
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 06 June 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.881510
This article is part of the Research TopicManagement of Peritoneal Surface Malignancies. (Cytoreductive Surgery, HIPEC, PIPAC, and Beyond)View all 11 articles
Objective: To determine prognosis for young female patients with peritoneal pseudomyxoma (PMP) of appendiceal origin and unilateral or bilateral ovaries preserved during cytoreductive surgery (CRS).
Methods: Clinical data of female patients treated with CRS with or without hyperthermic intraperitoneal chemotherapy (HIPEC) at the Aerospace Center Hospital, Beijing between January, 2009 and December, 2019 were retrospectively reviewed. Patients had no changes in the bilateral ovaries on gross pathological observations or biopsy during CRS, and normal ovarian function. The demographic and clinical characteristics and prognosis of women with ovaries preserved (ovarian preservation group) or resected (ovarian resection group) during CRS were compared. Independent prognostic factors for survival were identified using univariate and multivariate analysis.
Results: 40 patients were included in the final analysis. 19 patients chose ovarian preservation while 21 patients underwent ovarian resection. Completeness of cytoreduction (CCR) scores were CCR-0/1. There were significant differences in age (<40 vs. ≥40), symptoms, intraoperative HIPEC (Y vs. N), and histopathologic subtype of PMP (low-grade vs. high-grade) (p < 0.001) between patients in the ovarian preservation and ovarian resection groups. In the ovarian preservation group, median overall survival (OS) was 59 months (range, 53–65 months), and the 5-year survival rate was 37.9%. Median disease-free survival (DFS) was 13 months (range, 9–17 months), and the 5-year recurrence rate was 87.4%. In the ovarian resection group, the 5-year survival rate was 87.7%, and the 5-year recurrence rate was 18.3%. Median OS and median DFS were not reached. In patients with low-grade PMP, median DFS was significantly longer in patients with ovarian resection compared to ovarian preservation (p < 0.001). Univariate analysis showed histopathologic subtype of PMP (low-grade vs. high-grade, p < 0.001) was significantly associated with OS and DFS. On multivariate analysis, high-grade histopathologic subtype of PMP was an independent predictor of poor prognosis (OS and DFS).
Conclusion: Histopathologic subtype of PMP represents an independent predictor of prognosis in female patients with PMP of appendiceal origin and unilateral or bilateral ovaries preserved during CRS. These findings imply that ovarian preservation is a more suitable option for young females with low-grade PMP compared to high-grade PMP. Further prospective studies should be done investigating the role of resection of uninvolved ovaries in PMP.
Pseudomyxoma peritonei (PMP) is a rare clinical syndrome that occurs with an incidence of 2 cases per 100 million individuals (1, 2). Most PMP arise from perforation of a primary appendiceal cancer and seeding of tumor cells within the peritoneal cavity (3). The gold standard curative treatment for PMP is complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CCRS/HIPEC) (4, 5).
The majority of women with PMP have involvement of the ovaries due to direct invasion from the adjacent appendix or redistribution of PMP within the peritoneal cavity (6, 7); therefore, ovariectomy is often recommended. However, surgical menopause occurs after bilateral ovariectomy. This can have a negative impact on patient quality of life, especially in young women who wish to have children. There remains an unmet clinical need for effective strategies that preserve fertility in young women with PMP of appendiceal origin and to build consensus on management in cases where the ovaries appear normal during CRS. The objective of this study was to determine prognosis for female patients with PMP of appendiceal origin and unilateral or bilateral ovaries preserved during complete cytoreductive surgery (CCRS). Findings will inform clinicians who manage women with PMP.
The protocol for this study was approved by the Ethics committee of the Aerospace Center Hospital, Beijing, China (No. 20161109-ST-07). Written inform consent for publication of clinical data was obtained from all included patients.
Clinical data of patients with PMP treated at the Aerospace Center Hospital, Beijing between January, 2009 and December, 2019 were retrospectively reviewed. Inclusion criteria were: (1) female; (2) aged 20 to 60 years; (3) diagnosis of PMP of appendiceal origin on histology; (4) initial CRS (radical resection; completeness of cytoreduction [CCR] 0/1) performed at our hospital; and (5) no changes in the bilateral ovaries on gross pathological observations or biopsy during CRS, and normal ovarian function. Exclusion criteria were: (1) PMP derived from other organs or disease (e.g., colon, urachus, and pancreas); (2) previous removal of one or both ovaries; (3) incomplete medical records; or (4) loss to follow-up or death.
