
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 20 May 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.886135
 Mariarita Tarallo1*
Mariarita Tarallo1* Cristina Carruezzo1
Cristina Carruezzo1 Filippo Maria Dentice Di Accadia1
Filippo Maria Dentice Di Accadia1 Antonella Del Gaudio2
Antonella Del Gaudio2 Damiano Caruso2
Damiano Caruso2 Michela Polici2
Michela Polici2 Daniele Crocetti1
Daniele Crocetti1 Umberto Costi1
Umberto Costi1 Andrea Polistena1
Andrea Polistena1 Francesco Panzuto2,3
Francesco Panzuto2,3 Andrea Laghi2
Andrea Laghi2 Giuseppe Cavallaro1
Giuseppe Cavallaro1 Enrico Fiori1
Enrico Fiori1
Introduction: Multiple gastrointestinal stromal tumors (GISTs) are rare tumors. Differential diagnosis between metastatic and multiple GISTs represents a challenge for a proper workup, prediction prognosis, and therapeutic strategy.
Case presentation: We present the case of 67-year-old man with computed tomography (CT) evidence of multiple exophytic lesions in the abdomen, reaching diameters ranging from 1 to 9 cm, without any signs of organs infiltration, and resulting positive at 18F-FDG-PET/CT. Laparoscopic biopsy revealed multiple GISTs, and surgical resection by using an open approach was performed to achieve radicality. Moreover, an extensive review of the current literature was performed.
Results: Small GISTs (<5 cm) can be treated by the laparoscopic approach, while in the case of large GISTs (>5 cm), tumor location and size should be taken into account to reach the stage of radical surgery avoiding tumor rupture. For metastatic GISTs, Imatinib represents the first choice of treatment, and surgery should be considered only in a few selected cases when all lesions are resectable.
Conclusion: Sporadic multiple GISTs are a rare event, imaging findings are not specific for GISTs, and biopsy requires a secure diagnosis and proper management. In the case of large lesions, with a high risk of vessels injury, laparotomy excision should be considered to achieve radicality and to avoid tumor rupture.
Gastrointestinal stromal tumors (GISTs) are rare neoplasms (less than 1% of all gastrointestinal malignancies), representing the most common mesenchymal tumors of the gastrointestinal (GI) tract with an estimated annual incidence of 10–15 cases per million (1). GISTs usually appear as a single mass or, in rare occasions, as multiple lesions.
GISTs mostly occur in older individuals, having the median age of 55–65 years with a slightly male predominance (2). These tumors originate from the cells of Cajal, a site in muscularis propria and in the GI myenteric plexus, known as pacemaker cells of bowel peristalsis (3). The most common sites affected are the stomach (50%), small bowel (25%), colon-rectum (5%), and esophagus (<5%), and they may rarely occur in the omentum and mesentery (<5%), known as extra-GISTs (EGISTs) (4).
To date, initial diagnosis can be challenging because symptoms and signs are often mild and non-specific (e.g., nausea, vomiting, abdominal discomfort, and weight loss). However, in a few cases, GISTs can cause severe conditions such as bleeding, tumor rupture, dysphagia, and bowel or biliary obstruction (5). Severe symptoms are usually correlated to high-risk GIST according to Armed Forces Institute of Pathology risk classification, which is widely used to stratify patients (6, 7). According to the American Joint Committee of Cancer (AJCC) Staging Manual, 8th edition, tumor size, tumor location, and mitotic index are the key features correlated with patients’ prognosis (6–8).
Contrast enhanced computed tomography (CT) plays a primary role from diagnosis to follow-up; magnetic resonance imaging (MRI) represents a valid tool in selected cases (e.g., rectal GIST) (9). [18F]2-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography (18F-FDG-PET/CT) represents a valid imaging modality to assess early response to therapy, restaging, and follow-up.
In order to select the best surgical approach, an accurate examination for multiple GISTs should consider the localization of the masses and the size of the greater lesion. The aim of our study is to present a case of multiple GISTs with a dedicated focus on imaging findings and surgical management.
We present a case of a 67-year-old man with a past medical history of hypertension, hyperuricemia, and left inguinal hernioplasty, who presented to the Emergency Department with a new onset of asthenia, abdominal pain, hypochromic stools, and hyperchromic urine.
