
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 02 May 2022
Sec. Thoracic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.880007
This article is part of the Research Topic Non-Intubated Video-Assisted Thoracoscopic Surgery (NIVATS) View all 6 articles
 Pei-Hsing Chen1,2
Pei-Hsing Chen1,2 Jen-Hao Chuang3
Jen-Hao Chuang3 Tzu-Pin Lu4,5
Tzu-Pin Lu4,5 Wan-Ting Hung5,6,7
Wan-Ting Hung5,6,7 Hsien-Chi Liao6,8
Hsien-Chi Liao6,8 Tung-Ming Tsai3
Tung-Ming Tsai3 Mong-Wei Lin6
Mong-Wei Lin6 Ke-Cheng Chen6
Ke-Cheng Chen6 Hsao-Hsun Hsu6
Hsao-Hsun Hsu6 Jin-Shing Chen3,6*
Jin-Shing Chen3,6*
Introduction: In most developed countries, lung cancer is associated with the highest mortality rate among all cancers. The number of elderly patients with lung cancer is increasing, reflecting the global increase in aging population. Patients with impaired lung or cardiac function are at a high risk during intubated general anesthesia, which may preclude them from surgical lung cancer treatment. We evaluated the safety and survival of non-intubated video-assisted thoracoscopic surgery (VATS) versus those of intubated thoracoscopic surgery for surgical resection for lung cancer in older patients.
Methods: Patients aged ≥75 years who underwent non-intubated and intubated VATS resection with pathologically confirmed non-small cell lung cancer, using a combination of thoracic epidural anesthesia or intercostal nerve block and intra-thoracic vagal block with target-controlled sedation, from January 2011 to December 2019 were included. Ultimately, 79 non-intubated patients were matched to 158 patients based on age, sex, body mass index, family history, comorbidity index, pulmonary function (forced expiratory volume in one second/ forced vital capacity [%]), and disease stage. The endpoints were overall survival and recurrence progression survival.
Results: All patients had malignant lung lesions. Data regarding conversion data and the postoperative result were collected. Both groups had comparable preoperative demographic and cancer staging profiles. The anesthetic duration in the non-intubated group was shorter than that in the intubated group, which showed a significantly higher mean number of lymph nodes harvested (intubated vs non-intubated, 8.3 vs. 6.4) and lymph stations dissected (3.0 vs. 2.6). Intensive care unit (ICU) admission rate and postoperative ICU stay were significantly longer in the intubated group. The complication rate was higher and hospital stay were longer in the intubated group, but these differences were not significant (12% vs. 7.6%; p = .07, respectively).
Conclusions: In the elderly, non-intubated thoracoscopic surgery provides similar survival results as the intubated approach, although fewer lymph nodes are harvested. Non-intubated surgery may serve as an alternative to intubated general anesthesia in managing lung cancer in carefully selected elderly patients with a high risk of impaired pulmonary and cardiac function.
Due to prolonged human life expectancy and a steady increase in the aged population, the incidence of lung cancer has progressively increased in elderly patients (1, 2). Surgery is still considered the main curative treatment for lung cancer, even in the elderly (3). Older patients are more obviously affected by general anesthesia, resulting in alveolar barotrauma, volutrauma, atelectrauma, and hemodynamic instability in the perioperative period. Elderly patients generally have a poor cardiopulmonary function and a high possibility of decreased forced expiratory volume in one second (FEV1) and FEV1/forced vital capacity (FVC) ratio. Some elderly patients may be treated conservatively because of impaired pulmonary function and a relatively high incidence of cardiopulmonary complications.
Fortunately, rapid improvements in video-assisted thoracic surgery (VATS), including single-port thoracoscopic surgery (4, 5), preoperative localization (6), and the non-intubated method, have extended the surgical indication to include elderly patients. Non-intubated thoracoscopic surgery helps prevent ventilator-induced damage and consequently ventilation-perfusion (V/Q) mismatch and preserves functional residual capacity (FRC), which may decrease due to neuromuscular blockade and lack of spontaneous diaphragmatic contraction (7, 8).