A total of 40 patients were included in the final analysis. Patients were divided into two groups: ovarian preservation group, comprising 19 patients who retained at least one ovary during CRS, and ovarian resection group, comprising 21 patients who underwent bilateral ovariectomy during CRS (Figure 1).
Patients were treated with CRS with or without HIPEC. Patients underwent CRS to remove visible tumors (6). At our centre, criteria for considering patients for CRS include: (1) aged 20 to 75 years; (2) diagnosis of PMP with histopathologic subtype confirmed by two experienced pathologists; (3) normal liver and kidney function; (3) Eastern Cooperative Oncology Group (ECOG) performance status ≤1; (4) history of severe disease affecting other organs; and (5) presence of distant metastasis or another malignant tumor prior to CRS for PMP.
Patient’s care goals, personal values, and wishes were incorporated into HIPEC decision-making. For patients undergoing HIPEC, inflow and two outflow catheters were placed in the peritoneal cavity and connected to the HIPEC machine (Jilin Minda Company products, China, Model: RHL-2000B). Cisplatin 60–80 mg or mitomycin (20 mg/m2) was warmed to 41°C–42°C and circulated intraperitoneally for 60–90 min using a closed-abdomen technique.
Patients’ clinicopathological parameters were recorded, including gender; ECOG performance status; age at diagnosis of PMP; symptoms; time from diagnosis of PMP to CRS for PMP; presence/absence of mucus in the abdominal and/or pelvic cavity; intraoperative peritoneal cancer index (PCI); residual disease following CRS measured as CCR; intraoperative HIPEC; pathological grade of PMP; 5-year survival rate; 5-year recurrence rate; Median overall survival (OS); Median disease-free survival (DFS); and follow-up time.
Intraoperative PCI was determined based on tumor size and extent in nine regions in the abdomen and four regions in the small bowel, which were scored on a scale from 0 to 3 and summed (4).
CCR was scored as CCR-0, no macroscopic residual disease, CCR-1, residual disease <2.5 mm, CCR-2, residual disease 2.5 mm–2.5 cm, and CCR-3, residual disease >2.5 cm (5).
Pathological diagnosis was classified according to the 2016 Peritoneal Surface Oncology Group International (PSOGI) criteria (8) as acellular mucin (AC), low-grade mucinous carcinoma peritonei (LG-MCP), high-grade mucinous carcinoma peritonei (HG-MCP), or high-grade mucinous carcinoma peritonei with signet ring cells (HGMC-S).
OS was calculated from the date of CRS/HIPEC to the time of death or last follow-up. DFS was calculated from the date of CRS/ HIPEC to the time of recurrence or last follow-up.
Statistical analyses were performed using SPSS 24.0 (IBM Corporation, Armonk, NY, USA). Continuous data are expressed as medians and range (min, max). Categorical data are expressed as number and percentages. For categorical variables, data were compared using the χ2 or Fisher’s exact test. For continuous variables, normally distributed data were compared with the independent-sample t-test, and non-normally distributed data were compared the Mann-Whitney U test. Independent prognostic factors for survival were identified using univariate survival analysis, which was performed with the Kaplan-Meier method and the log-rank test, and multivariate analysis, which included statistically significant variables in a Cox proportional hazards model. All live patients were censored. P < 0.05 was considered statistically significant.
Among 963 patients with PMP who were treated at the Aerospace Center Hospital, Beijing between January, 2009 and December, 2019, 888 (92%) patients had PMP of appendiceal origin, including 436 (49%) female patients and 452 (52%) males. Among the female patients, 229 (52.5%) patients received a radical resection while 186 (42.7%) patients received palliative debulking surgery.
Patients who received a radical resection were eligible for this study. Of these, 70 patients with a history of oophprectomy and 119 (74.8%) patients with ovarian lesions identified during CRS, including 20 patients who had unilateral ovaries preserved, with macroscopic involvement of the other one, were excluded. Finally, 40 (25.2%) patients with bilateral normal ovaries identified during CRS were included in the analysis, including 19 patients who had unilateral (n = 4) or bilateral ovarian (n = 15) preservation (ovarian preservation group) during CRS and 21 patients who underwent bilateral ovarian resection (ovarian resection group) during CRS (Figure 1).
