
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 18 October 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.886129
 Mingli Su1,2,†
Mingli Su1,2,† Tingting Chen1,†
Tingting Chen1,† Qinghua Zhong1,2
Qinghua Zhong1,2 Dezheng Lin1,2
Dezheng Lin1,2 Wei Liu1,2
Wei Liu1,2 Yuping Su1
Yuping Su1 Jiaxin Deng1,2
Jiaxin Deng1,2 Jiawei Zhang1,2
Jiawei Zhang1,2 Jiancong Hu1,2*
Jiancong Hu1,2* Xuefeng Guo1,2*
Xuefeng Guo1,2*
Purpose: The aim of this study was to identify the effect of different injection times on pain during colonoscopy procedure.
Methods: In this retrospective study, the data of patients who underwent colonoscopy from June 2020 to September 2020 were assessed to investigate the effect of different injection time of sedative drugs (midazolam and dezocine). The primary endpoint was evaluating the pain intensity of the patients using visual analogue scale (VAS) immediately after colonoscopy .
Results: A total of 152 patients were eligible for this study. Of them, 76 received midazolam and dezocine injection 1 min prior to the colonoscopy procedure (the 1 Min group) and the other 76 patients received the injection 3 min prior to the procedure (the 3 Min group). The vital signs of all patients were stable except for one patient who was diagnosed with inflammatory bowel disease in the 3 Min group. A transient drop in blood pressure for this patient was observed during colonoscopy but returned to normal after general treatment. The two groups had similar rates of cecal intubation (84.21% vs. 90.97%, P = 0.22), addition of sedative drugs during procedure (2.63% vs. 5.26%, P = 0.68), and adequate bowel preparation (Boston Bowel Preparation Scale ≥6, 61.84% vs. 61.84%, P = 1.0). However, patients in the 3 Min group had significantly lower VAS than those in the 1 Min group [0 (0, 1) vs. 1 (0, 2), P = 0.041].
Conclusion: The timing of drug injection during conscious sedation may affect pain control during colonoscopy, with 3 min prior to the procedure showing lower VAS.
Colorectal cancer (CRC) is a global public health issue, with an estimation of 1.93 million new cases and 930,000 deaths worldwide in 2020 (1, 2). Detecting colorectal lesion by fecal immunochemical test (FIT) or colonoscopy has been shown to effectively decrease the incidence of CRC (3). FIT is convenient and economical, while colonoscopy has a greater CRC incidence reduction (4) and is currently regarded as the gold standard examination for colorectal lesion screening (5, 6). The primary goals of these screenings are to identify and aptly remove colorectal adenomas and lesions that are believed to lead to the development of CRC (7, 8).
Colonoscopy often presents as a painful and unpleasant procedure, which is one of its major barriers for willingness among the public (9). Among procedure-related pain factors, sedation could greatly reduce pain and discomfort of the examinee during colonoscopy. Recommendations for sedation methods in routine colonoscopy differ across countries and regions (10). Two methods of sedation are commonly used for colonoscopy test: one is conscious sedation with midazolam, and the other is modified anesthesia with propofol (11–15). The latter method could induce a deeper level of sedation with less patient movement and awareness. However, deep sedation colonoscopy is associated with higher cost and greater risk of sedation- and procedure-related complications (16). Furthermore, modified anesthesia can only be performed by an anesthesiologist, which is one of the main reasons for the lower sedation rate in China, compared to the United States and Europe (17). Different from propofol, conscious sedation with midazolam can be managed by the endoscopist and has similar sedative effect to propofol (15). However, a few studies have investigated the safety and effectiveness of conscious sedation. Fox example, the relationship between injection time and the efficiency of conscious sedation and analgesia remains unclear.
In this study, we evaluated the safety of sedation with midazolam and dezocine and compared the efficiency of different injection times (1 or 3 min before colonoscopy procedure) using the visual analogue scale (VAS) immediately after colonoscopy.
Patients older than 18 years who had undergone colonoscopy were eligible for inclusion, and patients with stage IV colorectal cancer, history of chronic pain, or who had incomplete or missing follow-up data were excluded. The patients were divided into two groups: group 1 (injection of midazolam and dezocine 1 min prior to colonoscopy) and group 2 (injection of midazolam and dezocine 3 min prior to colonoscopy). We retrospectively collected the clinical data of 200 people who underwent colonoscopy between June 2020 and September 2020 at the Six Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Propensity score matching was used to control selection bias and 152 patients were finally included in this study. The propensity score of being allocated to group 1 and group 2 was calculated using a multivariable logistic regression model with age and sex as covariates. The patients were matched using the nearest-neighbor method in a 1:1 ratio with a caliper width of 0.2 SD of the logit of the propensity score.