His complete blood count was normal, except for elevated values of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT).
The patient had already undergone contrast enhanced abdomen CT scan with a dedicated and optimized protocol (10), revealing two mass-like lesions, with diameters of 7 and 9 cm in the right iliac fossa and in the pelvis, respectively. These were characterized by moderate enhancement, rare hypodense areas due to the presence of necrotic- or cystic-changes, with focal spots of hemorrhages, and well-defined margins (Figure 1). The growth pattern was manly exophytic, without any signs of infiltration of organs and structures such as the ileum and vessels, which are closely adjacent. Other multiple similar smaller lesions were found on the left flank, with maximum diameters of 3 cm, and a 1-cm mass was found in the epigastric region. Furthermore, multiple pericentimetric solid nodules altered spleen parenchyma, with a high suspicion of metastatic nature.
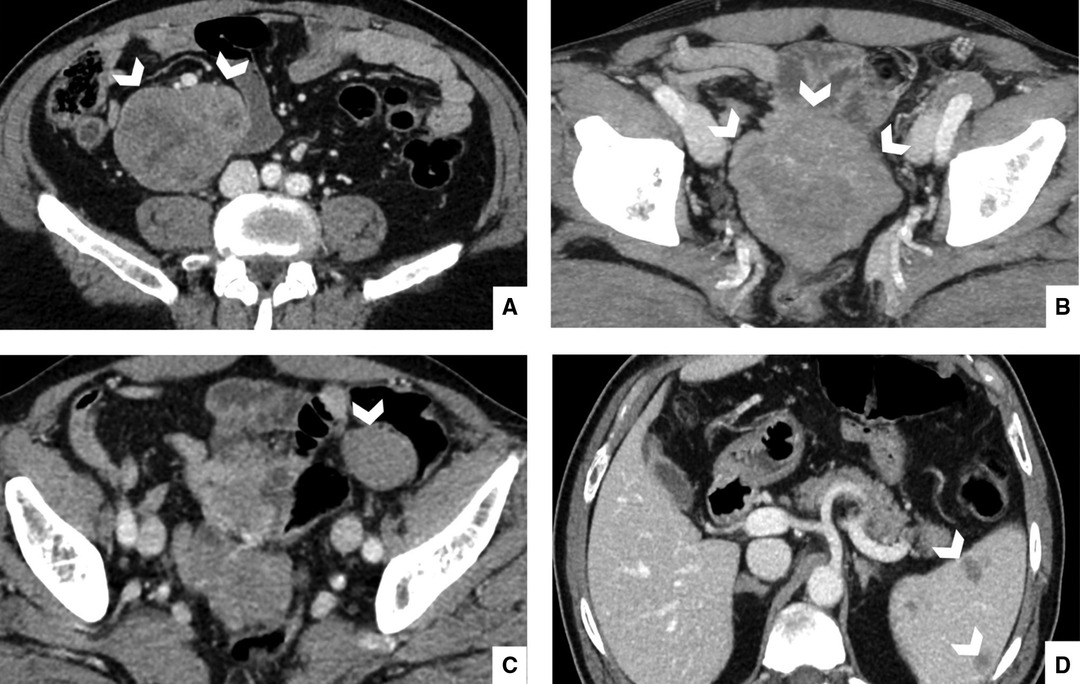
Figure 1. Portal phase, enhanced computed tomography of 67-year-old man, admitted to the Emergency Department with abdominal pain. Portal scans of the abdomen and pelvis shown in the right iliac fossa (A), a mass with moderate enhancement, measuring 7 cm, with hypodense areas. This mass had well-defined margins and appeared in contact with the ilium and vessels, but it did not infiltrate them. Another similar mass (B) appeared in the pelvic cavity in contact with the ileum; it measured a maximum diameter of 9 cm; this mass was also expansive but did not infiltrate adjacent structures. Other multiple smaller masses (C) were found on the right and left flank with maximum diameters of 3 cm. Multiple nodular pericentimetric formations (D) were present in the spleen, which were slightly hypodense.