Patients’ demographic and clinical characteristics are shown in Table 1. In the ovarian preservation group, patients’ median age was 37 years (range, 21–45 years). Median time from diagnosis of PMP to CRS was 1 month. PCI was <20 in 12 (63.2%) patients. Ovarian preservation was bilateral in 15 (78.9%) patients and the left ovary was preserved in 4 (21.1%) patients. CCR scores were CCR-0 in all patients. Pathological diagnosis showed low-grade disease in 10 (52.6%) patients and high-grade disease in 9 (47.4%) patients. In the ovarian resection group, patients’ median age was 53 years (range, 46–59 years). Median time from diagnosis of PMP to CRS was 1 month. PCI was <20 in 19 (90.5%) patients. CCR scores were CCR-0 in all patients. Pathological diagnosis showed low-grade disease in 19 (90.5%) patients. There were significant differences in age (<40 vs. ≥40), symptoms, intraoperative HIPEC (Y vs. N), and histopathologic subtype of PMP (low-grade vs. high-grade) (p < 0.05) between patients in the ovarian preservation and ovarian resection groups. There were no patients who needed secondary surgery because of serious complications in ovarian preservation group, but one patient underwent a second operation for urinary fistula in the ovarian resection group. No patients died within 90 days after CRS in two groups.
In the ovarian preservation group, most ovaries were preserved to maintain hormone production. 6 women were of child bearing age and wished to have children, and one patient was planning to undergo in vitro fertilization. At the end of follow-up, no patient achieved successful childbirth.
At the last follow-up in June 2021. In the ovarian preservation group, mean follow-up time was 63 months, 9 (47.4%) patients were alive. 10 patients experienced disease progression. Median OS was 59 months (range, 53–65 months), and the 5-year survival rate was 37.9%. Median DFS was 13 months (range, 9–17 months), and the 5-year recurrence rate was 87.4%. In the ovarian resection group, mean follow-up time was 31 months, 19 (90.5%) patients were alive. 2 patients experienced disease progression. The 5-year survival rate was 87.7%, and the 5-year recurrence rate was 18.3%. Median OS and median DFS were not reached (Figures 2A,B).
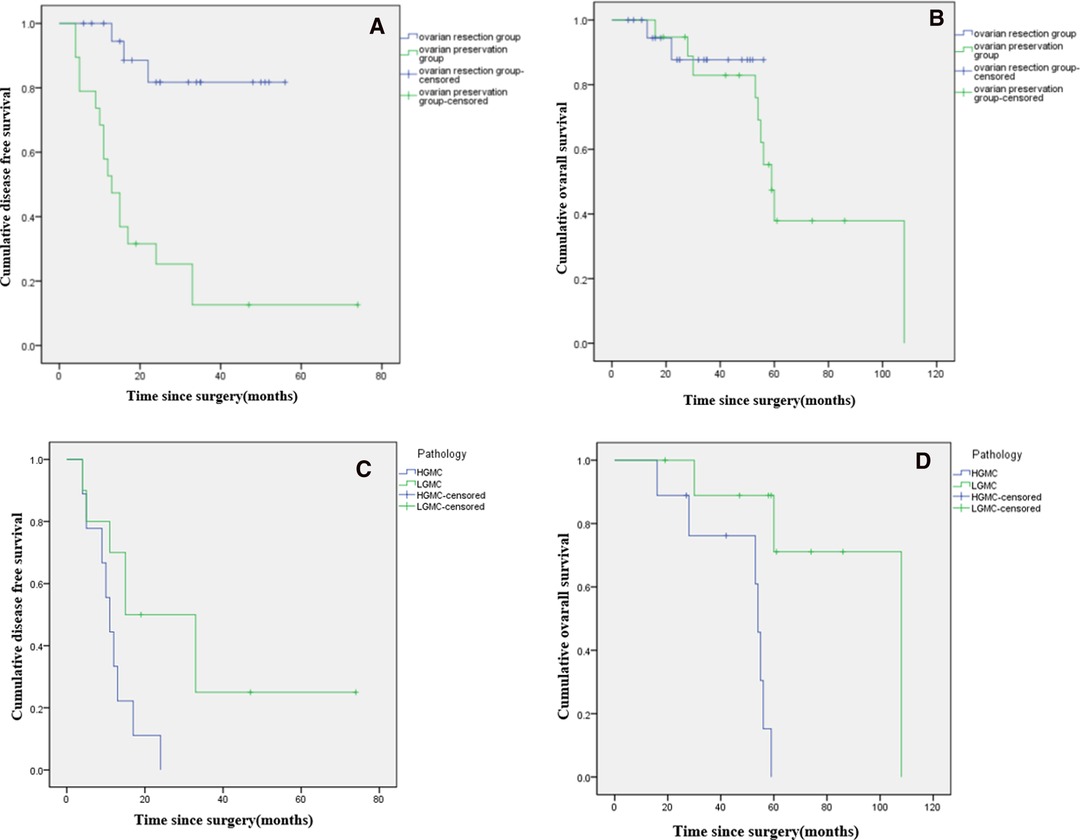
Figure 2. Kaplan-Meier curves showing disease free survival (A) and overall survival (B) of female patients with PMP of appendiceal origin and unilateral or bilateral ovaries preserved during CRS. Comparison of disease-free survival (C) and overall survival (D*) based on histopathologic subtype of PMP (low-grade vs. high-grade). (*: p < 0.05).
