- 1Department of Neurosurgery, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department of Neurosurgery, Affiliated Hospital of Hubei University of Medicine, First People’s Hospital of Xiangyang, Xiangyang, China
- 3Department of Paediatrics, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Central Hospital of Xiangyang, Xiangyang, China
Objective: This study aims to identify the predictors of postoperative hydrocephalus in patients with posterior fossa tumors (PFTs) and guide the management of perioperative hydrocephalus.
Methods: We performed a single-institution, retrospective analysis of patients who underwent resection of PFTs in our department over a 10-year period (2011–2021). A total of 682 adult patients met the inclusion criteria and were divided into either a prophylactic external ventricular drainage (EVD) group or a nonprophylactic-EVD group. We analyzed data for the nonprophylactic-EVD group by univariate and multivariate analyses to identify predictors of postoperative acute hydrocephalus. We also analyzed all cases by univariate and multivariate analyses to determine the predictors of postoperative ventriculoperitoneal (VP) shunt placement.
Results: Tumor infiltrating the midbrain aqueduct [P = 0.001; odds ratio (OR) = 9.8], postoperative hemorrhage (P < 0.001; OR = 66.7), and subtotal resection (P = 0.006; OR = 9.3) were independent risk factors for postoperative EVD. Tumor infiltrating the ventricular system (P < 0.001; OR = 58.5) and postoperative hemorrhage (P < 0.001; OR = 28.1) were independent risk factors for postoperative VP shunt placement.
Conclusions: These findings may help promote more aggressive monitoring and earlier interventions for postoperative hydrocephalus in patients with PFTs.
Introduction
Resection is the primary treatment measure for patients with posterior fossa tumors (PFTs), and hydrocephalus is a common perioperative complication (1, 2). Postoperative acute hydrocephalus often increases the length and cost of hospitalization and negatively affects the patient’s prognosis. Preoperative external ventricular drainage (EVD) and endoscopic third ventriculostomy (ETV) have been suggested to prevent postoperative acute hydrocephalus (3–5); however, the possibilities of additional brain-tissue damage and complications such as intracranial infection and hemorrhage also need to be considered (3, 6–9). Some studies found that the incidence of postoperative hydrocephalus in adult patients with PFTs was less than in juvenile PFT patients (10, 11) and hydrocephalus resolved in 96% of adult patients after tumor resection (2). Identifying adult patients at high risk of postoperative hydrocephalus and the placement of a prophylactic EVD is thus still controversial (3, 12, 13). Many studies have focused on this issue in pediatric patients (13–17), but few studies have considered adult patients (18). This study aimed to investigate the predictors of acute and persistent hydrocephalus development after resection in adult patients with PFTs.
Materials and Methods
Study Population and Data Collection
This retrospective descriptive cohort study investigated the incidence and predictors of postoperative acute and persistent hydrocephalus in consecutive patients who underwent surgery for PFT between January 2011 and February 2021 at the Department of Neurosurgery, Zhongnan Hospital of Wuhan University. A total of 762 patients aged >18 years who had previously been diagnosed with PFTs on the basis of outpatient computerized tomography (CT) or magnetic resonance imaging (MRI) were admitted to our department. The exclusion criteria were patients who underwent biopsy or did not receive tumor resection, patients who had a ventriculoperitoneal (VP) shunt or ETV performed before resection, and patients with a diagnosis of brain abscess. Clinical information was recorded for all enrolled patients, and the follow-up duration ranged from 90 days to 6 years.
Follow-up data were obtained from the last outpatient consultation or via telephone calls with patients if the follow-up was not appropriate for the specific patient. The data collected in this study were obtained from the hospital’s electronic database. The following information was recorded: patient characteristics, surgical procedure, placement of perioperative EVD, ETV, and VP shunt, and histological results. Tumor growth characteristics, tumor size, and the presence of preoperative hydrocephalus were determined via preoperative MRI scans. The extent of resection, postoperative hemorrhage, and presence of postoperative hydrocephalus was determined by postoperative CT and/or MRI scans.
The diagnostic criteria for preoperative hydrocephalus were imaging results indicating Evans’ index (maximum width between frontal horns divided by maximal width of inner table) >0.3 with signs of intracranial hypertension such as headache, nausea, and visual deterioration (19).
Prophylactic EVD Placement
The prophylactic EVD used in this study aimed to treat preoperative hydrocephalus, relieve intracranial hypertension, and prevent damage caused by acute hydrocephalus after resection. We considered both preoperative and intraoperative EVD as prophylactic EVD. The placement of an EVD device was decided by surgeons after a discussion of the case. The criteria for preoperative EVD placement were a radiographic diagnosis of hydrocephalus with severe clinical symptoms, such as frequent vomiting and a decreased level of consciousness at admission due to intracranial hypertension caused by hydrocephalus. If the patient’s decreased consciousness was caused by a brain stem tumor, cerebral herniation, or intracranial hypertension resulting from venous sinus compression, we usually chose to remove the lesion directly. However, in patients with mild clinical symptoms of intracranial hypertension, such as headache and nausea, we aimed to complete the preoperative preparation and perform surgery as soon as possible to relieve intracranial hypertension. We placed an intraoperative EVD as soon as the surgical procedure became difficult as a result of severe brain swelling.
Postoperative EVD Placement
The criteria for postoperative acute hydrocephalus were Evans’ index >0.3 and symptomatic intracranial hypertension leading to vomiting, nausea, or impaired consciousness (20). All patients with postoperative acute hydrocephalus received an EVD in our department. The time from EVD insertion to drainage removal was <14 days in all cases. The criteria for removal of the drain were patient remaining in stable condition while increasing the drainage height for several days and then closing the drain for at least 12 h and no evidence of hydrocephalus on CT images for 24 h before drain removal.
Postoperative VP Shunt Placement
The criterion for placement of a postresection VP shunt was failed EVD weaning or symptomatic persistent hydrocephalus necessitating permanent drainage.