In a previous study, we have proven the feasibility and safety of non-intubated thoracoscopic procedures in geriatric patients (9). Although perioperative outcomes are good, some studies have reported that non-intubated VATS might have the disadvantage of fewer resected lymph nodes compared with the intubated procedure (10). The influence of the operative method in oncological outcomes, especially in the elderly, however, remain unclear. The aim of this propensity-matched study was to discuss the oncological outcomes of non-intubated versus intubated thoracoscopic surgery for lung cancer in elderly patients (age ≥75 years).
This retrospective case-control study included 4420 patients who underwent pulmonary resection for non-small cell lung cancer (NSCLC) by a single surgical team at our institute between January 2011 and December 2019. Excluded from the study were patients <75 years of age, those with metastatic cancer (stage IV), those who did not intend to undergo curative treatment, and those with incomplete demographic data. Patients suitable for non-intubated VATS need to have a body mass index (BMI) of <25 kg/m2 and an American Society of Anesthesiologists Physical Status Classification score of <4. Patients who had prior thoracic surgery or those with difficult airway management were not included in the non-intubated approach.
The patients were compared based on the endpoints of overall survival and recurrence progression survival. Treatment response was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1) and based on systemic imaging, including positron emission tomography/computed tomography (CT), whole-body bone scan, brain/chest/abdominal contrast-enhanced CT, and brain magnetic resonance imaging.
This study was approved by the National Taiwan University Hospital Research Ethics Committee (approval number: 202110074RINA). The requirement for written informed consent was waived because of the retrospective nature of the study.
A total of 79 non-intubated and 359 intubated patients with stage I to stage III lung cancer according to the International Union Against Cancer/American Joint Committee on Cancer (8th edition) criteria were selected. To reduce selection bias, potential statistically significant (p < 0.3) predictors that were closely related to the non-intubated procedure were included. We matched patients with respect to age, sex, BMI, family history, comorbidity index, pulmonary function (FEV1/FVC[%]), and disease stage using the propensity score matching method (caliper width 0.1 of the standard deviation of the propensity score). The matching used a 1:2 ratio greedy (nearest-neighbor) approach with a maximum propensity score difference of ±1%. We calculated the standardized mean difference for each matched variable to check the success of propensity score matching. Ultimately, 79 non-intubated patients were matched to 158 patients. In total, 201 patients were excluded in the control group (Figure 1).

Figure 1. Algorithm for patient selection. Abbreviations: BMI, body mass index; FEV1/FVC, forced expiratory volume in 1 s/forced vital capacity ratio.
Categorical variable differences between the non-intubated and intubated groups were assessed using the chi-square or Fisher’s exact test. Survival was analyzed using the log-rank test and the Kaplan–Meier method. Complications were documented for each patient according to the Common Terminology Criteria for Adverse Events version 5.0. All comparisons were two-tailed, and a p-value of <.05 was considered significantly different. Cox proportional hazards regression analysis was used to calculate hazard ratios (HRs) with 95% confidence intervals.
Statistical analyses were performed using the SPSS software (version 22.0; IBM Corp., Armonk, NY, USA), R (version 3.4.3; R Foundation for Statistical Computing, Vienna, Austria), and R Studio (version 1.1.414; RStudio, PBC, Boston, MA, USA).
The anesthesia techniques used herein have been described previously (9, 11, 12). Sedation was performed using intravenous administration of propofol (10 mg/mL) followed by intravenous fentanyl (0.5–2 µg/kg) or rocuronium (0.6 mg/kg). Standard monitors included electrocardiograms, along with those that displayed pulse oximetry data, arterial blood pressure, body temperature, and urine output. The end-tidal carbon dioxide and respiratory rate were evaluated using a detector that was placed into one nostril or in front of the mouth. The sedation level was monitored using the frontal bispectral index (BIS Quatro; Aspect Medical System, Norwood, MA, USA) or the Ramsay sedation score. Sedation was maintained at a Ramsay sedation score of II or a bispectral index value of 40–60. A ventilation mask or high-flow nasal cannula was applied to maintain the patient’s oxygen saturation at <90% during the intraoperative period.
Regional anesthesia was administered using the thoracic epidural or intercostal blocks. The T5/T6 thoracic interspace was the target area for epidural catheter insertion to achieve a sensory block with target dermatomes from T2 to T9 with infusion of 2% lidocaine.