In the ovarian preservation group, patients were stratified by histopathologic subtype of PMP (LG-MCP, n = 10; HG-MCP, n = 9). Among patients with LG-MCP, median OS was 108 months, and the 5-year survival rate was 71.1%. Median DFS was 15 months (range, 0–30 months), and the 5-year recurrence rate was 75.0%. Among patients with HG-MCP, median OS was 54 months (range, 52–56 months), and the 5-year survival rate was 0%. Median DFS was 11 months (range, 8–14 months), and the 5-year recurrence rate was 100.0% (Figures 2C,D).
Among patients with LG-MCP (n = 29), median DFS was significantly longer in patients with ovarian resection compared to ovarian preservation (p < 0.001), but there was no significant difference in OS (p = 0.897). (Figures 3A,B). Among patients with HG-MCP (n = 11), there were no significant differences in DFS (p = 0.640) or OS (p = 0.315) between patients with ovarian preservation and ovarian resection (Figures 4A,B).
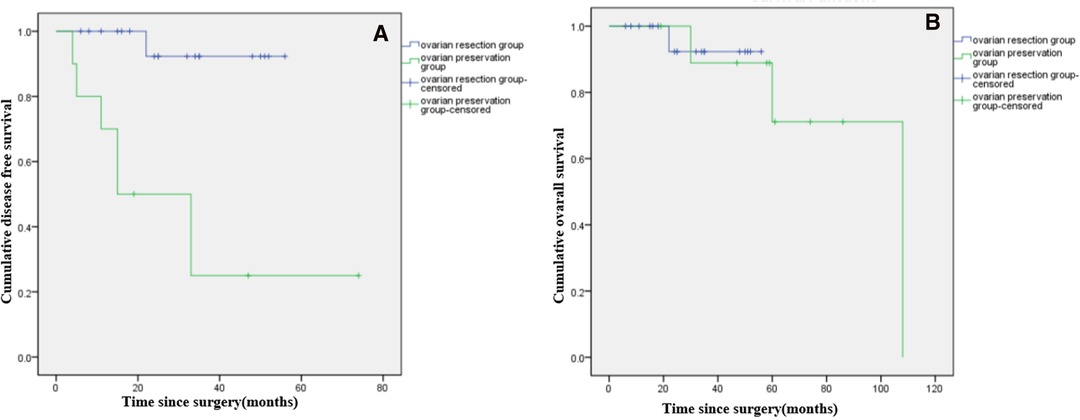
Figure 3. Kaplan-Meier curves showing disease free survival (A*) and overall survival (B) of female patients with low-grade PMP of appendiceal origin and unilateral or bilateral ovaries preserved during CRS. (*: p < 0.05).