Tumor Growth Characteristics
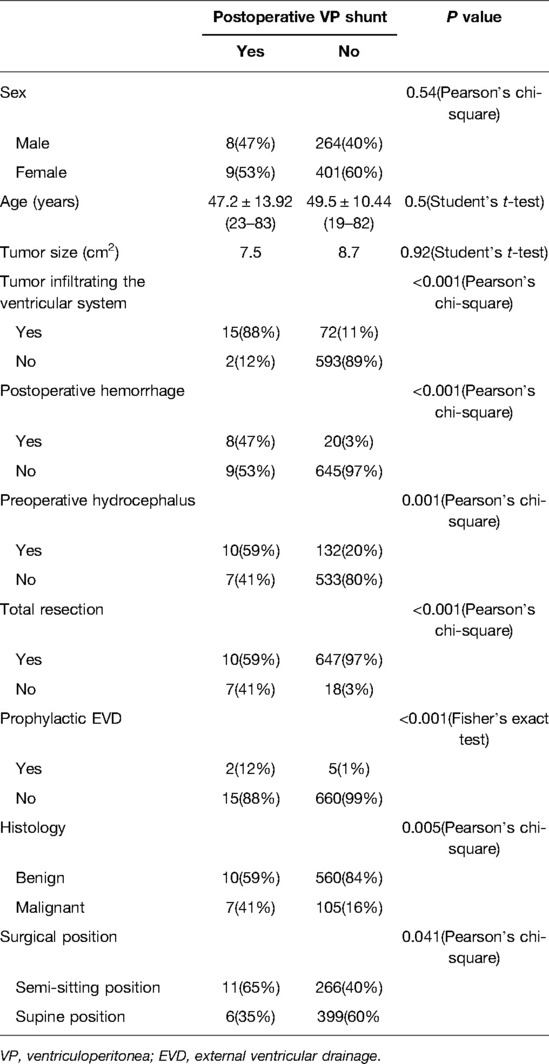
The ventricular system comprises a set of adjoining cavities that produce and circulate cerebrospinal fluid (CSF) within the brain (21). Ventricular infiltration was defined as a tumor infiltrating the brain tissue around the CSF circulation channel or invading the ventricular channel. In terms of spatial morphology, the CSF pathway tends to increase from the lower part upward to the lower half of the fourth ventricle and then decrease from the upper part of the fourth ventricle upward to the midbrain aqueduct. In addition, differences exist during surgery, with most type L2 tumors removable by elevating the cerebellum, while most type L3 and type L4 tumors require cerebellum incision. Based on the above characteristics and preoperative MRI scans, we classified each patient into the corresponding group according to the infiltration height of the tumor in the subtentorial ventricular system (Figure 1) as follows: L1, extra-axial tumor not infiltrating the ventricular system; L2, tumor infiltrating the ventricular system up to the lower half of the fourth ventricle (Figures 2A, B); L3: tumor infiltrating the ventricular system up to the upper half of the fourth ventricle (Figures 2C, D, 3D); and L4: tumor infiltrating the ventricular system up to the midbrain aqueduct, with invasion from the periphery (Figure 3A), invasion from below (Figure 2B), or endogenous tumor of the midbrain aqueduct (Figure 3C).
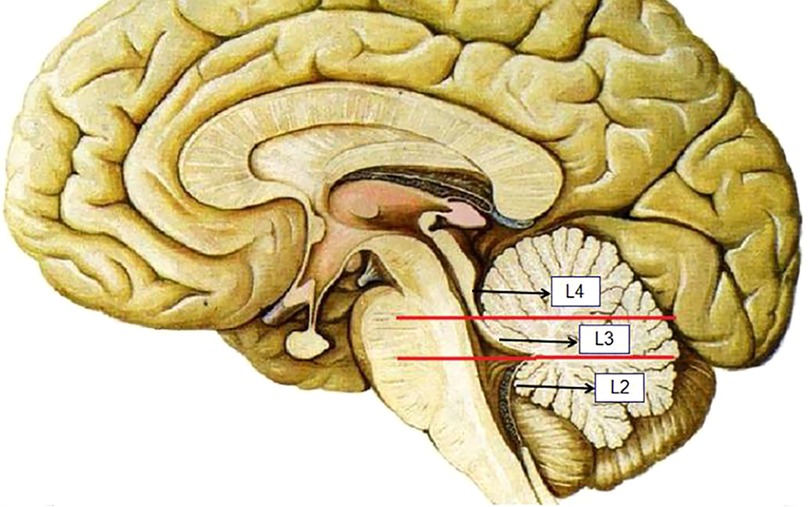
Figure 1. Section of L2, L3, and L4 according to the infiltration height of the tumor in the subtentorial ventricular system.
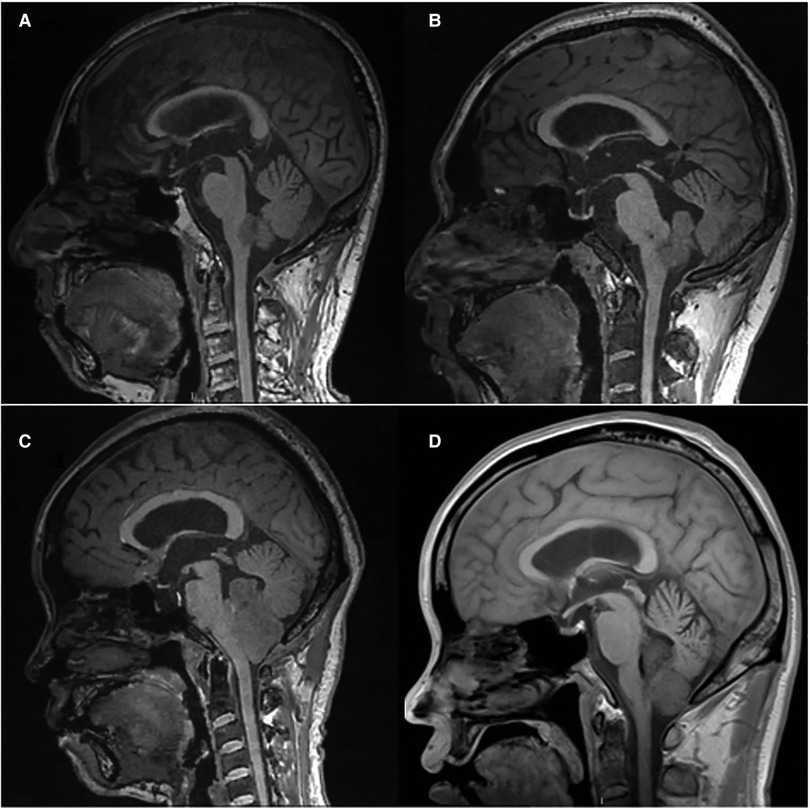
Figure 2. (A) and (B): sagittal (T1) views of type L2 tumor, (C) and (D): sagittal (T1) views of type L3 tumor.
Figure 3. (A): sagittal (T1) view of the tumor infiltration from the periphery; (B): sagittal (T1) view of the tumor infiltration from below; (C): sagittal (T1) view of the tumor endogenous from the ventricular system; (D): sagittal (T1) view of the tumor infiltration of the upper half of the fourth ventricle.
Extent of Resection
Total resection was defined as no residual tumor tissue seen by surgeons after resection and no reactive enhancement within 72 h after surgery on MRI (22). Tumor size was calculated using the maximum cross-sectional area of the tumor on MRI scans.
