Code 96825). The content and findings reported and illustrated are the sole deduction, view and responsibility of the researchers and do not reflect the official position and sentiments of the funders.
EEM: Research support from Haemonetics, Instrumentation Laboratory, Hemosonics, Stago, and Diapharma. HBM: Research support from Haemonetics and Instumentation Laboratory. MJK is a non-executive director and shareholder of Gknowmix (Pty) Ltd. EP is the managing director of BioCODE Technologies. MMW is on the speaker’s bureau for AstraZeneca.
In honor of the late Michael P. Chapman MD; mentor, colleague, and friend. We thank Dr. Arneaux Kruger for the blood sample collection of the patient diagnosed with Omicron.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mattingly AS, Rose L, Eddington HS, Trickey AW, Cullen MR, Morris AM, et al. Trends in US Surgical Procedures and Health Care System Response to Policies Curtailing Elective Surgical Operations During the COVID-19 Pandemic. JAMA Netw Open. (2021) 4(12):e2138038. doi: 10.1001/jamanetworkopen.2021.38038
2. Collaborative, Irish Surgical Research, Italian Society of Colorectal Surgery, Association of Surgeons in Training, and Transatlantic Australasian Retroperitoneal Sarcoma Working Group. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. (2021) 22(11):1507–17. doi: 10.1016/S1470-2045(21)00493-9
3. American College of Surgeons. COVID-19: recommendations for management of elective surgical procedures. Chicago: ACOS (2020).
4. American College of Surgeons. COVID-19: elective case triage guidelines for surgical care. Chicago: ACOS (2020).
5. Diaz A, Sarac BA, Schoenbrunner AR, Janis JE, Pawlik TM. Elective surgery in the time of COVID-19. Am J Surg. (2020) 219(6):900–2. doi: 10.1016/j.amjsurg.2020.04.014
6. American College of Surgeons. Joint statement: roadmap for resuming elective surgery after COVID-19 pandemic. Chicago: ACOS (2020).
7. Silvapulle E, Johnson D, Darvall JN. Risk stratification of individuals undergoing surgery after COVID-19 recovery. Br J Anaesth. (2021) 128(1):e37–9. doi: 10.1016/j.bja.2021.09.026
8. Nepogodiev D, Bhangu A, Glasbey JC, Li E, Omar OM, Simoes JF, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. (2020) 396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X
9. Reuben Abel J, Andreani L, Aprile V, Balestri R, Benettini G, Berrettini S, et al. SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia. (2022) 77(1):28–39. doi: 10.1111/anae.15563
10. COVIDSurg Collaborative, GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. (2021) 76(6):748–58. doi: 10.1111/anae.15458
11. El-Boghdadly K, Cook TM, Goodacre T, Kua J, Blake L, Denmark S, et al. SARS-CoV-2 infection, COVID-19 and timing of elective surgery: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. Anaesthesia. (2021) 76(7):940–6. doi: 10.1111/anae.15464
12. Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open. (2021) 4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568
13. ASA and ASPF Joint Statement on Elective Surgery and Anesthesia for Patients after COVID-19 Infection: American Society of Anesthesiologists and Anesthesia Patient Safety Foundation; 2021. Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2021/03/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection-rv [updated March 9, 2021].
14. Bui N, Coetzer M, Schenning KJ, O'Glasser AY. Preparing previously COVID-19-positive patients for elective surgery: a framework for preoperative evaluation. Perioper Med (Lond). (2021) 10(1):1. doi: 10.1186/s13741-020-00172-2
15. Mankarious M, Massand S, Potochny J. Considerations for Elective Surgery in the Post-COVID-19 Patient. Aesthet Surg J. (2021) 41(10):Np1347–8. doi: 10.1093/asj/sjab214
16. Giannis D, Allen SL, Tsang J, Flint S, Pinhasov T, Williams S, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. (2021) 137(20):2838–47. doi: 10.1182/blood.2020010529
17. Bunch CM, Thomas AV, Stillson JE, Gillespie L, Khan RZ, Zackariya N, et al. Preventing Thrombohemorrhagic Complications of Heparinized COVID-19 Patients Using Adjunctive Thromboelastography: A Retrospective Study. J Clin Med. (2021) 10(14):3097. doi: 10.3390/jcm10143097
18. Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. (2020) 69(30):993–8. doi: 10.15585/mmwr.mm6930e1
19. Carfì A, Bernabei R, Landi F. Persistent Symptoms in Patients After Acute COVID-19. JAMA. (2020) 324(6):603–5. doi: 10.1001/jama.2020.12603
20. Bareille M, Hardy M, Douxfils J, Roullet S, Lasne D, Levy JH, et al. Viscoelastometric Testing to Assess Hemostasis of COVID-19: A Systematic Review. J Clin Med. (2021) 10(8):1740. doi: 10.3390/jcm10081740
21. Hartmann J, Ergang A, Mason D, Dias JD. The Role of TEG Analysis in Patients with COVID-19-Associated Coagulopathy: A Systematic Review. Diagnostics. (2021) 11(2):172. doi: 10.3390/diagnostics11020172
22. Görlinger K, Almutawah H, Almutawaa F, Alwabari M, Alsultan Z, Almajed J, et al. The role of rotational thromboelastometry during the COVID-19 pandemic: a narrative review. Korean J Anesthesiol. (2021) 74(2):91. doi: 10.4097/kja.21006
23. Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA network open. (2020) 3(6):e2011192. doi: 10.1001/jamanetworkopen.2020.11192
24. Kreuziger LB, Lee AYY, Garcia D, DeSancho M, Connors JM. COVID-19 and VTE/Anticoagulation: Frequently Asked Questions American Society of Hematology website: American Society of Hematology; 2022. Available at: https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation [updated February 2, 2022].
25. Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Thromboprophylaxis in Patients with COVID-19. A Brief Update to the CHEST Guideline and Expert Panel Report. Chest. (2022) 12:S0012-3692(22)00250-1. doi: 10.1016/j.chest.2022.02.006
26. National Institutes of Health. Therapeutic Management of Hospitalized Adults With COVID-19 National Institutes of Health; 2022. Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults–therapeutic-management/?utm_source=site&utm_medium=home&utm_campaign=highlights [updated February 24, 2022].
27. Tu TM, Yi SJ, Koh JS, Saffari SE, Hoe RHM, Chen GJ, et al. Incidence of Cerebral Venous Thrombosis Following SARS-CoV-2 Infection vs mRNA SARS-CoV-2 Vaccination in Singapore. JAMA Netw Open. (2022) 5(3):e222940. doi: 10.1001/jamanetworkopen.2022.2940
28. Walsh MM, Khan R, Kwaan HC, Neal MD. Fibrinolysis Shutdown in COVID-19-Associated Coagulopathy: A Crosstalk among Immunity, Coagulation, and Specialists in Medicine and Surgery. J Am Coll Surg. (2021) 232(6):1003–1006. doi: 10.1016/j.jamcollsurg.2021.03.003
29. Meizoso JP, Moore HB, Moore EE. Fibrinolysis shutdown in COVID-19: clinical manifestations, molecular mechanisms, and therapeutic implications. J Am Coll Surg. (2021) 232(6):995–1003. doi: 10.1016/j.jamcollsurg.2021.02.019
30. Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circulation research. (2016) 118(9):1392–408. doi: 10.1161/CIRCRESAHA.116.306853
31. Kwaan HC, Lindholm PF. The Central Role of Fibrinolytic Response in COVID-19-A Hematologist's Perspective. Int J Mol Sci. (2021) 22(3):1283. doi: 10.3390/ijms22031283
32. Jayarangaiah A, Kariyanna PT, Chen X, Jayarangaiah A, Kumar A. COVID-19-associated coagulopathy: an exacerbated immunothrombosis response. Clin Appl Thromb Hemost. (2020) 26:1076029620943293. doi: 10.1177/1076029620943293
33. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. (2020) 18(8):1995–2002. doi: 10.1111/jth.14888
34. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. (2020) 46(6):1089–98. doi: 10.1007/s00134-020-06062-x
35. Nahum J, Morichau-Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, et al. Venous Thrombosis Among Critically Ill Patients With Coronavirus Disease 2019 (COVID-19). JAMA Netw Open. (2020) 3(5):e2010478. doi: 10.1001/jamanetworkopen.2020.10478
36. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) 191:145–7. doi: 10.1016/j.thromres.2020.04.013
37. Ribes A, Vardon-Bounes F, Mémier V, Poette M, Au-Duong J, Garcia C, et al. Thromboembolic events and Covid-19. Adv Biol Regul. (2020) 77:100735. doi: 10.1016/j.jbior.2020.100735
38. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. European heart journal. (2020) 41(32):3038–44. doi: 10.1093/eurheartj/ehaa623
39. Mosleh W, Chen K, Pfau SE, Vashist A. Endotheliitis and Endothelial Dysfunction in Patients with COVID-19: Its Role in Thrombosis and Adverse Outcomes. J Clin Med. (2020) 9(6):1862. doi: 10.3390/jcm9061862
40. Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. (2020) 24(1):353. doi: 10.1186/s13054-020-03062-7
41. Vrints CJM, Krychtiuk KA, Van Craenenbroeck EM, Segers VF, Price S, Heidbuchel H. Endothelialitis plays a central role in the pathophysiology of severe COVID-19 and its cardiovascular complications. Acta Cardiol. (2021) 76(2):109–24. doi: 10.1080/00015385.2020.1846921
42. Zhang J, Tecson KM, McCullough PA. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. (2020) 21(3):315–9. doi: 10.31083/j.rcm.2020.03.126
43. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5
44. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. (2020) 383(2):120–8. doi: 10.1056/NEJMoa2015432
45. Stahl K, Gronski PA, Kiyan Y, Seeliger B, Bertram A, Pape T, et al. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am J Respir Crit Care Med. (2020) 202(8):1178–81. doi: 10.1164/rccm.202007-2676LE
46. Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. (2020) 18(7):1548–55. doi: 10.1111/jth.14872
47. Coccheri S. COVID-19: The crucial role of blood coagulation and fibrinolysis. Intern Emerg Med. (2020) 15(8):1369–73. doi: 10.1007/s11739-020-02443-8
48. Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. (1995) 95(3):995–1001. doi: 10.1172/JCI117809
49. Fujimoto H, Gabazza EC, Hataji O, Yuda H, D'Alessandro-Gabazza CN, Nakano M, et al. Thrombin-activatable fibrinolysis inhibitor and protein C inhibitor in interstitial lung disease. Am J Respir Crit Care Med. (2003) 167(12):1687–94. doi: 10.1164/rccm.200208-905OC
50. Blasi A, von Meijenfeldt FA, Adelmeijer J, Calvo A, Ibañez C, Perdomo J, et al. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. Journal of Thrombosis and Haemostasis. (2020) 18(10):2646–53. doi: 10.1111/jth.15043
51. Hardy M, Michaux I, Lessire S, Douxfils J, Dogné J-M, Bareille M, et al. Prothrombotic disturbances of hemostasis of patients with severe COVID-19: A prospective longitudinal observational study. Thromb Res. (2021) 197:20–3. doi: 10.1016/j.thromres.2020.10.025
52. Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost. (2020) 18(9):2215–9. doi: 10.1111/jth.15016
53. Weiss E, Roux O, Moyer J-D, Paugam-Burtz C, Boudaoud L, Ajzenberg N, et al. Fibrinolysis Resistance: A Potential Mechanism Underlying COVID-19 Coagulopathy. Thromb Haemost. (2020) 120(9):1343–45. doi: 10.1055/s-0040-1713637
54. Ranucci M, Sitzia C, Baryshnikova E, Di Dedda U, Cardani R, Martelli F, et al. Covid-19-Associated Coagulopathy: Biomarkers of Thrombin Generation and Fibrinolysis Leading the Outcome. J Clin Med. (2020) 9(11):3487. doi: 10.3390/jcm9113487
55. Tsantes AE, Tsantes AG, Kokoris SI, Bonovas S, Frantzeskaki F, Tsangaris I, et al. COVID-19 Infection-Related Coagulopathy and Viscoelastic Methods: A Paradigm for Their Clinical Utility in Critical Illness. Diagnostics (Basel). (2020) 10(10):817. doi: 10.3390/diagnostics10100817
56. Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, et al. Fibrinolysis Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J Am Coll Surg. (2020) 231(2):193–203 e1. doi: 10.1016/j.jamcollsurg.2020.05.007
57. Ibañez C, Perdomo J, Calvo A, Ferrando C, Reverter J, Tassies D, et al. High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? Journal of thrombosis and thrombolysis. (2021) 51(2):308–12. doi: 10.1007/s11239-020-02226-0
58. Creel-Bulos C, Sniecinski R. Fibrinolysis Shutdown and Thrombosis in a COVID-19 ICU. Shock. (2021) 55(6):845–6. doi: 10.1097/SHK.0000000000001666
59. Moore HB, Moore EE. Temporal Changes in Fibrinolysis following Injury. Semin Thromb Hemost. (2020) 46(2):189–98. doi: 10.1055/s-0039-1701016
60. Moore H, Moore E, Gonzalez E, Chapman M, Chin T, Silliman C, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. (2014) 77(6):811–7; Discussion 7. doi: 10.1097/TA.0000000000000341
61. Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. Journal of the American College of Surgeons. (2016) 222(4):347–55. doi: 10.1016/j.jamcollsurg.2016.01.006
62. Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. Journal of the American College of Surgeons. (2017) 224(4):575–82. doi: 10.1016/j.jamcollsurg.2016.12.018
63. Bone RC, Francis PB, Pierce AK. Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med. (1976) 61(5):585–9. doi: 10.1016/0002-9343(76)90135-2
64. Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. (2006) 27(4):337–49. doi: 10.1055/s-2006-948288
65. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. (2000) 342(18):1334–49. doi: 10.1056/NEJM200005043421806
66. Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. (2017) 377(6):562–72. doi: 10.1056/NEJMra1608077
67. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. (2020) 15(5):700–4. doi: 10.1016/j.jtho.2020.02.010
68. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. (2020) 20(10):1135–40. doi: 10.1016/S1473-3099(20)30434-5
69. Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. (2020) 173(5):350–61. doi: 10.7326/M20-2566
70. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. (2020) 24:100434. doi: 10.1016/j.eclinm.2020.100434
71. Bastarache JA, Wang L, Geiser T, Wang Z, Albertine KH, Matthay MA, et al. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax. (2007) 62(7):608–16. doi: 10.1136/thx.2006.063305
72. Grau GE, de Moerloose P, Bulla O, Lou J, Lei Z, Reber G, et al. Haemostatic properties of human pulmonary and cerebral microvascular endothelial cells. Thromb Haemost. (1997) 77(3):585–90. doi: 10.1055/s-0038-1656009
73. MacLaren R, Stringer KA. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy. (2007) 27(6):860–73. doi: 10.1592/phco.27.6.860
74. Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. (2021) 88(1):15–27. doi: 10.1159/000512007
75. Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. (2020) 13(1):120. doi: 10.1186/s13045-020-00954-7
76. Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. (2020) 136(11):1330–41. doi: 10.1182/blood.2020007252
77. Grobler C, Maphumulo SC, Grobbelaar LM, Bredenkamp JC, Laubscher GJ, Lourens PJ, et al. Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci. (2020) 21(14):5168. doi: 10.3390/ijms21145168
78. Laubscher GJ, Lourens PJ, Venter C, Kell DB, Pretorius E. TEG(®), Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med. (2021) 10(22):5381. doi: 10.3390/jcm10225381
79. Bunch CM, Thomas AV, Stillson JE, Gillespie L, Lin KP, Speybroeck J, et al. Thromboelastography-Guided Anticoagulant Therapy for the Double Hazard of Thrombohemorrhagic Events in COVID-19: A Report of 3 Cases. Am J Case Rep. (2021) 22:e931080. doi: 10.12659/AJCR.931080
80. Thomas AV, Lin KP, Stillson JE, Bunch CM, Speybroeck J, Wiarda G, et al. A Case Series of Thromboelastography-Guided Anticoagulation in COVID-19 Patients with Inherited and Acquired Hypercoagulable States. Case Rep Med. (2021) 2021:5568982. doi: 10.1155/2021/5568982
81. Hulshof AM, Braeken DCW, Ghossein-Doha C, van Santen S, Sels JEM, Kuiper G, et al. Hemostasis and fibrinolysis in COVID-19 survivors 6 months after intensive care unit discharge. Res Pract Thromb Haemost. (2021) 5(6):e12579. doi: 10.1002/rth2.12579
82. Mauro A, De Grazia F, Lenti MV, Penagini R, Frego R, Ardizzone S, et al. Upper gastrointestinal bleeding in COVID-19 inpatients: Incidence and management in a multicenter experience from Northern Italy. Clin Res Hepatol Gastroenterol. (2021) 45(3):101521. doi: 10.1016/j.clinre.2020.07.025
83. Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. (2020) 29(8):104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984
84. Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JC, Fogerty AE, Waheed A, et al. COVID and Coagulation: Bleeding and Thrombotic Manifestations of SARS-CoV2 Infection. Blood. (2020) 136(4):489–500. doi: 10.1182/blood.2020006520
85. Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. (2021) 385(9):777–89. doi: 10.1056/NEJMoa2103417
86. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. (2021) 385(9):790–802. doi: 10.1056/NEJMoa2105911
87. Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. (2021) 11(3):e048391. doi: 10.1136/bmjopen-2020-048391
88. Yan Z, Yang M, Lai CL. Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans. Biomedicines. (2021) 9(8):966. doi: 10.3390/biomedicines9080966
89. Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. ‘Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. (2021) 76(4):396–8. doi: 10.1136/thoraxjnl-2020-215818
90. Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. (2021) 19(4):1064–70. doi: 10.1111/jth.15267
91. Sun B, Tang N, Peluso MJ, Iyer NS, Torres L, Donatelli JL, et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells. (2021) 10(2):386. doi: 10.3390/cells10020386.