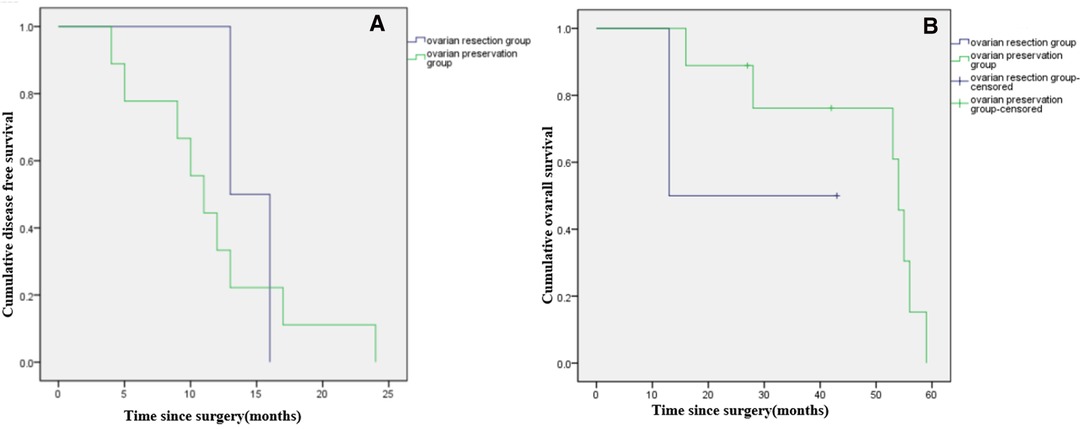
Figure 4. Kaplan-Meier curves showing disease free survival (A) and overall survival (B) of female patients with high-grade PMP of appendiceal origin and unilateral or bilateral ovaries preserved during CRS.
Univariate analysis showed histopathologic subtype of PMP (low-grade vs. high-grade, p < 0.05) was significantly associated with OS and DFS (Tables 2, 3). On multivariate analysis, high-grade histopathologic subtype of PMP was an independent predictor of poor prognosis (OS and DFS) (Tables 2, 3).
PMP is a clinical entity characterized by mucinous ascites, peritoneal soft-tissue implants, omental caking, and involvement of the gastrointestinal tract and ovaries. A distinguishing feature of PMP is its redistribution within the peritoneal cavity determined by normal flow of peritoneal fluid and gravity. In the present study, among the 963 patients with PMP who were treated at the Aerospace Center Hospital, Beijing between January, 2009, and December, 2019, 92% of patients had PMP of appendiceal origin. This rate is consistent with previously reported case series of patients with PMP treated with CRS/HIPEC, among which 89.6%–94% of patients with PMP had a primary appendiceal tumor (8–10). In other patients, PMP may originate from a tumor in the ovary, colon, small bowel, urachus, pancreas, bile duct, stomach, uterine cervix, fallopian tube, mesentery, kidney, extraovarian teratoma, or spleen.
In females, PMP of appendiceal origin usually metastasizes to the peritoneal surface of the ovaries and the uterus. At our center, the probability of ovarian involvement was 74.8% (119/159). A total hysterectomy with bilateral salpingo-oophorectomy may be recommended, regardless of pre-operative gynecologic organ involvement. However, young women may wish to avoid the associated iatrogenic surgical menopause and permanent infertility (11), which can have major psychosocial consequences and a negative impact on the quality of life of the patient and her family. In the present study, there was a significant difference in age between patients who chose to preserve their ovaries during CRS and those who underwent ovarian resection, with younger patients more likely to choose ovarian preservation.
Among the 19 patients with PMP of appendiceal origin and unilateral or bilateral ovaries preserved during CRS included in our study, 6 desired to have children. At the end of follow-up, only one patient was planning to undergo in vitro fertilization. Our findings showed that PMP recurrence rates rose rapidly two years post-CRS, implying that women who underwent CRS with/without HIPEC for PMP with ovarian preservation should attempt to conceive as soon as possible when the recommended waiting period following therapy is complete (12, 13).
In other literature, two small retrospective studies and 5 case reports have investigated the feasibility of ovarian preservation in patients with PMP of appendiceal origin (14–20). One study in four women aged 28–35 years with PMP who sought to maintain fertility adopted a strategy that involved laparoscopy for disease staging followed by appendicectomy, irrigation of the abdominal and pelvic cavity with water, and stripping of macroscopic disease from the peritoneal surface of the pelvis and the surface of the ovaries. All patients had a low-grade appendiceal mucinous neoplasm with acellular mucin or LG-MCP in the peritoneal cavity. After the procedure, all patients conceived and gave birth to healthy babies. After 12–29 months of follow-up, all women were well with no evidence of disease recurrence on radiology or laparoscopy (21). Other women have conceived following treatment with CCRS + HIPEC. In one study, women aged <41 years with peritoneal carcinomatosis of various origins who expressed a strong desire for future pregnancy were treated with CCRS + HIPEC. At least one ovary was preserved in 21 women. Of these, 4 women developed ovarian recurrence after a median follow-up of 32 months, and two women became pregnant (14). An international survey reported seven pregnancies in women with PMP, epithelial mesothelioma, or papillary mesothelioma who underwent genital organs-preserving CRS and HIPEC, with delivery of seven newborns. Bilateral ovaries were preserved in 5 women, the left ovary was preserved in one woman, and ovocytes were harvested and cryopreserved in one woman. All women were disease free at 42–106 months of follow-up (15). In a case study, a 28-year-old patient with PMP underwent CRS + HIPEC. Bilateral ovaries were preserved, and the woman spontaneously conceived 14 months after surgery. The pregnancy was uneventful (16).