Postoperative Hemorrhage
Postoperative hemorrhage was diagnosed from postoperative CT images. For patients with postoperative hemorrhage not flooding into the ventricle, we performed evacuation of the hematoma for patients with massive hemorrhage. For patients with postoperative intraventricular hemorrhage, we placed an EVD with or without evacuation of the intracranial hematoma.
Surgical Position
Patients with midline PFTs undergoing surgery at our center primarily adopted a semi-sitting position if a relevant patent foramen ovale was not detected, while patients with nonmidline PFTs primarily adopted a supine position.
Study Design
Patients were classified into either a nonprophylactic-EVD group or a prophylactic-EVD group. The primary and secondary endpoints of the study were to determine the predictors of postoperative acute hydrocephalus and persistent hydrocephalus, respectively. The age and tumor size were analyzed as median values. Tumor growth characteristics were divided into four categories, and each patient was assigned to one respective group to rule out bias. We dichotomized other clinical and radiological parameters. EVD was placed in all patients with postoperative acute hydrocephalus, and all enrolled patients who developed postoperative persistent hydrocephalus received a VP shunt. We analyzed the nonprophylactic-EVD group to identify the predictors of postoperative acute hydrocephalus to eliminate bias caused by other perioperative CSF drainage. We also analyzed all cases to determine the predictors of postoperative persistent hydrocephalus. In view of the small number of patients who received VP shunts, we reduced the stratification and included types L2, L3, and L4 tumors in one group of tumors infiltrating the ventricular system to reduce the bias.
This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University. The study did not involve patients’ personal or private information, and patient consent was therefore not required.
Statistical Analysis
Data analysis was performed using IBM SPSS Statistics (IBM releases 22 and 24). Parameters were compared between groups using Student’s t-test. Binary parameters were analyzed by χ2 tests. Multivariate logistic regression analysis was performed to identify independent predictors of EVD placement. Odds ratios (ORs) and 95% confidence intervals were calculated to assess the impacts of the variables. A P-value <0.05 was considered statistically significant.
Data Availability Statement: Data for this study will be provided upon reasonable request.
Results
Patient Characteristics
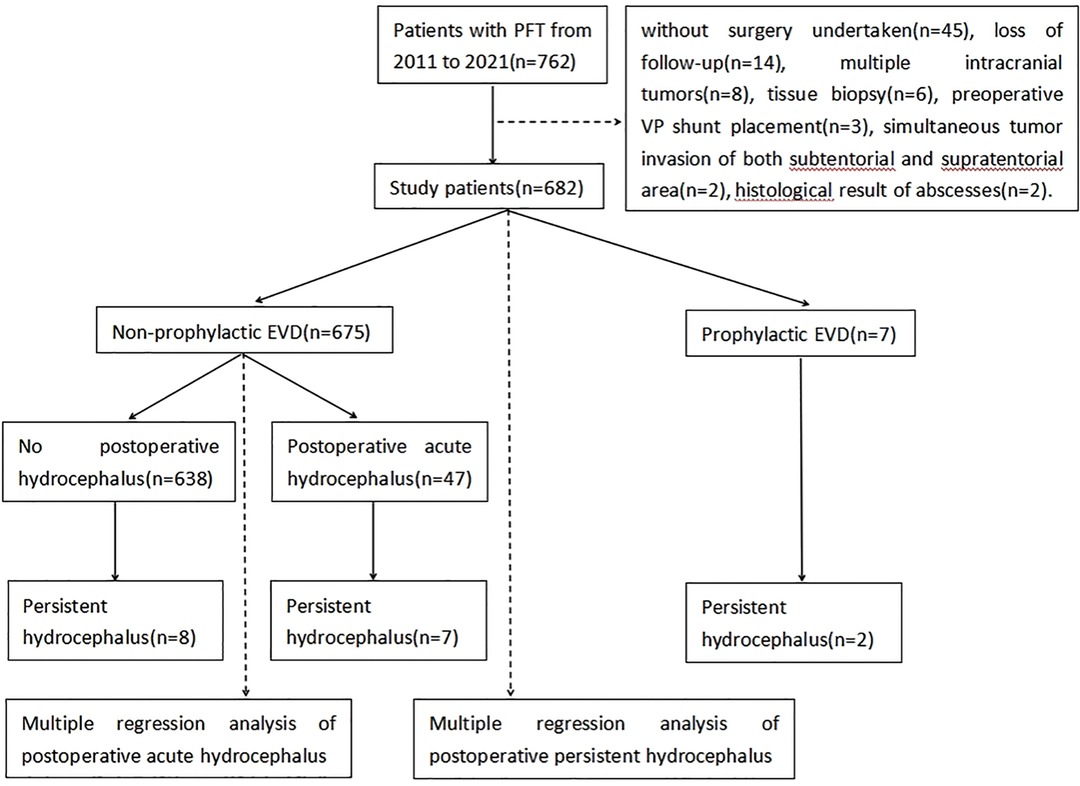
A total of 682 of 762 patients were finally enrolled in this study. Eighty patients were excluded in accordance with the inclusion criteria, including 42 patients without surgery, 14 patients who were lost to postoperative follow-up, eight patients with multiple intracranial tumors, six patients with biopsies, three patients with direct gamma-knife treatment, three patients with a preoperative VP shunt, two patients with simultaneous tumor invasion of both the subtentorial and supratentorial areas, and two patients with histological brain abscesses (Figure 4).
The average age of the 682 patients was 48.6 years (range 19–83 years). There were 272 (31%) men and 410 (69%) women. The pathological findings included 570 (84%) cases of benign tumors and 112 (16%) cases of malignant tumors.
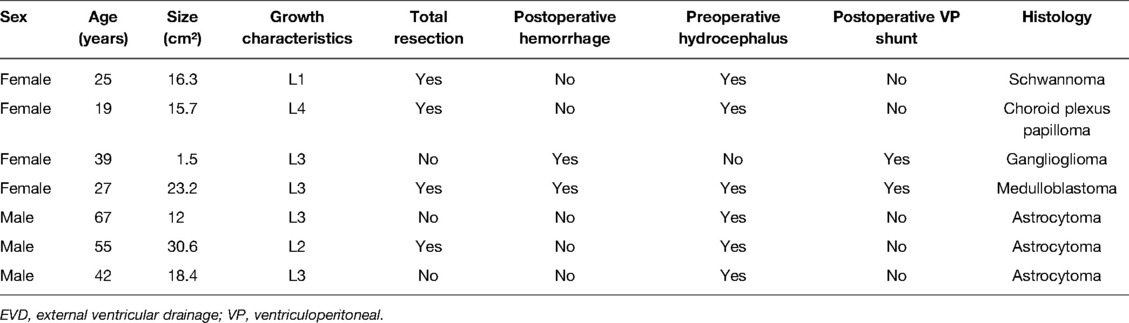
Seven of the enrolled patients (1%) received prophylactic EVD, including six patients with preoperative hydrocephalus who received preoperative EVD for severe intracranial hypertension and one patient without preoperative hydrocephalus who received EVD because of intraoperative hemorrhage and brain swelling. Two of the seven patients received a postoperative VP shunt due to EVD-weaning failure. Patient information is shown in Table 1.