92. Peluso MJ, Lu S, Tang AF, Durstenfeld MS, Ho HE, Goldberg SA, et al. Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis. (2021) 224(11):1839–48. doi: 10.1093/infdis/jiab490
93. Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. (2021) 19(10):2546–53. doi: 10.1111/jth.15490
94. Gorog DA, Storey RF, Gurbel PA, Tantry US, Berger JS, Chan MY, et al. Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol. (2022) 13:1–21. doi: 10.1038/s41569-021-00665-7
95. Lowe GD. Prediction of postoperative venous thrombosis using haemostasis tests. Int J Clin Lab Res. (1997) 27(3):153–7. doi: 10.1007/BF02912451
96. Emani S, Zurakowski D, Baird CW, Pigula FA, Trenor C 3rd, Emani SM. Hypercoagulability markers predict thrombosis in single ventricle neonates undergoing cardiac surgery. The Annals of thoracic surgery. (2013) 96(2):651–6. doi: 10.1016/j.athoracsur.2013.04.061
97. Taura P, Rivas E, Martinez-Palli G, Blasi A, Holguera JC, Balust J, et al. Clinical markers of the hypercoagulable state by rotational thrombelastometry in obese patients submitted to bariatric surgery. Surg Endosc. (2014) 28(2):543–51. doi: 10.1007/s00464-013-3203-1
98. Macfarlane RG, Biggs R. Fibrinolysis; its mechanism and significance. Blood. (1948) 3(10):1167–87. doi: 10.1182/blood.V3.10.1167.1167
99. Thachil J, Juffermans NP, Ranucci M, Connors JM, Warkentin TE, Ortel TL, et al. ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost. (2020) 18(9):2138–44. doi: 10.1111/jth.15004
100. Moore HB, Barrett CD, Moore EE, McIntyre RC, Moore PK, Talmor DS, et al. Is there a role for tissue plasminogen activator as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome? The Journal of Trauma and Acute Care Surgery. (2020) 88(6):1. doi: 10.1097/TA.0000000000002694
101. Barrett CD, Moore HB, Moore EE, Wang DJ, Hajizadeh N, Biffl WL, et al. Study of Alteplase for Respiratory Failure in SARS-CoV-2 COVID-19: A Vanguard Multicenter, Rapidly Adaptive, Pragmatic, Randomized Controlled Trial. Chest. (2022) 161(3):710–27. doi: 10.1016/j.chest.2021.09.024
102. Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. (2020) 18(8):1859–65. doi: 10.1111/jth.14929
103. Bradbury CA, McQuilten Z. Anticoagulation in COVID-19. Lancet. (2022) 399(10319):5–7. doi: 10.1016/S0140-6736(21)02503-4
104. Hozayen SM, Zychowski D, Benson S, Lutsey PL, Haslbauer J, Tzankov A, et al. Outpatient and inpatient anticoagulation therapy and the risk for hospital admission and death among COVID-19 patients. EClinicalMedicine. (2021) 41:101139. doi: 10.1016/j.eclinm.2021.101139
105. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. (2020) 18(7):1559–61. doi: 10.1111/jth.14849
106. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. (2020) 75(23):2950–73. doi: 10.1016/j.jacc.2020.04.031
107. Cohoon KP, Mahé G, Tafur AJ, Spyropoulos AC. Emergence of institutional antithrombotic protocols for coronavirus 2019. Research and Practice in Thrombosis and Haemostasis. (2020) 4(4):510–7. doi: 10.1002/rth2.12358
108. Stillson JE, Bunch CM, Gillespie L, Khan R, Wierman M, Pulvirenti J, et al. Thromboelastography-Guided Management of Anticoagulated COVID-19 Patients to Prevent Hemorrhage. Semin Thromb Hemost. (2021) 47(4):442–6. doi: 10.1055/s-0041-1723754
109. Ten Cate H. Surviving Covid-19 with Heparin? N Engl J Med. (2021) 385(9):845–6. doi: 10.1056/NEJMe2111151
110. Sollini M, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Gelardi F, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F]FDG-PET/CT study. Eur J Nucl Med Mol Imaging. (2021) 48(5):1460–6. doi: 10.1007/s00259-020-05084-3
111. Hartmann J, Walsh M, Grisoli A, Thomas AV, Shariff F, McCauley R, et al. Diagnosis and Treatment of Trauma-Induced Coagulopathy by Viscoelastography. Semin Thromb Hemost. (2020) 46(02):134–46. doi: 10.1055/s-0040-1702171
112. Walsh M, Thomas S, Kwaan H, Aversa J, Anderson S, Sundararajan R, et al. Modern methods for monitoring hemorrhagic resuscitation in the United States: Why the delay? J Trauma Acute Care Surg. (2020) 89(6):1018–22. doi: 10.1097/TA.0000000000002977
113. Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better indicator of postinjury hypercoagulable state than prothrombin time or activated partial thromboplastin time. J Trauma. (2009) 67(2):266–75; discussion 275-6. doi: 10.1097/TA.0b013e3181ae6f1c
114. Walsh M, Shreve J, Thomas S, Moore E, Moore H, Hake D, et al. Fibrinolysis in trauma:“myth,”“reality,” or “something in between”. Semin Thromb Hemost. (2017) 43(02):200–12. doi: 10.1055/s-0036-1597900
115. Moore HB, Moore EE, Neal MD, Sheppard FR, Kornblith LZ, Draxler DF, et al. Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications. Anesth Analg. (2019) 129(3):762–73. doi: 10.1213/ANE.0000000000004234
116. Stettler GR, Moore EE, Moore HB, Nunns GR, Silliman CC, Banerjee A, et al. Redefining postinjury fibrinolysis phenotypes using two viscoelastic assays. Journal of Trauma and Acute Care Surgery. (2019) 86(4):679–85. doi: 10.1097/TA.0000000000002165
117. Centers for Disease Control and Prevention. Omicron Variant: What You Need to Know CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html [updated March 29, 2022].