In our study, among all patients, median OS was 71 months (range, 54–88 months), and the 5-year survival rate was 37.9%. In a previous retrospective study of 2,289 patients from 16 specialized units who underwent CRS for PMP, 10- and 15-year survival rates were 63% and 59%, respectively, treatment-related mortality rate was 2%, and major operative complications occurred in 24% of patients (22). In another study of 42 patients who underwent CRS + HIPEC, 5-year survival rates after first and second CRS were 75.5% and 67.7%, respectively (23). In the present study, the incidence of serious complications and mortality rate was acceptable, but 5-year survival rate was comparatively low, potentially due to the distribution of pathological types. In the ovarian preservation group, 47.4% of patients had HG-MCP, which is associated with a poor prognosis. The ovary is a reproductive and endocrine organ that has a rich blood supply, which may promote tumor growth and metastasis. Consequently, we recommend ovarian resection during CRS in patients with HG-MCP. Meanwhile, the limitations section of our study provides context around our patient population, stating that the majority of patients were transferred to our institution from a local hospital in poor general condition, which increased their risk of mortality. Patients in this study were treated with CRS with or without HIPEC, and patient’s care goals, personal values, and wishes were incorporated into HIPEC decision-making. We believe that this treatment pathway is representative of the clinical situation in the real world.
In our study, among all patients, median DFS was 22 months (range, 12–32 months), and the 5- year recurrence rate was 87.4%, which are higher than reported elsewhere (24). Disparate findings between the present study and previous findings may be explained by differences in the patient populations. We included patients with LG-MCP or HG-MCP, while the previous report focused on patients with LG-MCP. Median DFS among our patients with LG-MCP was 32 months (range, 15–49 months), and the 5-year recurrence rate was 75.0%, which were comparable to the previous report. The present study was conducted at a referral center for myxoma, which may have led to selection bias favoring patients with more severe disease. Most notably, PMP is a rare disease; therefore, small sample size may have affected our findings.
In our study, there was no significant difference in OS in patients with LG-MCP or HG-MCP, whether ovariectomy was performed or not; however, in patients with LG-MCP, median DFS was significantly longer in patients with ovarian resection compared to ovarian preservation This suggests that ovarian preservation may increase risk for disease progression, but has little effect on the final prognosis of the patient. On multivariate analysis, high-grade histopathologic subtype of PMP was an independent predictor of poor prognosis (OS and DFS). This may be related to the growth pattern of the tumor cells and ovarian retention. Ovarian involvement is correlated with the peritoneal extent of PMP and tumor grade. Previous studies showed higher rates of ovarian invasion in patients with grade 2–3 PMP (25); specifically, 62% of ovaries were invaded in patients with grade-1 PMP, and 87.5% of ovaries were invaded in patients with grade 2–3 PMP (26). Other studies confirm these findings (27–30). Interestingly, in our study, neither PCI nor the use of HIPEC were independent predictors of prognosis. This may indicate that radical resection is more important than tumor burden and HIPEC in influencing prognosis.
This study was associated with several limitations. First, this was a retrospective study, and several clinical and histopathological data were lacking, such as the histology of the resected ovaries and intravenous chemotherapy regimen. The two groups were not homogenous to some extent. Second, most patients were transferred to our institution from a local hospital in poor general condition, which increased their risk of mortality. Last, the follow-up time was not long enough, especially for patients with preserved ovaries. Further large-scale studies are needed to confirm our results.
In conclusion, histopathologic subtype of PMP represents an independent predictor of prognosis in female patients with PMP of appendiceal origin and unilateral or bilateral ovaries preserved during CRS. These findings imply that ovarian preservation is a more suitable option for young females with low-grade PMP compared to high-grade PMP. However, the reported data were very limited and further prospective studies should be done investigating the role of resection of uninvolved ovaries in PMP.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
This study was approved by the Ethics committee of the Aerospace Center Hospital, Beijing, China (no. 20161109-ST-07). The patients provided written informed consent to participate in this study.