A total of 136/675 (20%) patients presented with preoperative hydrocephalus. The symptoms of intracranial hypertension were resolved or alleviated in 113 of these 136 (83%) patients with preoperative hydrocephalus without prophylactic EVD placement. A total of 47/675 (7%) patients developed postoperative acute hydrocephalus, and 9 (19%) of these patients received a VP shunt due to EVD-weaning failure. In addition to these 47 patients, 8 of the 628 (13%) patients subsequently developed chronic hydrocephalus and received a VP shunt. Finally, 55 patients developed hydrocephalus and received an EVD or/and VP shunt placement postoperatively. Twenty-four of 55 (44%) patients with postoperative hydrocephalus had ventricular system infiltration. Demographic data and clinical characteristics of patients with PFTs are shown in Table 2.
Predictors of Postoperative Acute Hydrocephalus
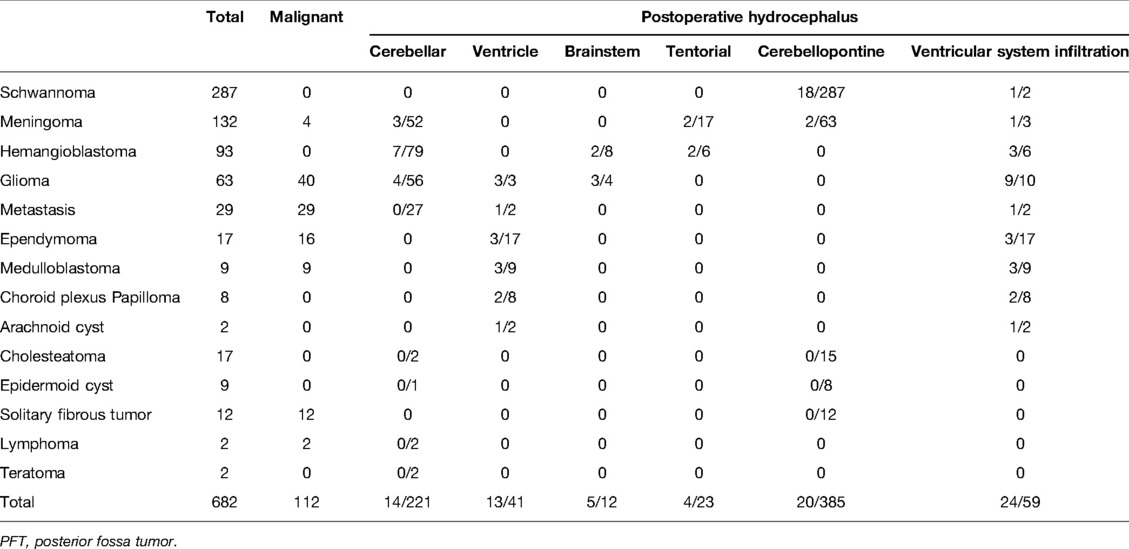
The nonprophylactic-EVD group included 675 patients [average age 48.6 years, range 19–83 years; 269 (40%) men and 406 (60%) women]. A total of 565 (84%) patients had benign tumors and 110 (16%) patients had malignant tumors; 23/136 (17%) patients with preoperative hydrocephalus received postoperative EVD due to unresolved or aggravated symptomatic hydrocephalus. Newly emerging acute hydrocephalus developed in 24/539 (4%) patients. The mean EVD-implantation time was 2.4 days (range 1–10 days). The highest morbidity rates in this group occurred in patients with schwannomas (42%) and meningiomas (20%). The rate of preoperative hydrocephalus was highest in patients with medulloblastomas (88%) and ependymomas (71%), while the rate of postoperative acute hydrocephalus was the highest in patients with choroid plexus papillomas (29%) and gliomas (15%). The rates of new acute hydrocephalus were the highest in patients with choroid plexus papillomas (29%) and hemangiomas (8%). The tumor type and incidence of perioperative hydrocephalus in the nonprophylactic-EVD group are shown in Table 3.
The incidence of postoperative acute hydrocephalus was 5% (32/594) in patients with tumors without ventricular system infiltration (L1), compared with 19% (15/81) in tumors with ventricular system infiltration (L2, L3, L4). Interestingly, none of the six cases of type L2 developed postoperative acute hydrocephalus. The incidences of postoperative hemorrhage were 56% (18/32) in cases of type L1 with postoperative acute hydrocephalus and 33% (5/15) in tumors with ventricular system infiltration (L2, L3, L4).
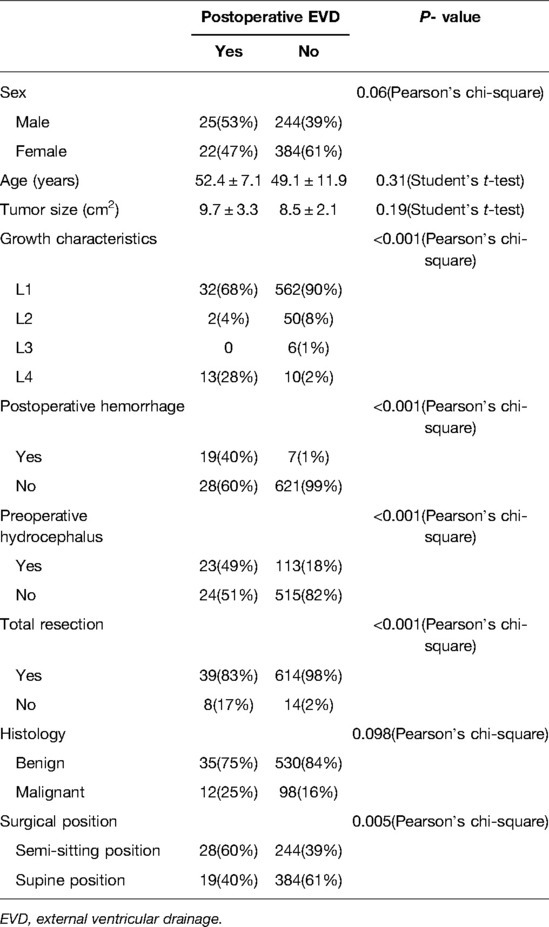
Univariate analysis showed that postoperative EVD due to acute hydrocephalus was significantly correlated with tumor growth characteristics (P < 0.001), resection degree, postoperative hemorrhage (P < 0.001), and preoperative hydrocephalus (P < 0.001) (Table 4). Multivariate analysis identified tumor type L4 (OR = 9.8), postoperative hemorrhage (OR = 66.7), and subtotal tumor resection (OR = 9.3) as independent risk factors for postoperative acute hydrocephalus (Table 5).