The monitor system was similar as that for the non-intubated patients (9, 11). Anesthesia was induced by the intravenous administration of the propofol, thiopental (3–5 mg/kg), rocuronium (0.6 mg/kg), or fentanyl. Sevoflurane and rocuronium were used for anesthesia maintenance. A standard left-sided double-lumen endotracheal tube was inserted for ventilation. Regional anesthesia was also administered in intubated patients. Patients were extubated immediately after surgery or in the intensive care unit.
The data of the 79 patients who underwent non-intubated surgery were compared to those of the 158 matched patients who underwent intubated surgery. Table 1 shows the demographic data before and after matching. The baseline characteristics were well balanced between the groups. Potential group differences were observed in age, BMI, family history, Charlson comorbidity index, pulmonary function (FVC), and stage before matching (Table 1). No differences were observed in the demographic data of the two groups after matching. The mean age of all the matched 237 patients was 79.3 years (79.3 ± 3.6 and 79.4 ± 3.7 for the intubated group and non-intubated group, respectively). A majority of the patients had an Eastern Cooperative Oncology Group performance status of 0 (intubated group, 76.3%, non-intubated group, 84.2%; female: intubated group, 54.4%, non-intubated group, 58.2%) and had early-stage disease (intubated group, 86.7%, non-intubated group, 87.3%). The mean comorbidity index was 5.9 for both groups.
Perioperative outcomes after matching are summarized in Table 2. All surgeries were performed via video-assisted thoracoscopy. The operation time was longer in the intubated group, but the difference was not significant. The percentage of lobectomies was higher in the intubated group than in the non-intubated group. No conversion in the operative method or from non-intubation to intubation was observed. No 30-day mortality was reported. The intubated group showed a significantly higher mean number of lymph nodes harvested (8.3 vs. 6.4) and lymph stations dissected (3.0 vs. 2.6). Intensive care unit (ICU) admission rate and postoperative ICU stay were significantly longer in the intubated group. The complication rate was higher in the intubated group, but the difference was not significant (12% vs. 7.6%). The hospital stays tended to be longer in the intubated group but not significant (p = .07).
The pathology results for both groups are presented in Table 3. The groups were similar in terms of histologic cancer cell type, pathological tumor stage, pathological nodal stage, and resection margin involvement. The pathological features, including differentiation, lymphovascular invasion, and visceral pleural invasion, also showed no significant differences.
There were 15 and 7 mortality events and 40 and 16 recurrences in the intubated and non-intubated groups, respectively. The median follow-up time for all matched patients was 45.5 months. The survival curves are presented in Figures 2 and 3. The overall survival and recurrence-free survival (RFS) rates were similar in both groups (p = .99 and p = .56, respectively).
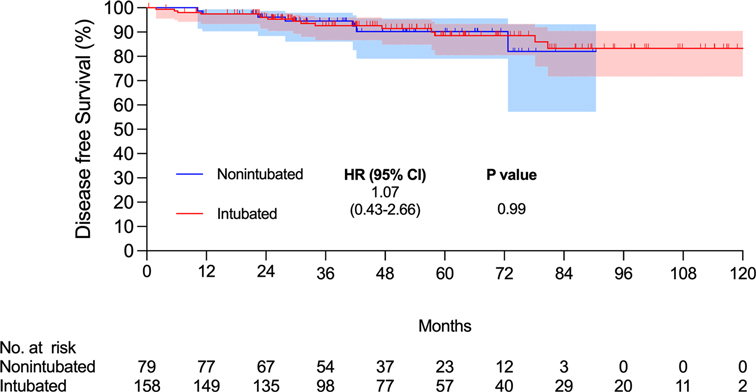
Figure 2. Kaplan–Meier overall survival curve. Abbreviations: CI, confidence interval; HR, hazard ratio.
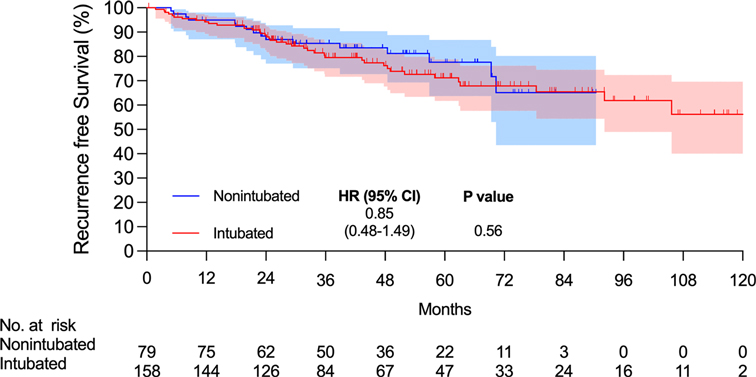
Figure 3. Kaplan–Meier recurrence-free survival curve. Abbreviations: CI, confidence interval; HR, hazard ratio.