The author contributions were as follows: FF and RM conceived and designed the experiments. DL and RM provided study material or patients. HT and YL collected and assembling data FF and HT analyzed and interpreted the data. FF and HT contributed to the draft of the manuscript. FF, RM, HT, YL, and DL revised the manuscript critically for important intellectual content. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
This research is supported by the “2021 Hygiene and Health Development Scientific Research Fostering Plan of Haidian District Beijing” and the project number is HP2021-04-50704.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Patrick-Brown TDJH, Carr NJ, Swanson DM, Larsen S, Mohamed F, Flatmark K. Estimating the prevalence of pseudomyxoma peritonei in Europe using a novel statistical method. Ann Surg Oncol. (2020) 28:252–7. doi: 10.1245/s10434-020-08655-8
2. Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia. (2017) 33:511–9. doi: 10.1080/02656736.2017.1310938
3. Rymer B, Forsythe RO, Husada G. Mucocoele and mucinous tumours of the appendix: A review of the literature. Int J Surg. (2015) 18:132–5. doi: 10.1016/j.ijsu.2015.04.052
4. Sugarbaker PH. Peritonectomy procedures. Ann Surg. (1995) 221(1):29–42. doi: 10.1097/00000658-199501000-00004
5. Ahmadi N, Kostadinov D, Sakata S, Ball WR, Gandhi J, Carr NJ, et al. Managing recurrent pseudomyxoma peritonei in 430 patients after complete cytoreduction and HIPEC: a dilemma for patients and surgeons. Ann Surg Oncol. (2021) 28(12):7809–20.34041626
6. Bignell MB, Mehta AM, Alves S. Impact of ovarian metastases on survival in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancy originating from appendiceal and colorectal cancer. Assoc Coloproctol G B Irel. (2018) 20:704–10. doi: 10.1111/codi.14057
7. Mehta AM, Bignell MB, Alves S, Dayal SP, Mohamed F, Cecil TD, et al. Risk of ovarian involvement in advanced colorectal or appendiceal tumors involving the peritoneum. Dis Colon Rectum. (2018) 60:691–6. doi: 10.1097/DCR.0000000000000791
8. Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González-Moreno S, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. (2016) 40:14–26. doi: 10.1097/PAS.0000000000000535
9. Baratti D, Kusamura S, Milione M, Pietrantonio F, Caporale M, Guaglio M, et al. Pseudomyxoma peritonei of extra-appendiceal origin: a comparative study. Ann Surg Oncol. (2016) 23:4222–30. doi: 10.1245/s10434-016-5350-9
10. Narasimhan V, Wilson K, Britto M, Warrier S, Lynch AC, Michael M, et al. Outcomes following cytoreduction and HIPEC for pseudomyxoma peritonei: 10-year experience. J Gastrointest Surg. (2020) 24:899–906. doi: 10.1007/s11605-019-04239-4
11. Papageorgiou D, Manatakis DK, Papakonstantinou K, Kyriazanos ID. A comprehensive review of childbearing after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Arch Gynecol Obstet. (2020) 302:793–9. doi: 10.1007/s00404-020-05687-z
12. Tan GHC, Chia CS, Tan SH, Soo KC, Teo MCC. Early recurrence after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Int J Clin Oncol. (2018) 23:989–98. doi: 10.1007/s10147-018-1301-8
13. Mercier F, Dagbert F, Pocard M, Goéré D, Quenet F, Wernert R, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. (2019) 3:195–202. doi: 10.1002/bjs5.97
14. Elias D, Duchalais E, Dartigues P, Duvillard P, Poirot C, Goéré D. A new policy regarding ovarian resection in young women treated for peritoneal carcinomatosis. Ann Surg Oncol. (2013) 20:1837–42. doi: 10.1245/s10434-013-2879-8
15. Ortega-Deballon P, Glehen O, Levine E, Piso P, Sugarbaker PH, Hayes-Jordan A, et al. Childbearing after hyperthermic intraperitoneal chemotherapy: results from an international survey. Ann Surg Oncol. (2013) 18:2297–301. doi: 10.1245/s10434-011-1595-5