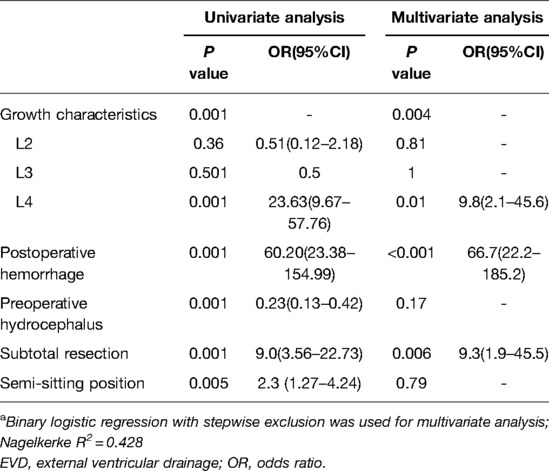
Table 5. Univariate and multivariate analyses of the association between postoperative EVD and factors.a
Predictors of Postoperative VP Shunt
Overall, 17/682 (3%) patients received postoperative VP shunt placement, including two (12%) patients who initially received postoperative ETV, followed by VP shunt placement for unresolved obstructive hydrocephalus. The mean implantation time was 62.4 days (range 14–191 days). In univariate analysis, postoperative VP shunt placement due to persistent hydrocephalus was significantly correlated with tumor growth characteristics (P < 0.001), postoperative hemorrhage (P = 0.001), preoperative hydrocephalus (P = 0.001), subtotal resection (P = 0.001), tumor pathology (P = 0.005), and surgical position (P = 0.04) (Table 6).
In multivariate analysis, both tumor infiltrating the ventricular system (OR = 58.5) and postoperative hemorrhage (OR = 28.1) were independent risk factors for postoperative VP shunt placement (Table 7).
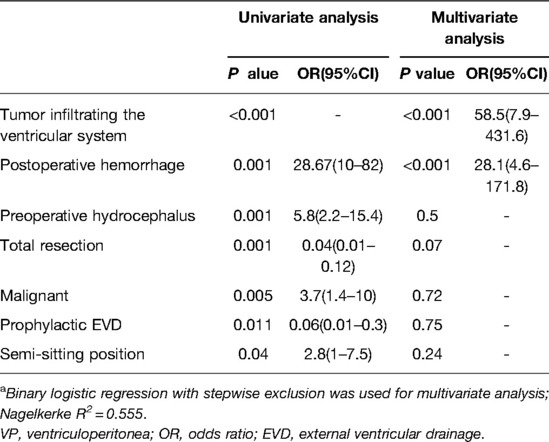
Table 7. Univariate and multivariate analyses of the association between postoperative VP shunt placement and factors.a
Discussion
Postoperative symptomatic hydrocephalus is a severe complication after resection of PFT, but there is still no consensus on the placement of a prophylactic EVD. The current study evaluated the incidence of perioperative hydrocephalus in adult patients with PFTs, with the aim of identifying the predictors of postoperative hydrocephalus requiring CSF drainage, and determined the value of prophylactic EVD. The incidence of preoperative hydrocephalus in adult patients with PFTs in our study was 20.8% and the incidence of persistent postoperative hydrocephalus was 3.6%, compared with rates of 21.4% and 2.1%, respectively, reported by Marx et al. (2) The ratio is much higher in pediatric patients (1), largely due to the different distribution of PFTs by histology in pediatric and adult patients. In our study, schwannomas and meningiomas accounted for the highest proportions of PFTs. Numerous studies have shown that these tumors rank in the top three PFTs in terms of morbidity (2, 18, 23). The symptoms of intracranial hypertension can be resolved or alleviated in most patients with preoperative hydrocephalus, and ETV or EVD prior to PFT surgery is not routinely recommended in adult patients.
In our study, the incidences of preoperative hydrocephalus were the highest in patients with medulloblastomas and ependymomas. These tumors had similar incidences and ranked second after metastasis among all PFTs in the study by Marx et al. (2). This is related to the corresponding tumor growth characteristics, with an increased risk of obstructing the CSF circulation pathway as the tumor volume increases. Preoperative hydrocephalus was not a significant predictor of postoperative hydrocephalus requiring drainage in our study. This was in contrast to the study by Won et al., who found that pilocytic astrocytoma and preoperative hydrocephalus were independent risk factors for the development of postoperative hydrocephalus in adult patients (11).
The incidence of postoperative hydrocephalus was the highest in patients with choroid plexus papillomas (29%) in our research. Two of the seven patients with choroid plexus papilloma who did not receive prophylactic EVD developed acute obstructive hydrocephalus after resection due to postoperative hemorrhage and cerebral edema, and one of these two also received a VP shunt due to EVD-weaning failure. Previous research suggested that plexus papilloma tumors showed the most significant association with VP shunt dependence (24), given that these tumors often invade the ventricle compartments, and ventricle entry has been reported to increase the risk of postoperative hydrocephalus. However, the incidence of plexus papilloma tumors was not high in another study (2). However, the current and previous studies all included small numbers of these patients, and further studies with larger data volumes are needed to clarify this.
In our study, postoperative acute hydrocephalus was relatively common in patients with choroid plexus papillomas, gliomas, and medulloblastomas, all of which are located near the midline. The incidence of postoperative acute hydrocephalus was 5% in tumors without ventricular system infiltration compared with 19% for tumors infiltrating the ventricular system. The incidence of postoperative acute hydrocephalus was thus lower in tumors without ventricular system infiltration and was closely related to postoperative hemorrhage, which is known to increase the risk of hydrocephalus. Some studies have also suggested that the incidence of postoperative hydrocephalus is higher in tumors located near the midline (25). Chen et al. analyzed fourth ventricular tumors and considered the upward growth of tumors as an independent risk factor for postoperative CSF drainage (26). Tumor growth was a significant factor in the current univariate analysis, while type L4 tumors were an independent risk factor for postoperative acute hydrocephalus in multivariate analysis, using type L1 tumors as a reference. Type L2 tumors are anatomically distant from the midbrain aqueduct, and releasing CSF, elevating the cerebellum, or even sacrificing portions of cerebellar tissue during surgery can make it easier to obtain adequate surgical space. Although type L3 tumors are located adjacent to the midbrain aqueduct, the space of the midbrain aqueduct above the lesion is often dilated, secondary to intracranial hypertension. Compared with type L2 and L3 tumors, resection of type L4 tumors is more difficult and can cause more severe brain-tissue damage. In addition, the midbrain aqueduct is a relatively narrow part of the ventricular system anatomically and is thus more susceptible to stenosis or obstruction from postoperative brain swelling, residual hematoma formation, the mass effect, and migration of hemostatic materials (26).