118. Boscolo A, Spiezia L, Correale C, Sella N, Pesenti E, Beghetto L, et al. Different Hypercoagulable Profiles in Patients with COVID-19 Admitted to the Internal Medicine Ward and the Intensive Care Unit. Thromb Haemost. (2020) 120(10):1474–7. doi: 10.1055/s-0040-1714350
119. Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. (2021) 20(1):172. doi: 10.1186/s12933-021-01359-7
120. Scott L, Hsiao NY, Moyo S, Singh L, Tegally H, Dor G, et al. Track Omicron's spread with molecular data. Science. (2021) 374(6574):1454–5. doi: 10.1126/science.abn4543
121. Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. (2022) 399(10323):437–46. doi: 10.1016/S0140-6736(22)00017-4
122. Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA. (2022) 327(7):639–51. doi: 10.1001/jama.2022.0470
123. Brown WA, Moore EM, Watters DA. Mortality of patients with COVID-19 who undergo an elective or emergency surgical procedure: a systematic review and meta-analysis. ANZ J Surg. (2021) 91(1–2):33–41. doi: 10.1111/ans.16500
124. American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetricians-gynecologists, gynecology. Washington, DC: ACOG (2021). Available from: https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology [updated August 09, 2021].
125. Joint Statement on Re-introduction of Hospital and Office-based Procedures for the Practicing Urogynecologist and Gynecologist. J Minim Invasive Gynecol. (2020) 27(5):1030–2. doi: 10.1016/j.jmig.2020.05.019
126. Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy. London: RCOG (2021).
127. Lott A, Urish KL. Recommendations Regarding Safety of Elective Surgery During COVID-19 Pandemic. Rosemont, IL: AAOS (2020). Available at: https://www.aaos.org/about/covid-19-information-for-our-members/guidance-for-elective-surgery/recommendations-regarding-safety-of-elective-surgery-during-covid-19/.
128. Naam NM. Message from the AAHS President: Nash Naam, MD. Beverly, MA: American Association for Hand Surgery (2020).
129. AAHKS. CMS Provides Regional Guidance on Reopening. Rosemont, IL: American Association of Hip and Knee Surgeons (2020).
130. Surgeons ACo. Local Resumption of Elective Surgery Guidance. Chicago, IL: American College of Surgeons (2020).
131. Kort NP, Barrena EG, Bédard M, Donell S, Epinette JA, Gomberg B, et al. Resuming elective hip and knee arthroplasty after the first phase of the SARS-CoV-2 pandemic: the European Hip Society and European Knee Associates recommendations. Knee Surg Sports Traumatol Arthrosc. (2020) 28(9):2730–46. doi: 10.1007/s00167-020-06233-9
132. Executive Council of ASMBS. Safer through surgery: American Society for Metabolic and Bariatric Surgery statement regarding metabolic and bariatric surgery during the COVID-19 pandemic. Surg Obes Relat Dis. (2020) 16(8):981–2. doi: 10.1016/j.soard.2020.06.003
133. American Society of General Surgeons. ASGS COVID-19 Position Statement. Mendota Heights, MN: ASGS (2020).
134. American College of Surgeons. COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. Available at: https://www.facs.org/covid-19/clinical-guidance/triage [updated March 17, 2020].
135. American College of Surgeons. COVID 19: Elective Case Triage Guidelines for Surgical Care. Available at: https://www.facs.org/-/media/files/covid19/guidance_for_triage_of_nonemergent_surgical_procedures.ashx (accessed January 14, 2020)
136. Centers for Medicare & Medicaid Services. Non-Emergent, Elective Medical Services, and Treatment Recommendations. Available at: https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf [updated April 7, 2020].
137. American Society of Transplant Surgeons. Transplant Capacity in the COVID-19 Era and Early Vaccine Recommendations. Arlington, VA: ASTS (2020). Available at: https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/transplant-capacity-in-the-covid-19-era#.YdiiqBNuc-S [updated December 03, 2020].
138. Society of American Gastrointestinal Endoscopic Surgeons. SAGES recommendations regarding surgical management of gastric cancer patients during the response to the COVID-19 crisis Los Angeles. CA: SAGES (2020). Available at: https://www.sages.org/sages-recommendations-surgical-management-gastric-cancer-covid-19-crisis/ [updated April 11, 2020].
139. European Association of Neurosurgical Societies. EANS advice: Triaging non-emergent neurosurgical procedures during the COVID-19 outbreak. EANS (2020).