16. Kyser K, Bidus MA, Rodriguez M, Rose GS, Elkas JC. Spontaneous pregnancy following cytoreduction with peritonectomy and hyperthermic intraperitoneal chemotherapy. Gynecol Oncol. (2006):198–200. doi: 10.1016/j.ygyno.2005.08.045
17. Violette C, Kim T, Shandley L, Lee R, Staley C, Winer J, et al. Fertility after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a call to action. J Surg Oncol. (2021) 123:1045–9. doi: 10.1002/jso.26387
18. Campanati RG, Hanan B, de Souza SS, Gomes da Silva R. Pregnancy after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei. Case Rep Surg. (2014) 2014:694912. doi: 10.1155/2014/694912
19. Turkmen O, Karalok A, Basaran D, Kimyon GC, Tasci T, Ureyen I, et al. Fertility-sparing surgery should be the standard treatment in patients with malignant ovarian germ cell tumors. J Adolesc Young Adult Oncol. (2017) 6(2):270–76. doi: 10.1089/jayao.2016.0086
20. de Assis V, Saunders R. In vitro fertilization pregnancy following treatment of difuse malignant peritoneal mesothelioma. J Case Rep Images Obstet Gynecol. (2019) 5:1. doi: 10.5348/100043Z08VA2019CR
21. Sheehan LA, Mehta AM, Sawan S, Dayal SP, Mohamed F, Moran BJ, et al. Preserving fertility in pseudomyxoma peritonei, a novel approach. Pleura and Peritoneum. (2017) 2(1):33–6. doi: 10.1515/pp-2016-0024
22. Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early and long-term outcome data on 2298 patients with pseudomyxoma peritonei of appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. (2012) 30:2449–56. doi: 10.1200/JCO.2011.39.7166
23. Kitai T, Yamanaka K. Repeat cytoreduction and hyperthermic intraperitoneal chemotherapy for recurrent peritoneal carcino-matosis of appendiceal origin. Int J Clin Oncol. (2018) 23:298–304. doi: 10.1007/s10147-017-1217-8
24. Filippi F, Kusamura S, Martinelli F. Fertility preservation in women with peritoneal surface malignancies: a case series. Eur J Surg Oncol. (2021) 47(11):2948–51. doi: 10.1016/j.ejso.2021.03.259
25. Evers DJ, Verwaal VJ. Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg. (2011) 98:287–92. doi: 10.1002/bjs.7303
26. Elias D, Duchalais E, David A, Dartigues P, Duvillard P, Poirot C, et al. Comprehensive study of ovarian etastases in young women with peritoneal pseudomyxoma: is a preservation of fertility possible? Eur J Surg Oncol (EJSO). (2013) 39:748e753. doi: 10.1016/j.ejso.2013.03.005
27. Zhang W, Tan C, Xu M, Wu X. Primary appendiceal mucinous neoplasm: gynecological manifestations, management, and prognosis. Gynecologic Oncology. (2019) 11:030. doi: 10.1016/j.ygyno.2019.11.030
28. Huang Y, Alzahrani NA, Chua TC, Morris DL. Histological subtype remains a significant prognostic factor for survival outcomes in patients with appendiceal mucinous neoplasm with peritoneal dissemination. Dis Colon Rectum. (2013) 60:360–7. doi: 10.1097/DCR.0000000000000719
29. Carr NJ, Finch J, Ilesley IC, Chandrakumaran K, Mohamed F, Mirnezami A, et al. Pathology and prognosis in pseudomyxoma peritonei: a review of 274 cases. J Clin Pathol. (2012) 65:919–23. doi: 10.1136/jclinpath-2012-200843
Keywords: pseudomyxoma peritonei of appendiceal origin, ovarian involvement, CRS and HIPEC, prognostic prediction, female
Citation: Fu F, Tang H, Lu Y, Lu D and Ma R (2022) Prognosis for Young Females with Pseudomyxoma Peritonei of Appendiceal Origin and Unilateral or Bilateral Ovaries Preserved During Cytoreductive Surgery. Front. Surg. 9:881510. doi: 10.3389/fsurg.2022.881510
Received: 22 February 2022; Accepted: 5 May 2022;
Published: 6 June 2022.
Edited by:
Amine Souadka, National Institute of Oncology, MoroccoReviewed by:
Francesco Fleres, University of Messina, AOU G Martino, ItalyCopyright © 2022 Fu, Tang, Lu, Lu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruiqing Ma bWFydWlxaW5nMjAxNEAxMjYuY29t Dongmei Lu NDIxODA0MzIzQHFxLmNvbQ==
†These authors share first authorship
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.