Won et al. found that placing patients in a semi-sitting position could reduce the incidence of postoperative hydrocephalus (18). This position has the advantage of improving the outflow of blood and protein-rich CSF during surgery. However, the semi-sitting position was not an independent predictor of postoperative hydrocephalus in the current study. This difference may be due to their routine selection of the semi-sitting position, while we select this position for most midline tumors and partial nonmidline tumors.
Total resection was an independent protective factor for postoperative acute hydrocephalus in our study. Residual tumors may have a mass effect, which is particularly evident for tumors that infiltrate the ventricles. Clinically, tumors with unclear margins and a rich blood supply are more difficult to resect totally. Postoperative local cerebral edema around these tumors can be severe, and the risk of postoperative cerebral hemorrhage is higher, leading to a higher incidence of postoperative acute obstructive hydrocephalus. However, total resection is not appropriate for all PFTs. Won et al. suggested that total resection of intraparenchymal tumors was a protective factor for CSF drainage (18). Some studies have suggested that postoperative acute hydrocephalus is related to brain swelling caused by the delayed opening of the venous sinus after the recovery of blood supply to the compressed brain tissues (27, 28). For tumors without venous sinus infiltration or compression, total resection can provide more space to alleviate the effects caused by brain swelling. However, for tumors infiltrating the venous sinus, we often select subtotal resection because previous studies suggested that treating subtentorial meningiomas with total venous sinus resection was a risk factor for postoperative acute hydrocephalus (29, 30).
Postoperative hemorrhage is a serious complication, with a reported incidence in PFTs of 3%, most of which are substantial tumors (31). The incidence of postoperative hemorrhage in the current study was 4% and usually occurred within 24 h after surgery. Supratentorial hemorrhage has a mass effect and postoperative hemorrhage near the midline can block the CSF pathway, thus causing acute obstructive hydrocephalus. However, the incidence of postoperative hemorrhage can be effectively reduced by appropriate management of intracranial pressure, perioperative blood pressure, and abnormal coagulation. The incidence of postoperative persistent hydrocephalus in the present study was 2%, compared with 6% (11, 32) reported by Marx et al. and 10% by Won et al. (18).
Tumor infiltration of the ventricular system was an independent risk factor for VP shunt placement after resection, consistent with the conclusion that postoperative hydrocephalus is more likely to occur when tumors are located near the midline (13). This may be due to differences in tumor location and histological characteristics; compared with type L1 tumors, tumors infiltrating the ventricular system are usually associated with increased risks of intraventricular hemorrhage and postoperative hydrocephalus (33). Furthermore, recurrence of these tumors can be more prone to cause obstruction of the CSF pathway.
The current study did not consider if prophylactic EVD could reduce the occurrence of postoperative persistent hydrocephalus. Radiographic upward herniation is common in patients with obstructive hydrocephalus associated with a PFT. It is advisable to be cautious when placing a preoperative EVD in patients with a PFT causing radiographic upward herniation, and this intervention should be reserved for patients with severe symptomatic obstructive hydrocephalus, in whom tumor resection is not immediately feasible. It is also reasonable to maintain a relatively high level of drainage about the tragus to avoid sudden or excessive CSF outflow (34). This is one reason why prophylactic EVD is not generally placed in our center. Lumbar drainage placement is another controversial method of prophylactic CSF drainage. The potential risk of down-herniation cannot be ignored, and removal of the CSF from below the tentorium was proposed to increase the pressure gradient between the supratentorial and lumbar cistern compartments, promoting transtentorial herniation. Pneumocephalus enhanced this pressure gradient, thus causing clinical herniation syndrome (35). Excessive drainage due to EVD can cause localized hydrocephalus and increase the risk of shunt dependence (36). Some studies support the use of postoperative ETV, which has been reported to be effective in cases of EVD-weaning failure as an alternative to VP shunt placement (37). The success rate of ETV for the avoidance of shunt placement was 72% in adult patients with obstructive hydrocephalus due to aqueductal stenosis (38), which was higher than in pediatric patients (39); however, there is currently no consensus on this issue (40, 41). According to Srinivasan et al., the incidence of postoperative complications with ETV was 9%, and the rate of ETV failure was higher in postresection cases with posterior fossa ependymomas (42).
The propositions for early and direct VP shunt placement differ. We recommend initial EVD insertion when postoperative acute hydrocephalus occurs, but seven of 47 (15%) patients in the current study with postoperative acute hydrocephalus subsequently received a VP shunt due to EVD-weaning failure. The proposed mechanism of acute hydrocephalus involves increased resistance of CSF flow caused by local cerebral edema or blood clots in the ventricular system. In contrast, chronic hydrocephalus may be caused by persistent obstruction of the arachnoid granulations by blood-breakdown products and their inflammatory reactions (43). In patients with postoperative acute hydrocephalus, the incidence of persistent hydrocephalus can be reduced by using EVD to get through the cerebral edema period and drain intraventricular blood clots, thus avoiding the complications of shunt implantation. According to our institutional standards, no subdural, epidural, or intratumoral drainage tubes are placed after PFT resection, and repeated irrigation with saline after resection is therefore necessary to find the source of a hemorrhage and clear the residual blood before suturing.
The association between the degree of resection and the development of postoperative persistent hydrocephalus is controversial. Total resection was reported to reduce the occurrence of long-term postoperative hydrocephalus (44), possibly as a result of relieving the obstructive effect or slow growth kinetics. Re-resection can be selected in cases of symptomatic obstructive hydrocephalus caused by tumor recurrence.
Limitations
This study had some limitations. First, total resection was a protective factor for postoperative persistent hydrocephalus in this study; however, the postoperative follow-up time varied from 3 months to 6 years, and it is therefore unclear if the patients with a short follow-up time might go on to develop hydrocephalus in the future. This might lead to potential bias in the results. Second, the study included few cases of ETV, and our ability to evaluate ETV was thus limited. Third, the prophylactic-EVD group only included seven patients. To reduce bias, we did not analyze this group separately, but we did include prophylactic EVD as a covariable in the analysis of all samples. Fourth, bias of surgical position selection based on the tumor location may have affected the analysis. Finally, differences in surgical procedures and perioperative management exist in different medical centers, which may influence the results.