140. Hassan A, Arora RC, Lother SA, Adams C, Bouchard D, Cook R, et al. Ramping Up the Delivery of Cardiac Surgery During the COVID-19 Pandemic: A Guidance Statement From the Canadian Society of Cardiac Surgeons. Can J Cardiol. (2020) 36(7):1139–43. doi: 10.1016/j.cjca.2020.04.030
141. Haft JW, Atluri P, Ailawadi G, Engelman DT, Grant MC, Hassan A, et al. Adult Cardiac Surgery During the COVID-19 Pandemic: A Tiered Patient Triage Guidance Statement. The Annals of thoracic surgery. (2020) 110(2):697–700. doi: 10.1016/j.athoracsur.2020.04.003
143. Jongkind V, Earnshaw JJ, Bastos Gonçalves F, Cochennec F, Debus ES, Hinchliffe R, et al. Update of the European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia in Light of the COVID-19 Pandemic, Based on a Scoping Review of the Literature. Eur J Vasc Endovasc Surg. (2022) 63(1):80–9. doi: 10.1016/j.ejvs.2021.08.028
144. American Society of Plastic Surgeons. Important Update to Considerations for the Continuation or Resumption of Elective Surgery. Available at: https://www.plasticsurgery.org/for-medical-professionals/covid19-member-resources/resumption-of-elective-surgery (accessed February 3, 2022).
145. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos A-M, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (2012) 141(2):e691S–36S. doi: 10.1378/chest.11-2300
146. Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green Top Guideline. (2015), no. 37a.
147. Dargaud Y, Rugeri L, Vergnes MC, Arnuti B, Miranda P, Negrier C, et al. A risk score for the management of pregnant women with increased risk of venous thromboembolism: a multicentre prospective study. Br J Haematol. (2009) 145(6):825–35. doi: 10.1111/j.1365-2141.2009.07698.x
148. Danilenko-Dixon DR, Heit JA, Silverstein MD, Yawn BP, Petterson TM, Lohse CM, et al. Risk factors for deep vein thrombosis and pulmonary embolism during pregnancy or post partum: a population-based, case-control study. Am J Obstet Gynecol. (2001) 184(2):104–10. doi: 10.1067/mob.2001.107919
149. Ettema HB, Kollen BJ, Verheyen CC, Büller HR. Prevention of venous thromboembolism in patients with immobilization of the lower extremities: a meta-analysis of randomized controlled trials. J Thromb Haemost. (2008) 6(7):1093–8. doi: 10.1111/j.1538-7836.2008.02984.x
150. Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost. (2008) 6(6):905–12. doi: 10.1111/j.1538-7836.2008.02961.x
151. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. (2006) 194(5):1311–5. doi: 10.1016/j.ajog.2005.11.008
152. Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. (1999) 94(4):595–9. doi: 10.1016/s0029-7844(99)00308-7
153. Macklon NS, Greer IA. Venous thromboembolic disease in obstetrics and gynaecology: the Scottish experience. Scott Med J. (1996) 41(3):83–6. doi: 10.1177/003693309604100305
154. Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. Bjog. (2001) 108(1):56–60. doi: 10.1111/j.1471-0528.2001.00004.x
155. Blondon M, Perrier A, Nendaz M, Righini M, Boehlen F, Boulvain M, et al. Thromboprophylaxis with low-molecular-weight heparin after cesarean delivery. Thromb Haemost. (2010) 103(1):129–37. doi: 10.1160/TH09-06-0349
156. Kobayashi T, Nakabayashi M, Ishikawa M, Adachi T, Kobashi G, Maeda M, et al. Pulmonary thromboembolism in obstetrics and gynecology increased by 6.5-fold over the past decade in Japan. Circ J. (2008) 72(5):753–6. doi: 10.1253/circj.72.753
157. Liew-Spilger AE, Sorg NR, Brenner TJ, Langford JH, Berquist M, Mark NM, et al. Viscoelastic Hemostatic Assays for Postpartum Hemorrhage. J Clin Med. (2021) 10(17):3946. doi: 10.3390/jcm10173946
158. Seeho S, Nassar N. Thromboprophylaxis after caesarean: when even the ‘experts’ disagree. BJOG. (2016) 123(13):2163. doi: 10.1111/1471-0528.13740
159. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet Gynecol. (2018) 132(1):e1–17. doi: 10.1097/AOG.0000000000002712
160. Overcash RT, Somers AT, LaCoursiere DY. Enoxaparin dosing after cesarean delivery in morbidly obese women. Obstet Gynecol. (2015) 125(6):1371–6. doi: 10.1097/AOG.0000000000000873
161. He Z, Morrissey H, Ball P. Review of current evidence available for guiding optimal Enoxaparin prophylactic dosing strategies in obese patients—actual weight-based vs fixed. Crit Rev Oncol Hematol. (2017) 113:191–4. doi: 10.1016/j.critrevonc.2017.03.022
162. Hagopian JC, Riney JN, Hollands JM, Deal EN. Assessment of bleeding events associated with short-duration therapeutic enoxaparin use in the morbidly obese. Ann Pharmacother. (2013) 47(12):1641–8. doi: 10.1177/1060028013506404
163. Bates SM, Middeldorp S, Rodger M, James AH, Greer I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis. (2016) 41(1):92–128. doi: 10.1007/s11239-015-1309-0
164. Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardeny O, Greene MF, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. (2021) 181(5):714–7. doi: 10.1001/jamainternmed.2020.9241
165. Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. (2021) 137(4):571. doi: 10.1097/AOG.0000000000004339