The second step of diagnosis was 18F-FDG-PET/CT, in which almost all lesions showed a vivid uptake (SUV Max 7.3), reflecting an intense metabolic activity, with the exception of splenic lesions (Figure 2).
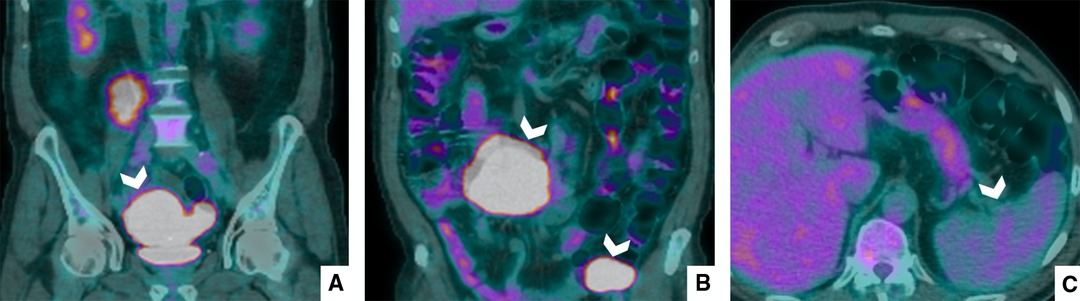
Figure 2. PET/CT with 18F-FDG. PET/CT shows that all lesions (A,B) had intense metabolic activity; however, the splenic nodular areas (C) did not pick up FDG.
Then, for the final diagnosis, a biopsy of the pelvic mass was performed. Histological data demonstrated a neoplastic proliferation, with fibrous septa, showing a lobulated appearance, formed by cells with an elongated, hyperchromatic nucleus, with a proliferation index of more than 10%, without evidence of atypical mitosis; the cytoplasm was poorly definable and weakly eosinophilic. Neoplastic cells resulted positive for the reactions set up with anti-vimentin, anti-CD117, anti-CD99, anti-BCL2, and focally anti-EMA, while negative data emerged from the tests for antiprotein S100, antipancytokeratin, antismooth muscle actin, antidesmin, anti-CD34, anti-CD31, antipodoplanin, anti-HMB45, anticytokeratin 6/6, and anti-WT1.
At first, the patient underwent surgery with the laparoscopic technique. The exploration of abdominal cavity revealed a 7-cm mass in the right flank, close to the mesentery, a 4-cm mass in the left iliac fossa, and a smaller mass inside the omentum in the epigastric region. The omental mass was removed and analyzed by extemporaneous histological examination that reported a mesenchymal neoplasm. Consequently, complete surgical excision remained the main goal, and a midline laparotomy was carried out. During ileum mobilization, the 9-cm mass shown by CT was detected in the pelvis in tight contact with the ileum. The mass was mobilized with the intestinal loops and removed with segmental ileal loop resection. The 7-cm mass was shown close to the superior mesenteric vessels, so its complete removal required cautious handling. The mass on the left iliac region was also completely removed. All visible masses were completely removed, taking care to avoid tumor rupture (Figure 3). The definitive histological examination established that the diagnosis was multiple GISTs with spindle and epithelioid cells. Neoplastic cells were positive for DOG-1 and c-Kit. The marker of proliferation Ki-67 was 5%–10%.