Conclusion
Postoperative acute hydrocephalus is associated with tumor infiltration of the midbrain aqueduct, which can be determined by preoperative imaging studies, and prophylactic EVD is recommended for patients with this tumor characteristic. Postoperative persistent hydrocephalus is associated with tumor infiltration of the upper part of the infratentorial ventricular system, and prophylactic EVD is useless in these cases, but postoperative EVD insertion can significantly decrease the incidence of shunt dependence in patients with postoperative acute hydrocephalus. Total resection and measures to avoid postoperative hemorrhage can reduce the incidences of postoperative acute and persistent hydrocephalus. The results of this study and the identified predictors may provide insights and help manage patients with PFTs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongnan Hospital of Wuhan University. Because this study did not involve patients’ personal identity and private information, it did not require patients’ consent. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceptualization: CZ; data curation: CZ, TZ; formal analysis: LG, TZ; methodology: ZL, JC; project administration: TZ; resources: JC; software: CZ; writing—original draft: CZ, TZ; writing—review and editing: ZL, JC. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Leonie McKinlay, DVM, and Susan Furness, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of drafts of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin CT, Riva-Cambrin JK. Management of posterior fossa tumors and hydrocephalus in children: a review. Childs Nerv Syst. (2015) 31(10):1781–9. doi: 10.1007/s00381-015-2781-8
2. Marx S, Reinfelder M, Matthes M, Schroeder H, Baldauf J. Frequency and treatment of hydrocephalus prior to and after posterior fossa tumor surgery in adult patients. Acta Neurochir (Wien). (2018) 160(5):1063–71. doi: 10.1007/s00701-018-3496-x
3. Taylor WA, Todd NV, Leighton SE. CSF drainage in patients with posterior fossa tumours. Acta Neurochir (Wien). (1992) 117(1–2):1–6. doi: 10.1007/BF01400627
4. Di Rocco F, Jucá CE, Zerah M, Sainte-Rose C. Endoscopic third ventriculostomy and posterior fossa tumors. World Neurosurg. (2013) 79(2 Suppl):S18.e15–9. doi: 10.1016/j.wneu.2012.02.018
5. Sainte-Rose C, Cinalli G, Roux FE, Maixner R, Chumas PD, Mansour M, et al. Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg. (2001) 95(5):791–7. doi: 10.3171/jns.2001.95.5.0791
6. Goel A. Whither preoperative shunts for posterior fossa tumours? Br J Neurosurg. (1993) 7(4):395–9. doi: 10.3109/02688699309103494
7. Schmid UD, Seiler RW. Management of obstructive hydrocephalus secondary to posterior fossa tumors by steroids and subcutaneous ventricular catheter reservoir. J Neurosurg. (1986) 65(5):649–53. doi: 10.3171/jns.1986.65.5.0649
8. Tamburrini G, Massimi L, Caldarelli M, Di Rocco C. Antibiotic impregnated external ventricular drainage and third ventriculostomy in the management of hydrocephalus associated with posterior cranial fossa tumours. Acta Neurochir (Wien). (2008) 150(10):1049–55. discussion 1055–6. doi: 10.1007/s00701-008-0022-6
9. Bouras T, Sgouros S. Complications of endoscopic third ventriculostomy: a systematic review. Acta Neurochir Suppl. (2012) 113:149–53. doi: 10.1007/978-3-7091-0923-6_30
10. Dewan MC, Lim J, Shannon CN, Wellons JR. The durability of endoscopic third ventriculostomy and ventriculoperitoneal shunts in children with hydrocephalus following posterior fossa tumor resection: a systematic review and time-to-failure analysis. J Neurosurg Pediatr. (2017) 19(5):578–84. doi: 10.3171/2017.1.PEDS16536
11. Won SY, Dubinski D, Behmanesh B, Bernstock JD, Seifert V, Konczalla J, et al. Management of hydrocephalus after resection of posterior fossa lesions in pediatric and adult patients-predictors for development of hydrocephalus. Neurosurg Rev. (2020) 43(4):1143–50. doi: 10.1007/s10143-019-01139-8
12. Papo I, Caruselli G, Luongo A. External ventricular drainage in the management of posterior fossa tumors in children and adolescents. Neurosurgery. (1982) 10(1):13–5. doi: 10.1097/00006123-198201000-00002
13. Culley DJ, Berger MS, Shaw D, Geyer R. An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery. (1994) 34(3):402–7. discussion 407–8. doi: 10.1227/00006123-199403000-00003
14. Foreman P, McClugage SR, Naftel R, Griessenauer CJ, Ditty BJ, Agee BS, et al. Validation and modification of a predictive model of postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr. (2013) 12(3):220–6. doi: 10.3171/2013.5.PEDS1371
15. Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. The natural history of ventriculomegaly and tonsillar herniation in children with posterior fossa tumours – an MRI study. Pediatr Neurosurg. (2003) 39(5):246–53. doi: 10.1159/000072869
16. Gopalakrishnan CV, Dhakoji A, Menon G, Nair S. Factors predicting the need for cerebrospinal fluid diversion following posterior fossa tumor surgery in children. Pediatr Neurosurg. (2012) 48(2):93–101. doi: 10.1159/000343009
17. Helmbold LJ, Kammler G, Regelsberger J, Fritzsche FS, Emami P, Schüller U, et al. Predictive factors associated with ventriculoperitoneal shunting after posterior fossa tumor surgery in children. Childs Nerv Syst. (2019) 35(5):779–88. doi: 10.1007/s00381-019-04136-w
18. Won SY, Gessler F, Dubinski D, Eibach M, Behmanesh B, Herrmann E, et al. A novel grading system for the prediction of the need for cerebrospinal fluid drainage following posterior fossa tumor surgery. J Neurosurg. (2019) 132(1):296–305. doi: 10.3171/2018.8.JNS181005
19. Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelsø C. Estimated ventricle size using Evans index: reference values from a population-based sample. Eur J Neurol. (2017) 24(3):468–74. doi: 10.1111/ene.13226
20. Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A, et al. The insertion and management of external ventricular drains: an evidence-based consensus statement: a statement for healthcare professionals from the neurocritical care society. Neurocrit Care. (2016) 24(1):61–81. doi: 10.1007/s12028-015-0224-8