166. Servante J, Swallow G, Thornton JG, Myers B, Munireddy S, Malinowski AK, et al. Haemostatic and thrombo-embolic complications in pregnant women with COVID-19: a systematic review and critical analysis. BMC Pregnancy Childbirth. (2021) 21(1):108. doi: 10.1186/s12884-021-03568-0
167. Wilson D, Cooke E, McNally M, Wilson H, Yeates A, Mollan R. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury. (2001) 32(10):765–70. doi: 10.1016/S0020-1383(01)00139-5
168. Gary JL, Schneider PS, Galpin M, Radwan Z, Munz JW, Achor TS, et al. Can thrombelastography predict venous thromboembolic events in patients with severe extremity trauma? J Orthop Trauma. (2016) 30(6):294–8. doi: 10.1097/BOT.0000000000000523
169. Brown W, Lunati M, Maceroli M, Ernst A, Staley C, Johnson R, et al. Ability of Thromboelastography to Detect Hypercoagulability: A Systematic Review and Meta-Analysis. J Orthop Trauma. (2020) 34(6):278–86. doi: 10.1097/BOT.0000000000001714
170. Iyengar KP, Jain VK, Vaish A, Vaishya R, Maini L, Lal H. Post COVID-19: Planning strategies to resume orthopaedic surgery -challenges and considerations. J Clin Orthop Trauma. (2020) 11(Suppl 3):S291–5. doi: 10.1016/j.jcot.2020.04.028
171. Donell ST, Thaler M, Budhiparama NC, Buttaro MA, Chen AF, Diaz-Ledezma C, et al. Preparation for the next COVID-19 wave: The European Hip Society and European Knee Associates recommendations. Knee Surg Sports Traumatol Arthrosc. (2020) 28(9):2747–55. doi: 10.1007/s00167-020-06213-z
172. Ding BTK, Decruz J, Kunnasegaran R. Time-sensitive ambulatory orthopaedic soft-tissue surgery paradigms during the COVID-19 pandemic. Int Orthop. (2020) 44(8):1531–8. doi: 10.1007/s00264-020-04606-w
173. Kaidi AC, Held MB, Boddapati V, Trofa DP, Neuwirth AL. Timing and tips for total hip arthroplasty in a critically ill patient with coronavirus disease 2019 and a femoral neck fracture. Arthroplast Today. (2020) 6(3):566–70. doi: 10.1016/j.artd.2020.07.006
174. Giuffrida M, Cozzani F, Rossini M, Bonati E, Del Rio P. How COVID-19 pandemic has changed elective surgery: the experience in a general surgery unit at a COVID-hospital. Acta Biomed. (2020) 91(4):e2020152. doi: 10.23750/abm.v91i4.10301
175. Navarra G, Komaei I, Currò G, Angrisani L, Bellini R, Cerbone MR, et al. Bariatric surgery and the COVID-19 pandemic: SICOB recommendations on how to perform surgery during the outbreak and when to resume the activities in phase 2 of lockdown. Updates Surg. (2020) 72(2):259–68. doi: 10.1007/s13304-020-00821-7
176. Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. (2020) 20(7):1800–8. doi: 10.1111/ajt.15941
177. Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and Kidney Transplantation. N Engl J Med. (2020) 382(25):2475–7. doi: 10.1056/NEJMc2011117
178. Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation. (2021) 105(1):37–55. doi: 10.1097/TP.0000000000003523
179. Romagnoli R, Gruttadauria S, Tisone G, Maria Ettorre G, De Carlis L, Martini S, et al. Liver transplantation from active COVID-19 donors: A lifesaving opportunity worth grasping? Am J Transplant. (2021) 21(12):3919–25. doi: 10.1111/ajt.16823
180. Kates OS, Fisher CE, Rakita RM, Reyes JD, Limaye AP. Use of SARS-CoV-2-infected deceased organ donors: Should we always “just say no?”. Am J Transplant. (2020) 20(7):1787–94. doi: 10.1111/ajt.16000
181. Turaga KK, Girotra S. Are We Harming Cancer Patients by Delaying Their Cancer Surgery During the COVID-19 Pandemic? Ann Surg. (2020) 2:10.1097/SLA.0000000000003967. doi: 10.1097/SLA.0000000000003967
182. Aziz H, Filkins A, Kwon YK. Review of COVID-19 Outcomes in Surgical Patients. Am Surg. (2020) 86(7):741–5. doi: 10.1177/0003134820934395
183. Francis N, Dort J, Cho E, Feldman L, Keller D, Lim R, et al. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc. (2020) 34(6):2327–31. doi: 10.1007/s00464-020-07565-w
184. Arezzo A, Francis N, Mintz Y, Adamina M, Antoniou SA, Bouvy N, et al. EAES Recommendations for Recovery Plan in Minimally Invasive Surgery Amid COVID-19 Pandemic. Surg Endosc. (2021) 35(1):1–17. doi: 10.1007/s00464-020-08131-0
185. Arimappamagan A, Vilanilam G, Pandey P. Is Elective Neurosurgery Justified During COVID-19 Pandemic? Neurol India. (2021) 69(1):21–5. doi: 10.4103/0028-3886.310113
186. Bernstein M (Editorial). Neurosurgical priority setting during a pandemic: COVID-19. J Neurosurg. (2020) 17:1–2. doi: 10.3171/2020.4.JNS201031
187. Patel ZM, Fernandez-Miranda J, Hwang PH, Nayak JV, Dodd R, Sajjadi H, et al. Letter: Precautions for Endoscopic Transnasal Skull Base Surgery During the COVID-19 Pandemic. Neurosurgery. (2020) 87(1):e66–7. doi: 10.1093/neuros/nyaa125
188. Givi B, Schiff BA, Chinn SB, Clayburgh D, Iyer NG, Jalisi S, et al. Safety Recommendations for Evaluation and Surgery of the Head and Neck During the COVID-19 Pandemic. JAMA Otolaryngol Head Neck Surg. (2020) 146(6):579–84. doi: 10.1001/jamaoto.2020.0780
189. Kessler RA, Zimering J, Gilligan J, Rothrock R, McNeill I, Shrivastava RK, et al. Neurosurgical management of brain and spine tumors in the COVID-19 era: an institutional experience from the epicenter of the pandemic. J Neurooncol. (2020) 148(2):211–9. doi: 10.1007/s11060-020-03523-7