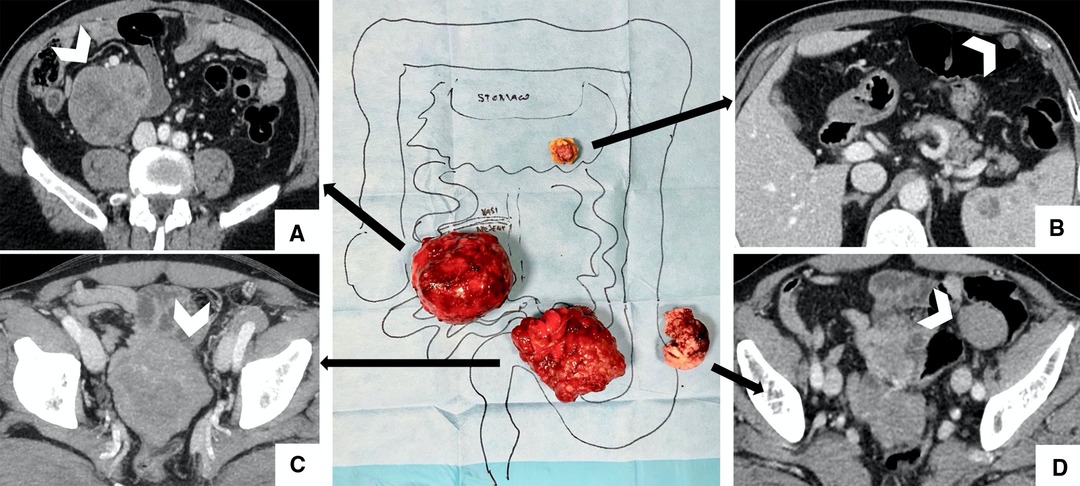
Figure 3. Surgical findings with imaging correlation. (A) Complete removal of the 7-cm mass is shown in the right iliac flank, close to the superior mesenteric vessels; (B) Centimetric omental mass was removed and analyzed by extemporaneous histological examination; (C) 9-cm mass (shown at CT in the pelvis) removed with segmental ileal loop resection to perform an en-bloc removal; (D) 4-cm mass in the left iliac region was completely removed.
The patient underwent adjuvant therapy with Imatinib. CT and PET/CT scan after 6 months showed a full resolution of the clinical picture, with no uptake of 18-FDG upon PET/CT scan. The patient is still receiving adjuvant therapy, and a CT scan is scheduled at 12 months’ time (6 months from the last one).
We described the case of a patient affected by multiple GISTs, with intense abdominal pain and hypochromic stools and hyperchromic urine, who underwent surgical procedure upon admission in the Emergency Department. Our intent was to achieve the radical resection of all lesions without considering the neoadjuvant therapeutic option due to the emergency and symptomatic presentation of the patient. Postoperative histological examination and mutational panel analysis revealed multiple GISTs.
GISTs are usually solitary lesions, but in extremely rare cases, multiple GISTs may be detected in one or more organs. Single GISTs are often characterized by good prognosis in comparison with multiple GISTs, according to recent evidence (11).
With regard to radiologic findings, GISTs are usually represented as heterogeneous-enhancing mesenteric lesions with hemorrhagic or necrosis areas or cystic components. Many lesions present as isolated masses in the mesentery entering the differential diagnosis of GISTs, in particular: desmoid fibromatosis (DF), sclerosis mesenteritis (SM), lymphoma, neuroendocrine tumor (NET), and liposarcoma. DF is a soft tissue mass mainly composed of mesenchymal tissue. Upon unenhanced CT, it has a homogenous density and enhances slightly heterogeneously with intravenous contrast. SM presents with the characteristic “Halo sign”, a hyperdense mass with surrounding mesenteric vessels and soft tissue nodules, and frequently contains calcifications. A pseudocapsule can be present, with a dense band with normal fat surrounding the inflamed lesion. The classical CT appearance of mesenteric lymphoma is a “sandwich” appearance: an extensive homogeneously enhancing lymphadenopathy and occasional central necrosis enveloping the mesenteric vessels with preserved perivascular fat borders. Usually, NETs are hypervascular lesions with fibrous adhesive bands with outward radiating peritumoral vessels and contain coarse calcifications. Liposarcoma generally shows a non-homogeneous lesion with fat density intermixed with zones of denser tissue; the borders are poorly defined and often infiltrating. Contrast uptake is heterogeneous and progressive (12, 13).
One of the main challenges is the differential diagnosis of multiple sporadic GISTs when non-syndromic, which are often misdiagnosed as metastatic GISTs (14). In fact, multiple GISTs could often occur in the case of Carney’s triad, Carney–Stratakis syndrome, and neurofibromatosis type I (15, 16); nevertheless, multicentricity alone is not an evidence of a syndromic or hereditary disease (17). In multiple GISTs, each lesion is characterized by different mutational panels, while metastatic GISTs show the same genetic pattern in all lesions. In fact, some authors state that a genetic analysis of KIT/PDGFRA could be helpful for differential diagnosis (17, 18).