Demographic data including age, gender, cardiovascular disease history, diagnosis, history of colonoscopy screening, history of previous abdominal surgery, Boston Bowel Preparation Scale (BBPS), addition of sedative drugs during procedure, cecal intubation rate (CIR), resection of polyp, VAS, change in vital signs [blood pressure, pulse, and peripheral blood oxygen saturation (SPO2)], satisfaction of colonoscopy, and postoperative complications were collected.
Patients who were inpatients and outpatients received colonoscopy according to their diagnosis and clinical need. All patients received regular bowel preparation instructions at their appointment to ensure proper preparation for colonoscopy. Colonoscopy was performed by several experienced endoscopists. All patients were assessed to tolerate conscious sedation and analgesia and were divided into group 1 or group 2. Midazolam (0.05 mg/kg) and dezocine (5.0 mg) were intravenously injected by nurses from the endoscopic department 1 or 3 min prior to the beginning of colonoscopy. Vital signs including pulse, blood pressure, and blood oxygen saturation were recorded every 5 min during the procedure.
To evaluate the quality of bowel preparation, the BPPS criteria was used. It ranges from a score of 0–9 and divides the colon into three regions (including right colon, transverse colon, and left colon) whereby each region receives a score from 0 to 3 respectively (18). An adequate bowel preparation is usually defined as a BBPS ≥6.
VAS was used to measure the pain intensity of patients during the colonoscopy procedure (19). Patients would be transferred from the examination room to the resuscitation room after finishing colonoscopy procedure and then were asked to place a line perpendicular to the VAS line to present their pain intensity. The score was determined by measuring the distance between the “no pain” anchor and the patient's mark, ranging from 0 to 10.
IBM SPSS (version 25.0 for Windows; SPSS, Chicago, IL, United States) was used for data analysis. Measurement data are expressed as the mean ± SD or median (minimum, maximum). The Mann–Whitney rank sum tests or t-tests were used to compare the measurement data between groups. The differences between rates were tested by the Chi-square test or Fisher exact tests, where appropriate. Differences were considered statistically significant for P values <0.05.
In this study, a total of 152 patients who underwent colonoscopy with conscious sedation between June 2020 and September 2020 were finally included after propensity score matching. Their basic characteristics are shown in Table 1. Of them, 76 patients received an injection of midazolam and dezocine 1 min prior to colonoscopy (defined as group 1) and the other 76 patients were 3 min prior to the procedure (defined as group 2). In group 1 and group 2, 48 and 39 male patients were included, respectively. The differences between the two groups are presented in Table 1. The two groups had similar characteristics of gender, age, cardiovascular disease history, history of colonoscopy procedure, and diagnosis. Benign perianal disease and colorectal polyp were the two most diagnosed in both groups.
Table 2 illustrates the comparison of clinical outcomes of patients between the two groups. Patients in both groups had high satisfaction with the procedure. The two groups had similar rates of cecal intubation (84.21% vs. 90.97%, P = 0.22), addition of sedative drugs during the procedure (2.63% vs. 5.26%, P = 0.68), and adequate bowel preparation (BBPS ≥6, 61.84% vs. 61.84%, P = 1.0). However, patients in group 2 had a significantly lower VAS than those in group 1 [0 (0, 1) vs. 1 (0, 2), P = 0.041].
To further investigate the safety of the procedure, we have illustrated the changes in arterial blood pressure, pulse, and SPO2 in Figure 1. The vital signs of all patients remained stable during the procedure except for one patient in group 2 who had inflammatory bowel disease (IBD). There was a transient drop in blood pressure for this patient during the procedure and it quickly returned to normal after general treatment without interrupting the endoscopic procedure. Moreover, no patient had any postoperative complications such as intestinal bleeding, intestinal perforation, and bowel obstruction.