This propensity-matched case study confirmed that non-intubated thoracoscopic surgery is associated with similar OS and RFS rates in elderly patients aged ≥75 years with pathologically proven NSCLC. Compared to the intubated group, the non-intubated group showed satisfactory perioperative results, as in previous studies (9, 11, 13–15). Although there were fewer lymph nodes harvested in the non-intubated group, it did not influence the long-term results compared to the intubated group. To the best of our knowledge, this is the first report revealing oncologic outcomes in elderly patients with lung cancer undergoing non-intubated thoracoscopic pulmonary resection.
Owing to prolonged life expectancy, lung cancer has become a leading cause of death in the elderly worldwide. Pulmonary resection for lung cancer in elderly patients is still considered an option for first-line treatment. Several studies have demonstrated the feasibility and safety of pulmonary resection in the elderly (16–21). However, this requires careful patient selection and skilled surgery. Fortunately, rapid improvements in thoracoscopy, including single-port surgery (22), preoperative and intraoperative localization techniques (6, 23, 24). wedge resection for subcentimeter lung cancer, and non-intubated thoracoscopic surgery have facilitated pulmonary resection in the elderly. Non-intubated thoracoscopic surgery could be considered a part of enhanced recovery after surgery protocols. It has the benefits of minimizing airway injury, residual neuromuscular blockade, and ventilator-induced lung complications. Elderly individuals are considered to have poor FEV1 and FEV1/FVC ratios compared with the young population. Non-intubated thoracoscopic surgery can help prevent V/Q mismatch and maintain better FRC; it has also extended the criteria for surgery, as opposed to conservative treatment. Advantages such as the faster resumption of postoperative feeding, shorter duration of antibiotic use, shorter hospital stay, and fewer inflammatory responses have been reported for non-intubated thoracoscopic surgery. This may lead to a better recovery course in elderly patients (25, 26).
Our previous report demonstrated the feasibility and safety of non-intubated thoracoscopic surgery in a geriatric population, even when performing lobectomy (9). Although non-intubated thoracoscopic surgery has potential advantages of lower perioperative complications and faster recovery, debates regarding its oncological outcomes and true benefit remain (27). Alghamdi et al. compared 62 patients who underwent VATS lobectomy using the non-intubated and intubated approaches. In that study, although the operative time was shorter in the non-intubated group, the number of harvested lymph nodes in the non-intubated group were significantly fewer than those in the intubated group (13 vs. 18, p = .003) (10). In our previous analysis comparing non-intubated uniportal segmentectomy with the intubated approach, the non-intubated group had fewer lymph nodes harvested. This result may be due to the activated cough reflex during non-intubated surgery, which makes it difficult to perform lymph node dissection, especially in male patients with higher BMI (12).
To date, few studies have focused on the survival of patients undergoing non-intubated thoracoscopic surgeries. Wang et al. conducted an analysis of patients with early-stage disease who underwent lobectomy (11). The results showed similar OS and RFS rates for non-intubated and intubated lobectomy. However, to our knowledge, no report has focused on non-intubated thoracoscopic surgery in the geriatric population. Our study also showed no obvious survival benefit in the elderly group; although the number of harvested lymph nodes was fewer in the non-intubated group, the OS and RFS were similar after matching. In addition, perioperative outcomes, such as hospital stay, ICU admission rate, and ICU stay were better in the non-intubated group. The postoperative complication rate was lower for all complications and for complications with a grade >2. Similar survival results in the elderly may support the application of non-intubated thoracoscopic surgery in this subgroup of patients.
To the best of our knowledge, our study is the first to evaluate and compare, using the propensity score matching method, the long-term survival of elderly patients with resectable lung cancer undergoing non-intubated and intubated thoracoscopic surgery. Factors related to the choice of surgical procedure, lymph node dissection, and possible influence on survival, such as age, sex, BMI, family history, pulmonary function, and disease stage, were used to balance the groups. In addition, the comorbidity index was used to ensure that the baseline values in the two groups were equal.