To date, some authors have published their personal experiences in the form of case reports or small case series of multiple GISTs. Shen et al. describe how they classified patients based on genetic analysis of the specimen, starting from 44 initial diagnosis of multiple GISTs to 27 truly multiple sporadic GISTs. They performed mutation analysis with genomic DNA isolated from formalin-fixed paraffin-embedded tissues; all patients underwent surgery with the removal of all lesions (19). Li et al. state that radical surgery is the primary treatment for multiple GISTs, and that it is important to evaluate the number and sites of tumors for the removal of all lesions. They reported a personal record of 20 patients affected by multiple GISTs (17).
The current guidelines do not define a structured workflow for these patients (9) due the limited scientific evidence about multiple sporadic GISTs (19). However, a surgical procedure with a radical approach may be considered as a first treatment option for multiple non-metastatic GISTs. However, Imatinib, a tyrosine kinase inhibitor of KIT/PDGFRA, is considered the first option for metastatic and/or unresectable GISTs (20).
According to the size and the number of lesions, GISTs are categorized into three groups: small GISTs, large GISTs, and metastatic GISTs (21). In general, small GISTs (<5 cm) are resected with R0 pathology results, without the need for lymphadenectomy, also in consideration of expansive growth and the extremely rare occurrence of nodal metastasis. Today, the laparoscopic approach can be considered the standard procedure, because it has proved to be less invasive and is characterized by shorter postoperative time of recovery in comparison with conventional laparotomy (21). Recently, Nishida et al. reviewed the literature with the aim of summing up the main guidelines about the treatment of small GISTs, and they observed that there is no global consensus (21). In fact, Japanese and Asian GIST guidelines recommend surgical resection for gastric GISTs <2 cm (22, 23), while the National Comprehensive Cancer Network (NCCN) guidelines recommend surgical resection only for small gastric GISTs with high-risk features (24). However, all the main guidelines, including the NCCN and ESMO guidelines (1, 24), suggest the surgical approach for treating small rectal GISTs due to the different clinical behaviors and prognostic outcomes.
Large GISTs are considered tumors bigger than 5 cm, and complete surgical excision should be considered (9, 24). The main goal of surgery is to achieve radicality without the risk of tumor rupture, representing an adverse prognostic factor (25). In such a scenario, laparoscopy can also be performed for large GISTs, but it can be performed with accurate surgical planning by considering tumor location and size, with a specific focus on tumors greater than 8 cm, for which the chance to use the laparoscopic approach seems to be limited (21). In case of large GISTs with an infiltrating growth pattern, surgical resection is recommended with the object to preserve as much as possible the functionality of the organs involved (26).
When GISTs are not eligible for radical surgery, neoadjuvant target therapy, Imatinib, could be considered as a chance to reduce the size of tumors (27). The group of Şentürk demonstrated in 151 GIST patients that radical resection of the tumor is the ideal treatment, while Imatinib therapy should be administered in large GISTs considered unresectable (28). With regard to metastatic GISTs, Imatinib represents the first option, while surgery should be considered only in a few selected cases when all lesions are resectable (21, 27, 29). The introduction of the tyrosine kinase inhibitor in the management of GISTs has radically improved the outcome of patients with high disease burden (30). In case of unresectable metastatic disease, target therapy should be continued indefinitely (9).
With regard to follow-up, there is no consensus on the optimal routine for patients affected by GISTs. Programs differ across institutions. The last updated guidelines reported that high-risk patients should undergo abdominal CT scan or MRI every 3–6 months for 3 years during adjuvant therapy, then every 3 months for 2 years (on cessation of adjuvant therapy), then every 6 months until 5 years, and annually for an additional 5 years; low-risk patients should undergo abdominal CT scan or MRI every 6–12 months for 5 years. (9).
Multiple GISTs need to be differentiated from metastatic GISTs for ensuring the appropriate therapeutic management and outcome prediction. The surgical approach may be considered as the first treatment option for multiple GISTs, whereas Imatinib is mandatory for metastatic GISTs. According to the guidelines, small GISTs (<5 cm) can be treated by laparoscopy, while for large GISTs, the surgical approach should consider tumor location and size. For multiple GISTs, the dimensions of the lesions should be evaluated; we suggest surgical planning with a specific focus on the biggest lesion. In our case, after a first consideration of the laparoscopic approach, a conversion to laparotomy was chosen due to lesion dimensions and the close relation of the lesions with the vessels, to avoid tumor rupture.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB). The patients/participants provided their written informed consent to participate in this study.