21. Stratchko L, Filatova I, Agarwal A, Kanekar S. The ventricular system of the brain: anatomy and normal variations. Semin Ultrasound CT MR. (2016) 37(2):72–83. doi: 10.1053/j.sult.2016.01.004
22. Lescher S, Schniewindt S, Jurcoane A, Senft C, Hattingen E. Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg Focus. (2014) 37(6):E3. doi: 10.3171/2014.9.FOCUS14479
23. Chen T, Ren Y, Wang C, Huang B, Lan Z, Liu W, et al. Risk factors for hydrocephalus following fourth ventricle tumor surgery: a retrospective analysis of 121 patients. PLoS One. (2020) 15(11):e0241853. doi: 10.1371/journal.pone.0241853
24. Won S, Dubinski D, Behmanesh B, Bernstock JD, Seifert V, Konczalla J, et al. Management of hydrocephalus after resection of posterior fossa lesions in pediatric and adult patients—predictors for development of hydrocephalus. Neurosurg Rev. (2020) 43(4):1143–50. doi: 10.1007/s10143-019-01139-8
25. Hosainey S, Lassen B, Hald JK, Helseth E, Meling TR. Risk factors for new-onset shunt-dependency after craniotomies for intracranial tumors in adult patients. Neurosurg Rev. (2018) 41(2):465–72. doi: 10.1007/s10143-017-0869-1
26. McLaurin RL. Disadvantages of the preoperative shunt in posterior fossa tumors. Clin Neurosurg. (1983) 30:286–92. doi: 10.1093/neurosurgery/30.CN_suppl_1.286
27. Ktari O, Frassanito P, Gessi M, Bianchi F, Tamburrini G, Massimi L. Gelfoam migration: a potential cause of recurrent hydrocephalus. World Neurosurg. (2020) 142:212–7. doi: 10.1016/j.wneu.2020.06.214
28. Bateman GA, Fiorentino M. Childhood hydrocephalus secondary to posterior fossa tumor is both an intra- and extraaxial process. J Neurosurg Pediatr. (2016) 18(1):21–8. doi: 10.3171/2016.1.PEDS15676
29. Gessler F, Bruder M, Duetzmann S, Tritt S, Bernstock JD, Seifert V, et al. Risk factors governing the development of cerebral vein and dural sinus thrombosis after craniotomy in patients with intracranial tumors. J Neurosurg. (2018) 128(2):373–9. doi: 10.3171/2016.11.JNS161871
30. Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O. True petroclival meningiomas: results of surgical management. J Neurosurg. (2014) 120(1):40–51. doi: 10.3171/2013.8.JNS13535
31. Seifert V. Clinical management of petroclival meningiomas and the eternal quest for preservation of quality of life: personal experiences over a period of 20 years. Acta Neurochir (Wien). (2010) 152(7):1099–116. doi: 10.1007/s00701-010-0633-6
32. Dubey A, Sung WS, Shaya M, Patwardhan R, Willis B, Smith D, et al. Complications of posterior cranial fossa surgery—an institutional experience of 500 patients. Surg Neurol. (2009) 72(4):369–75. doi: 10.1016/j.surneu.2009.04.001
33. Garton T, Keep RF, Wilkinson DA, Strahle JM, Hua Y, Garton HJ, et al. Intraventricular hemorrhage: the role of blood components in secondary injury and hydrocephalus. Transl Stroke Res. (2016) 7(6):447–51. doi: 10.1007/s12975-016-0480-8
34. Braksick SA, Himes BT, Snyder K, Van Gompel JJ, Fugate JE, Rabinstein AA. Ventriculostomy and risk of upward herniation in patients with obstructive hydrocephalus from posterior fossa mass lesions. Neurocrit Care. (2018) 28(3):338–43. doi: 10.1007/s12028-017-0487-3
35. Açikbaş SC, Akyüz M, Kazan S, Tuncer R. Complications of closed continuous lumbar drainage of cerebrospinal fluid. Acta Neurochir (Wien). (2002) 144(5):475–80. doi: 10.1007/s007010200068
36. Ang BT, Steinbok P, Cochrane DD. Etiological differences between the isolated lateral ventricle and the isolated fourth ventricle. Childs Nerv Syst. (2006) 22(9):1080–5. doi: 10.1007/s00381-006-0046-2
37. Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C. Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst. (2008) 24(12):1405–12. doi: 10.1007/s00381-008-0699-0
38. Amini A, Schmidt RH. Endoscopic third ventriculostomy in a series of 36 adult patients. Neurosurg Focus. (2005) 19(6):E9. doi: 10.3171/foc.2005.19.6.9
39. Mohanty A, Biswas A, Satish S, Vollmer DG. Efficacy of endoscopic third ventriculostomy in fourth ventricular outlet obstruction. Neurosurgery. (2008) 63(5):905–13. discussion 913–4. doi: 10.1227/01.NEU.0000333262.38548.E1
40. Srinivasan HL, Foster MT, van Baarsen K, Hennigan D, Pettorini B, Mallucci C. Does pre-resection endoscopic third ventriculostomy prevent the need for post-resection CSF diversion after pediatric posterior fossa tumor excision? A historical cohort study and review of the literature. J Neurosurg Pediatr. (2020):1–10. doi: 10.3171/2019.12.PEDS19539
41. Marx S, El DA, Manwaring J, El RE, Fleck S, Fritsch M, et al. Endoscopic third ventriculostomy before posterior fossa tumor surgery in adult patients. J Neurol Surg A Cent Eur Neurosurg. (2018) 79(2):123–9. doi: 10.1055/s-0037-1608786
42. Lam S, Harris DA, Lin Y, Rocque BG, Ham S, Pan IW. Outcomes of endoscopic third ventriculostomy in adults. J Clin Neurosci. (2016) 31:166–71. doi: 10.1016/j.jocn.2016.03.004
43. Kuo LT, Lu HY, Tsai JC, Tu YK. Prediction of shunt dependency after intracerebral hemorrhage and intraventricular hemorrhage. Neurocrit Care. (2018) 29(2):233–40. doi: 10.1007/s12028-018-0532-x
Keywords: PFT, hydrocephalus, adult, evd, VP shunt
Citation: Zhang C, Zhang T, Ge L, Li Z and Chen J (2022) Management of Posterior Fossa Tumors in Adults Based on the Predictors of Postoperative Hydrocephalus. Front. Surg. 9:886438. doi: 10.3389/fsurg.2022.886438
Received: 28 February 2022; Accepted: 9 May 2022;
Published: 1 June 2022.
Edited by:
Philipp Taussky, University of Utah, United StatesReviewed by:
Michel Roethlisberge, University Hospital of Basel, SwitzerlandPaul Krafft, University of South Florida, United States
Ivo Peto, University of South Florida, United States
Copyright © 2022 Zhang, Zhang, Ge, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jincao Chen amluY2Fvem5AMTYzLmNvbQ== Zhengwei Li bGl6aGVuZ3dlaTEwMDZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Chengda Zhang
Chengda Zhang Tingbao Zhang
Tingbao Zhang Lingli Ge3
Lingli Ge3 Zhengwei Li
Zhengwei Li Jincao Chen
Jincao Chen