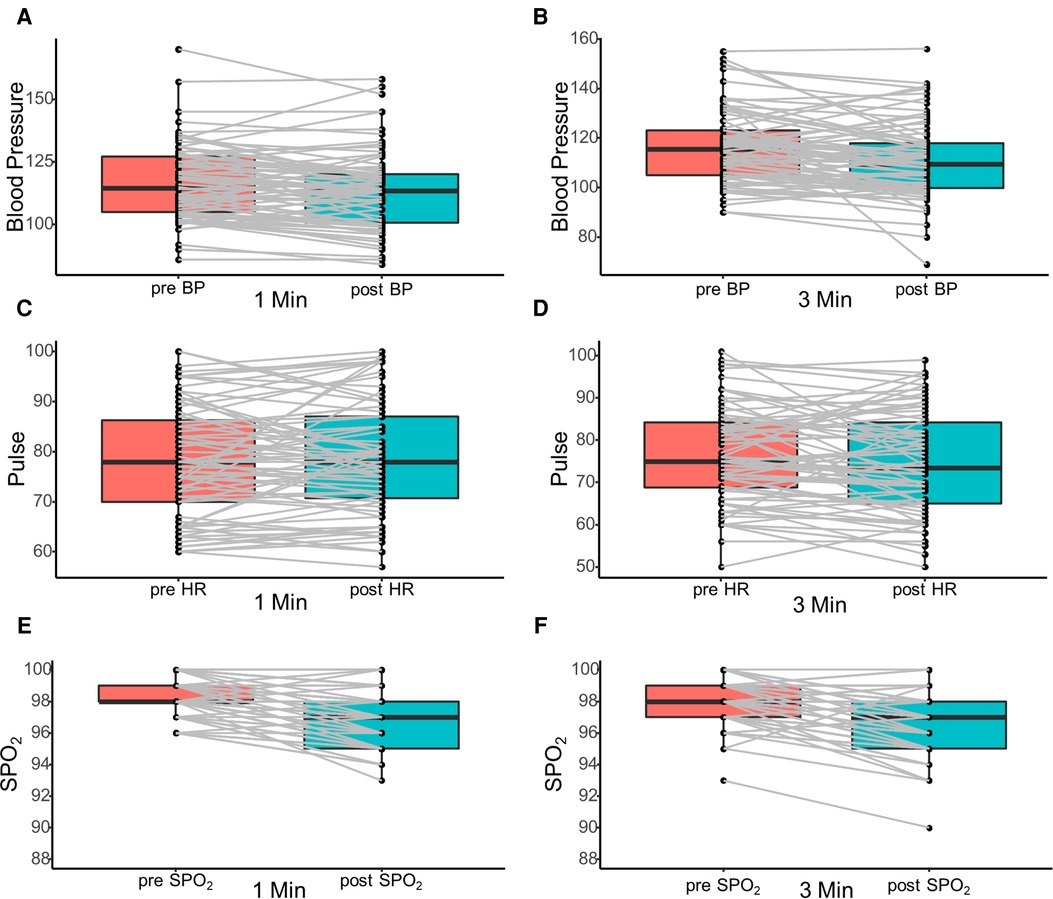
Figure 1. Illustration of the changes in arterial blood pressure (A,B), pulse (C,D), and SPO2 (E,F) before and after colonoscopy procedure. SPO2, peripheral blood oxygen saturation.
In this study, we found that conscious sedation and analgesia was safe and had a satisfactory level of comfort during the procedure, with a high satisfaction score (approximately 86.84%) and low rate of need for additional pain medication. In addition, intravenous injection of sedative drugs 3 min before the procedure had significantly better pain control than 1 min (P = 0.041). Patients’ pain and fear of colonoscopy are among the reasons for reluctance to colonoscopy (17). Main sedation during colonoscopy includes propofol sedation and conscious sedation with midazolam, which could decrease procedure-related pain and discomfort (15). Furthermore, clear and satisfying visualization under colonoscopy due to the patient's cooperation has led to increased satisfaction. The overall sedation rate for colonoscopy is only 47.9% in China, which is much lower than in the United States (>98.0%), Greece (78.0%), and Germany (91.0%) (17). Previous studies have reported that propofol sedation has some disadvantages, including severe respiratory depression and hypotension (10, 22). Conscious sedation with midazolam is efficient and safe and has been used widely during colonoscopy (21). Cancer burden has major economic implications both on the patient-level and country-level (22). Here, we found that compared with propofol, sedation administration by a nurse under endoscopist supervision during conscious sedation could help lower the costs of examination to the patient and might also lead to greater willingness for this procedure (10). However, the timing of drug injection for better pain control remains unclear. Thus, in this study, the 1 and 3 min injection times of sedation prior to colonoscopy were investigated. The results suggest that 3 min injection prior to the procedure had significantly lower VAS than 1 min (P = 0.041). The onset time of a sedative analgesic such as midazolam is relatively slower than propofol. This may be the reason why 3 min injection administration had better pain control effect than 1 min. Further studies are needed to see if lower VAS vary based on time and level of sedation administered.
Several factors including sedation, adequate bowel preparation (based on BBPS ≥6), previous abdominal surgery, and endoscopists’ experience were identified in previous studies to be associated with pain during procedure (23, 24). In this study, the procedural technique was the same for each endoscopist in both the 1 and 3 min groups at the same academic teaching institution. The rate of adequate bowel preparation (BBPS ≥6) for all patients was 60.9%, which did not have lower VAS than the patients with BBPS <6 (P = 0.76).