MT, AL, and EF designed the study. CC, FMDdA, ADG, and DC collected and analyzed the data. DC, UC, and AP wrote the manuscript. MP, FP, and GC reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29(Suppl 4):iv267. doi: 10.1093/annonc/mdy320
2. Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. (2002) 38(Suppl 5):S39–51. doi: 10.1016/S0959-8049(02)80602-5
3. Liu X, Chu KM. Molecular biomarkers for prognosis of gastrointestinal stromal tumor. Clin Transl Oncol. (2019) 21(2):145–51. doi: 10.1007/s12094-018-1914-4
4. Catarci M, Balla A, Coppola L. Unusual presentation of small bowel GIST: diffuse omental & mesenteric sarcomatosis. J Surg Case Rep. (2020) 2020(9):rjaa341. doi: 10.1093/jscr/rjaa341
5. Watson GA, Kelly D, Melland-Smith M, Gleeson J, McEntee G, Kelly CM, et al. Get the GIST? An overview of gastrointestinal stromal tumours. Ir J Med Sci. (2016) 185(2):319–26. doi: 10.1007/s11845-016-1410-1
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. (2006) 130(10):1466–78. doi: 10.5858/2006-130-1466-GSTROM
7. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. (2006) 23(2):70–83. doi: 10.1053/j.semdp.2006.09.001
8. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67(2):93–9. doi: 10.3322/caac.21388
9. Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2021) 33(1):20–33. doi: 10.1016/j.annonc.2021.09.005
10. Caruso D, Rosati E, Panvini N, Rengo M, Bellini D, Moltoni G, et al. Optimization of contrast medium volume for abdominal CT in oncologic patients: prospective comparison between fixed and lean body weight-adapted dosing protocols. Insights Imaging. (2021) 12(1):40. doi: 10.1186/s13244-021-00980-0
11. Wu H, Li C, Li H, Shang L, Jing HY, Liu J, et al. Clinicopathological characteristics and longterm survival of patients with synchronous multiple primary gastrointestinal stromal tumors: a propensity score matching analysis. World J Gastroenterol. (2021) 27(36):6128–41. doi: 10.3748/wjg.v27.i36.6128
12. Diab R, Virarkar M, Saleh M, Elsheif S, Javadi S, Bhosale P, et al. Imaging spectrum of mesenteric masses. Abdom Radiol (NY). (2020) 45(11):3618–36. doi: 10.1007/s00261-020-02535-1
13. Dufay C, Abdelli A, Le Pennec V, Chiche L. Mesenteric tumors: diagnosis and treatment. J Visc Surg. (2012) 149(4):e239–51. doi: 10.1016/j.jviscsurg.2012.05.005
14. Díaz-Delgado M, Hernández-Amate A, Sánchez-León M, Pereira-Gallardo S, Prieto-Sánchez E, Jiménez-Sáenz M, et al. Multiple non-metastatic gastrointestinal stromal tumors. Differential features. Rev Esp Enferm Dig. (2010) 102(8):489–97. doi: 10.4321/S1130-01082010000800006
15. Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumors. Pathol Int. (2006) 56(1):1–9. doi: 10.1111/j.1440-1827.2006.01924.x
16. Gheorghe G, Bacalbasa N, Ceobanu G, Ilie M, Enache V, Constantinescu G, et al. Gastrointestinal stromal tumors - a mini review. J Pers Med. (2021) 11(8). doi: 10.3390/jpm11080694
17. Li K, Tjhoi W, Shou C, Yang W, Zhang Q, Liu X, et al. Multiple gastrointestinal stromal tumors: analysis of clinicopathologic characteristics and prognosis of 20 patients. Cancer Manag Res. (2019) 11:7031–8. doi: 10.2147/CMAR.S197560
18. Gasparotto D, Rossi S, Bearzi I, Doglioni C, Marzotto A, Hornick JL, et al. Multiple primary sporadic gastrointestinal stromal tumors in the adult: an underestimated entity. Clin Cancer Res. (2008) 14(18):5715–21. doi: 10.1158/1078-0432.CCR-08-0622