The limitations of this study include its retrospective nature and the fact that the data were collected by a single surgical team. The primary problem in the comparison was selection bias, which we tried to minimize by using propensity score matching to eliminate confounding factors. Moreover, adenocarcinoma was the dominant cell type in the pathology. The recent rapid progression of systemic treatment using epidermal growth factor receptors and anaplastic lymphoma kinase may significantly prolong survival. Our results should be interpreted carefully in populations with other predominant cell types. Further studies should be conducted using a prospective design to reinforce this conclusion.
Despite these limitations, this study showed that non-intubated thoracoscopic surgery provides similar survival results in the elderly as the intubated approach, although fewer lymph nodes were harvested. With preferable perioperative outcomes, non-intubated thoracoscopic surgery may be beneficial in carefully selected elderly patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Conception and Methodology : P-HC, T-PL, J-SC; Project administration: J-SC; Data curation: K-CC, H-CL, W-TH, T-MT, M-WL, H-HH; Software: P-HC, T-PL; Formal analysis and/or interpretation of data: P-HC, T-PL; Writing - original draft: P-HC; Writing - review & editing: P-HC, J-SC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thanks to the support of National Taiwan University Hospital Surgery Department for their assistance with statistical analyses.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. (2007) 25:5570–7. doi: 10.1200/JCO.2007.12.5435
2. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. (2009) 27:2758–65. doi: 10.1200/JCO.2008.20.8983
3. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv1–iv21. doi: 10.1093/annonc/mdx222
4. Gonzalez-Rivas D, Fernandez R, Mdl T, Rodriguez JL, Fontan L, Molina F. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiov Thorac Surg. (2014) 19:552–5. doi: 10.1093/icvts/ivu209
5. Galvez C, Navarro-Martinez J, Bolufer S, Lirio F, Mafe JJ, Rivera MJ, et al. Benefits of awake uniportal pulmonary resection in a patient with a previous contralateral lobectomy. Ann Transl Med. (2014) 2:93. doi: 10.3978/j.issn.2305-5839.2014.05.07
6. Chen PH, Hsu HH, Yang SM, Tsai TM, Tsou KC, Liao HC, et al. Preoperative dye localization for thoracoscopic lung surgery: hybrid versus computed tomography room. Ann Thorac Surg. (2018) 106:1661–7. doi: 10.1016/j.athoracsur.2018.07.030
7. Liu YJ, Hung MH, Hsu HH, Chen JS, Cheng YJ. Effects on respiration of nonintubated anesthesia in thoracoscopic surgery under spontaneous ventilation. Ann Transl Med. (2015) 3:107. doi: 10.3978/j.issn.2305-5839.2015.04.15
8. Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med. (2005) 31:1327–35. doi: 10.1007/s00134-005-2761-7
9. Wu CY, Chen JS, Lin YS, Tsai TM, Hung MH, Chan KC, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg. (2013) 95:405–11. doi: 10.1016/j.athoracsur.2012.10.082
10. Alghamdi ZM, Lynhiavu L, Moon YK, Moon MH, Ahn S, Kim Y, et al. Comparison of non-intubated versus intubated video-assisted thoracoscopic lobectomy for lung cancer. J Thorac Dis. (2018) 10:4236–43. doi: 10.21037/jtd.2018.06.163
11. Wang ML, How CH, Hung MH, Huang HH, Hsu HH, Cheng YJ, et al. Long-term outcomes after nonintubated versus intubated thoracoscopic lobectomy for clinical stage I non-small cell lung cancer: a propensity-matched analysis. J Formos Med Assoc. (2021) 120:1949–56. doi: 10.1016/j.jfma.2021.04.018