Similarly, the quality of bowel preparation, the use of sedation, and the endoscopist's experience were the main factors influencing CIR (25). Conscious sedation provides the endoscopists time to focus on the examination and not be distracted by a patient’s incorporation. No significant differences were noted considering the CIR between two groups in this study (P = 0.22). However, patients with adequate bowel preparation (BBPS ≥6) had a higher rate of reaching the cecum than other patients (P = 0.001). An adequate bowel preparation could decrease the procedure time and make endoscope insertion easier, thereby having a higher rate of cecal intubation and better procedure tolerance (26). Furthermore, adequate bowel preparation may facilitate complete gas removal during colonoscope withdrawal, thereby contributing to pain control during the procedure.
Except for pain during the procedure, several factors such as previous experience with colonoscopy and appropriate level of sleep were also associated with satisfaction of the procedure (13). In this study, 59.2% patients had previous experience with colonoscopy, which had a similar satisfaction of colonoscopy comparing with those who received colonoscopy for the first time (P = 0.516). In addition, patients who underwent polyp resection during colonoscopy had similar median VAS scale with others. It was reported that patients with inflammatory bowel disease favored propofol sedation over conscious sedation (16). Moreover, IBD patients (n = 15) presented similar outcomes compared to non-IBD patients (n = 137) in our study (P = 0.75).
The limitation of this study was its retrospective nature and single institution data with relatively small sample size. Large prospective large-sample-size and multicenter studies are required to further evaluate the timing of drug injection and outcomes.
The timing of drug injection during conscious sedation may affect the pain control during colonoscopy, with 3 min prior to the procedure showing lower VAS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by institutional ethics board of the Sixth Affiliated Hospital, Sun Yat-sen University (NO. 2021ZSLYEC-028). We confirmed that we have obtained ethical approval to conduct the study as well as permission from dataset and was conducted in accordance with the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
MS, TC, JH, and XG conceived this study. MS, TC, and YS retrieved the data, did the analysis, and drafted this manuscript. QZ, DL, WL, JD, JZ, JH, and XG revised this manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Planning Project of Guangzhou (201803010074) and the Six Affiliated Hospital of Sun Yat-sen University Clinical Research 1010 Program [grant number 1010 PY(2020)-63].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun Lond Engl. (2021) 41(10):1037–48. doi: 10.1002/cac2.12197
3. Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. (2020) 158(2):418–32. doi: 10.1053/j.gastro.2019.06.043
4. Buskermolen M, Cenin DR, Helsingen LM, Guyatt G, Vandvik PO, Haug U, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a microsimulation modelling study. Br Med J. (2019) 367:l5383. doi: 10.1136/bmj.l5383
5. Waghray A, Jain A, Waghray N. Colorectal cancer screening in African Americans: practice patterns in the United States. Are we doing enough? Gastroenterol Rep. (2016) 4(2):136–40. doi: 10.1093/gastro/gow005
6. Liao Y, Li S-M, Chen C-Y, He X, Lin F, Wang J-P, et al. Screening for colorectal cancer in Tianhe, Guangzhou: results of combining fecal immunochemical tests and risk factors for selecting patients requiring colonoscopy. Gastroenterol Rep. (2018) 6(2):132–6. doi: 10.1093/gastro/gox030
7. Li D-H, Liu X-Y, Huang C, Deng C-N, Zhang J-L, Xu X-W, et al. Pathological analysis and endoscopic characteristics of colorectal laterally spreading tumors. Cancer Manag Res. (2021) 13:1137–44. doi: 10.2147/CMAR.S286039
8. Shen J, Wu Y-L, Feng X-S, Liang F, Mo M, Cai B-X, et al. Assessing individual risk for high-risk early colorectal neoplasm for pre-selection of screening in Shanghai, China: a population-based nested case-control study. Cancer Manag Res. (2021) 13:3867–78. doi: 10.2147/CMAR.S301185
9. Hayman CV, Vyas D. Screening colonoscopy: the present and the future. World J Gastroenterol. (2021) 27(3):233–9. doi: 10.3748/wjg.v27.i3.233
10. Dossa F, Megetto O, Yakubu M, Zhang DDQ, Baxter NN. Sedation practices for routine gastrointestinal endoscopy: a systematic review of recommendations. BMC Gastroenterol. (2021) 21(1):22. doi: 10.1186/s12876-020-01561-z
11. Nakshabendi R, Berry AC, Munoz JC, John BK. Choice of sedation and its impact on adenoma detection rate in screening colonoscopies. Ann Gastroenterol. (2016) 29(1):50–5.