190. Panciani PP, Saraceno G, Zanin L, Renisi G, Signorini L, Fontanella MM. Letter: COVID-19 Infection Affects Surgical Outcome of Chronic Subdural Hematoma. Neurosurgery. (2020) 87(2):e167–71. doi: 10.1093/neuros/nyaa140
191. Ozoner B, Gungor A, Hasanov T, Toktas ZO, Kilic T. Neurosurgical Practice During Coronavirus Disease 2019 (COVID-19) Pandemic. World Neurosurg. (2020) 140:198–207. doi: 10.1016/j.wneu.2020.05.195
192. Liu H, Wang Z, Sun H, Teng T, Li Y, Zhou X, et al. Thrombosis and Coagulopathy in COVID-19: Current Understanding and Implications for Antithrombotic Treatment in Patients Treated With Percutaneous Coronary Intervention. Front Cardiovasc Med. (2020) 7:599334. doi: 10.3389/fcvm.2020.599334
193. Mahmud E, Dauerman HL, Welt FGP, Messenger JC, Rao SV, Grines C, et al. Management of Acute Myocardial Infarction During the COVID-19 Pandemic: A Position Statement From the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). J Am Coll Cardiol. (2020) 76(11):1375–84. doi: 10.1016/j.jacc.2020.04.039
194. Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. (2020) 120(6):998–1000. doi: 10.1055/s-0040-1710018
195. Roh DJ, Eiseman K, Kirsch H, Yoh N, Boehme A, Agarwal S, et al. Hypercoagulable viscoelastic blood clot characteristics in critically ill coronavirus disease 2019 patients and associations with thrombotic complications. J Trauma Acute Care Surg. (2021) 90(1):e7–12. doi: 10.1097/TA.0000000000002963
196. Aires RB, Soares A, Gomides APM, Nicola AM, Teixeira-Carvalho A, da Silva DLM, et al. Thromboelastometry demonstrates endogenous coagulation activation in nonsevere and severe COVID-19 patients and has applicability as a decision algorithm for intervention. PLoS One. (2022) 17(1):e0262600. doi: 10.1371/journal.pone.0262600
197. Krieger JB, Jennelle. The Use of ECMO in Patients with Cardiopulmonary Failure Due to COVID-19, American College of Cardiology (2020). Available at: https://www.acc.org/latest-in-cardiology/articles/2020/08/03/12/44/the-use-of-ecmo-in-patients-with-cardiopulmonary-failure-due-to-covid-19 (accessed February 6, 2022).
198. Hassan A, Arora RC, Adams C, Bouchard D, Cook R, Gunning D, et al. Cardiac Surgery in Canada During the COVID-19 Pandemic: A Guidance Statement From the Canadian Society of Cardiac Surgeons. Can J Cardiol. (2020) 36(6):952–5. doi: 10.1016/j.cjca.2020.04.001
199. Dorfman R, Saadat S, Gupta N, Roostaeian J, Da Lio A. The COVID-19 Pandemic and Plastic Surgery: Literature Review, Ethical Analysis, and Proposed Guidelines. Plast Reconstr Surg. (2020) 146(4):482e–93e. doi: 10.1097/PRS.0000000000007268
200. Ozturk CN, Kuruoglu D, Ozturk C, Rampazzo A, Gurunian Gurunluoglu R. Plastic Surgery and the COVID-19 Pandemic: A Review of Clinical Guidelines. Ann Plast Surg. (2020) 85(2S Suppl 2):S155–60. doi: 10.1097/SAP.0000000000002443
201. Nguyen AX, Gervasio KA, Wu AY. COVID-19 Recommendations From Ophthalmic and Plastic Reconstructive Surgery Societies Worldwide. Ophthalmic Plast Reconstr Surg. (2020) 36(4):334–45. doi: 10.1097/IOP.0000000000001776
202. Al-Benna S. Failure of Free Flaps in Head and Neck Oncology Surgery in COVID-19 Patients. Plast Reconstr Surg. (2021) 147(5):900e–1e. doi: 10.1097/PRS.0000000000007847
203. Benmoussa N, Alkhashnam H. Reply: Failure of Free Flaps in Head and Neck Oncology Surgery in COVID-19 Patients. Plast Reconstr Surg. (2021) 147(5):901e. doi: 10.1097/PRS.0000000000007849
204. Marsee MK, Shariff FS, Wiarda G, Watson PJ, Sualeh AH, Brenner TJ, et al. Use of Thromboelastography and Rotational Thromboelastometry in Otolaryngology: A Narrative Review. J Clin Med. (2022) 11(4):1119. doi: 10.3390/jcm11041119
Keywords: COVID-19, elective surgical procedure, immunothrombosis, obstetrics, orthopedic procedures, venous thromboembolism, thrombophilia, fibrinolysis
Citation: Bunch CM, Moore EE, Moore HB, Neal MD, Thomas AV, Zackariya N, Zhao J, Zackariya S, Brenner TJ, Berquist M, Buckner H, Wiarda G, Fulkerson D, Huff W, Kwaan HC, Lankowicz G, Laubscher GJ, Lourens PJ, Pretorius E, Kotze MJ, Moolla MS, Sithole S, Maponga TG, Kell DB, Fox MD, Gillespie L, Khan RZ, Mamczak CN, March R, Macias R, Bull BS and Walsh MM (2022) Immuno-Thrombotic Complications of COVID-19: Implications for Timing of Surgery and Anticoagulation. Front. Surg. 9:889999. doi: 10.3389/fsurg.2022.889999
Received: 4 March 2022; Accepted: 5 April 2022;
Published: 4 May 2022.
Edited by:
Philip Frank Stahel, Rocky Vista University, United StatesReviewed by:
Robert W. Cross, University of Texas Medical Branch at Galveston, United States Paolo Bernante, University of Bologna, ItalyCopyright © 2022 Bunch, Moore, Moore, Neal, Thomas, Zackariya, Zhao, Zackariya, Brenner, Berquist, Buckner, Wiarda, Fulkerson, Huff, Kwaan, Lankowicz, Laubscher, Lourens, Pretorius, Kotze, Moolla, Sithole, Maponga, Kell, Fox, Gillespie, Khan, Mamczak, March, Macias, Bull and Walsh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark M. Walsh bWFya3dhbHNobWRAZ21haWwuY29t
†Deceased
Speciality section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.