19. Shen YY, Ma XL, Yang LX, Zhao WY, Tu L, Zhuang C, et al. Clinicopathologic characteristics, diagnostic clues, and prognoses of patients with multiple sporadic gastrointestinal stromal tumors: a case series and review of the literature. Diagn Pathol. (2020) 15(1):56. doi: 10.1186/s13000-020-00939-7
20. Lopes LF, Bacchi CE. Imatinib treatment for gastrointestinal stromal tumour (GIST). J Cell Mol Med. (2010) 14(1–2):42–50. doi: 10.1111/j.1582-4934.2009.00983.x
21. Nishida T, Yoshinaga S, Takahashi T, Naito Y. Recent progress and challenges in the diagnosis and treatment of gastrointestinal stromal tumors. Cancers (Basel). (2021) 13(13). doi: 10.3390/cancers13133158
22. Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. (2008) 13(5):416–30. doi: 10.1007/s10147-008-0798-7
23. Nishida T. Asian consensus guidelines for gastrointestinal stromal tumor: what is the same and what is different from global guidelines. Transl Gastroenterol Hepatol. (2018) 3(11). doi: 10.21037/tgh.2018.01.07
24. Zhao WY, Zhao G, Wang M. Updates and interpretations of the NCCN Clinical Practice Guidelines (2019 6th version) on gastrointestinal stromal tumor. Zhonghua Wei Chang Wai Ke Za Zhi. (2020) 23(9):866–71. doi: 10.3760/cma.j.cn.441530-20200731-00454
25. Zhou YB. Definition of tumor rupture in gastrointestinal stromal tumor. Zhonghua Wei Chang Wai Ke Za Zhi. (2021) 24(9):762–8. doi: 10.3760/cma.j.cn.441530-20210304-00097
26. Crocetti D, Sapienza P, Cisano C, Tarallo M, Polistena A, Venturini L, et al. Pancreas preserving surgery for duodenal gastrointestinal stromal tumor removal. Minerva Chir. (2016) 71(5):281–5.
27. Yang J, Feng F, Li M, Sun L, Hong L, Cai L, et al. Surgical resection should be taken into consideration for the treatment of small gastric gastrointestinal stromal tumors. World J Surg Oncol. (2013) 11:273. doi: 10.1186/1477-7819-11-273
28. Şentürk M, Yıldırım MA, Çakır M, Kişi Ö. Clinicopathologic and surgical characteristics study of 151 cases of GIST. J Gastrointest Cancer. (2021) 52(2):542–6. doi: 10.1007/s12029-020-00414-y
29. Tattersall HL, Gronchi A. Surgical resection as an adjuvant to tyrosine kinase inhibitors in metastatic GIST: association or causation? Are we any closer to an answer? Eur J Surg Oncol. (2018) 44(9):1287–8. doi: 10.1016/j.ejso.2018.06.006
Keywords: multiple gastrointestinal stromal tumors, gastrointestinal stromal tumor, computed tomography, surgical resection, minimally invasive surgery
Citation: Tarallo M, Carruezzo C, Dentice Di Accadia FM, Del Gaudio A, Caruso D, Polici M, Crocetti D, Costi U, Polistena A, Panzuto F, Laghi A, Cavallaro G and Fiori E (2022) A Case Report of Multiple Gastrointestinal Stromal Tumors: Imaging Findings, Surgical Approach, and Review of the Literature. Front. Surg. 9:886135. doi: 10.3389/fsurg.2022.886135
Received: 28 February 2022; Accepted: 25 April 2022;
Published: 20 May 2022.
Edited by:
Dimitrios Schizas, National and Kapodistrian University of Athens, GreeceReviewed by:
Maximos Frountzas, National and Kapodistrian University of Athens, GreeceCopyright © 2022 Tarallo, Carruezzo, Dentice di Accadia, Del Gaudio, Caruso, Polici, Crocetti, Costi, Polistena, Panzuto, Laghi, Cavallaro and Fiori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariarita Tarallo bWFyaWFyaXRhLnRhcmFsbG9AdW5pcm9tYTEuaXQ=
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.