12. Metwally M. Conscious or unconscious: the impact of sedation choice on colon adenoma detection. World J Gastroenterol. (2011) 17(34):3912. doi: 10.3748/wjg.v17.i34.3912
13. Paspatis GA, Tribonias G, Manolaraki MM, Konstantinidis K, Chainaki I, Theodoropoulou A, et al. Deep sedation compared with moderate sedation in polyp detection during colonoscopy: a randomized controlled trial. Colorectal Dis. (2011) 13(6):e137–44. doi: 10.1111/j.1463-1318.2011.02555.x
14. Lin OS, La Selva D, Kozarek RA, Weigel W, Beecher R, Gluck M, et al. Nurse-administered propofol continuous infusion sedation: a new paradigm for gastrointestinal procedural sedation. Am J Gastroenterol. (2021) 116(4):710–6. doi: 10.14309/ajg.0000000000000969
15. Dossa F, Medeiros B, Keng C, Acuna SA, Baxter NN. Propofol versus midazolam with or without short-acting opioids for sedation in colonoscopy: a systematic review and meta-analysis of safety, satisfaction, and efficiency outcomes. Gastrointest Endosc. (2020) 91(5):1015–26.e7. doi: 10.1016/j.gie.2019.12.047
16. Steenholdt C, Jensen JT, Brynskov J, Møller A, Limschou AC, Konge L, et al. Patient satisfaction of propofol versus midazolam and fentanyl sedation during colonoscopy in inflammatory bowel disease. Clin Gastroenterol Hepatol. (2022) 20(3):559–68.e5. doi: 10.1016/j.cgh.2020.10.037
17. Zhou S, Zhu Z-Y, Dai W-B, Qi S-Y, Tian W-T, Zhang Y-Z, et al. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. (2021) 127(1):56–64. doi: 10.1016/j.bja.2021.01.028
18. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston Bowel Preparation Scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. (2009) 69(3 Pt 2):620–5. doi: 10.1016/j.gie.2008.05.057
19. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. (2011) 63(S11):S240–52. doi: 10.1002/acr.20543
20. Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Risk stratification and safe administration of propofol by registered nurses supervised by the gastroenterologist: a prospective observational study of more than 2000 cases. Gastrointest Endosc. (2003) 57(6):664–71. doi: 10.1067/mge.2003.191
21. Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, et al. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. (2003) 58(3):317–22. doi: 10.1067/s0016-5107(03)00001-4
22. Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun Lond Engl. (2020) 40(5):205–10. doi: 10.1002/cac2.12025
23. Bugajski M, Wieszczy P, Hoff G, Rupinski M, Regula J, Kaminski MF. Modifiable factors associated with patient-reported pain during and after screening colonoscopy. Gut. (2018) 67(11):1958–64. doi: 10.1136/gutjnl-2017-313905
24. Holme O, Bretthauer M, de Lange T, Seip B, Huppertz-Hauss G, Høie O, et al. Risk stratification to predict pain during unsedated colonoscopy: results of a multicenter cohort study. Endoscopy. (2013) 45(9):691–6. doi: 10.1055/s-0033-1344239
25. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. (2011) 140(1):65–72. doi: 10.1053/j.gastro.2010.09.006
Keywords: colonoscopy, conscious sedation and analgesia, injection time, visual analogue scale, pain
Citation: Su M, Chen T, Zhong Q, Lin D, Liu W, Su Y, Deng J, Zhang J, Hu J and Guo X (2022) Choice of injection time of conscious sedation and its impact on pain control in colonoscopy. Front. Surg. 9:886129. doi: 10.3389/fsurg.2022.886129
Received: 28 February 2022; Accepted: 20 September 2022;
Published: 18 October 2022.
Edited by:
Gabriel Sandblom, Karolinska Institutet (KI), SwedenReviewed by:
Audrius Dulskas, National Cancer Institute (Lithuania), Lithuania© 2022 Su, Chen, Zhong, Lin, Liu, Su, Deng, Zhang, Hu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiancong Hu aHVqaWFuY0BtYWlsLnN5c3UuZWR1LmNu Xuefeng Guo Z3VveGZlbmdAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Abbreviations CRC, colorectal cancer; FIT, fecal immunochemical test; IBD, inflammatory bowel disease; VAS, visual analogue scale; BBPS, Boston Bowel Preparation Scale; CIR, cecal intubation rate; SPO2, peripheral blood oxygen saturation.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.