12. Hung WT, Hung MH, Wang ML, Cheng YJ, Hsu HH, Chen JS. Nonintubated thoracoscopic surgery for lung tumor: seven years’ experience with 1025 cases. Ann Thorac Surg. (2019) 107:1607–12. doi: 10.1016/j.athoracsur.2019.01.013
13. Liu HY, Hsu HH, Tsai TM, Chiang XH, Lu TP, Chang CH, et al. Nonintubated versus intubated uniportal thoracoscopic segmentectomy for lung tumors. Ann Thorac Surg. (2021) 111:1182–1189. doi: 10.1016/j.athoracsur.2020.06.058
14. Hung WT, Hsu HH, Hung MH, Cheng YJ, Huang PM, Chen JS. Comparison of perioperative outcomes between intubated and nonintubated thoracoscopic surgery in children. J Formos Med Assoc. (2021. doi: 10.1016/j.jfma.2021.03.029
15. Liu HY, Chiang XH, Hung MH, Wang ML, Lin MW, Cheng YJ, et al. Nonintubated uniportal thoracoscopic segmentectomy for lung cancer. J Formos Med Assoc. (2020) 119:1396–1404. doi: 10.1016/j.jfma.2020.03.021
16. Zaatar M, Stork T, Valdivia D, Mardanzai K, Stefani D, Collaud S, et al. Minimal-invasive approach reduces cardiopulmonary complications in elderly after lung cancer surgery. J Thorac Dis. (2020) 12:2372–9. doi: 10.21037/jtd.2020.03.73
17. de Ruiter JC, Heineman DJ, Daniels JM, van Diessen JN, Damhuis RA, Hartemink KJ. The role of surgery for stage I non-small cell lung cancer in octogenarians in the era of stereotactic body radiotherapy in the Netherlands. Lung Cancer. (2020) 144:64–70. doi: 10.1016/j.lungcan.2020.04.005
18. Okami J. Treatment strategy and decision-making for elderly surgical candidates with early lung cancer. J Thorac Dis. (2019) 11:S987–97. doi: 10.21037/jtd.2019.04.01
19. Driessen EJM, Schulkes KJG, Dingemans AC, van Loon JGM, Hamaker ME, Aarts MJ, et al. Patterns of treatment and survival among older patients with stage III non-small cell lung cancer. Lung Cancer. (2018) 116:55–61. doi: 10.1016/j.lungcan.2017.12.013
20. Detillon DDEMA, Veen EJ. Postoperative outcome after pulmonary surgery for non-small cell lung cancer in elderly patients. Ann Thorac Surg. (2018) 105:287–93. doi: 10.1016/j.athoracsur.2017.07.032
21. Shirvani SM, Jiang J, Chang JY, Welsh J, Likhacheva A, Buchholz TA, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non–small cell lung cancers in the elderly. JAMA Surg. (2014) 149:1244–53. doi: 10.1001/jamasurg.2014.556
22. Gonzalez-Rivas D, Fieira E, Mendez L, Garcia J. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg. (2012) 42:e169–71. doi: 10.1093/ejcts/ezs482
23. Tsai TM, Chiang XH, Liao HC, Tsou KC, Lin MW, Chen KC, et al. Computed tomography-guided dye localization for deeply situated pulmonary nodules in thoracoscopic surgery. Ann Transl Med. (2019) 7:31. doi: 10.21037/atm.2019.01.29
24. Lin MW, Tseng YH, Lee YF, Hsieh MS, Ko WC, Chen JY, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg. (2016) 152:535–44.e2. doi: 10.1016/j.jtcvs.2016.04.052
25. Liu J, Cui F, Li S, Chen H, Shao W, Liang L, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov. (2015) 22:123–30. doi: 10.1177/1553350614531662
26. Vanni G, Tacconi F, Sellitri F, Ambrogi V, Mineo TC, Pompeo E. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg. (2010) 90:973–8. doi: 10.1016/j.athoracsur.2010.04.070
Keywords: lung cancer surgery, thoracoscopy/VATS, thoracoscopic surgery, nonintubated surgery, uniportal thoracoscopic surgery, elderly
Citation: Chen PH, Chuang JH, Lu TP, Hung WT, Liao HC, Tsai TM, Lin MW, Chen KC, Hsu HH and Chen JS (2022) Non-Intubated Versus Intubated Video-Assisted Thoracic Surgery in Patients Aged 75 Years and Older: A Propensity Matching Study. Front. Surg. 9:880007. doi: 10.3389/fsurg.2022.880007
Received: 20 February 2022; Accepted: 11 April 2022;
Published: 2 May 2022.
Edited by:
Long Jiang, First Affiliated Hospital of Guangzhou Medical University, ChinaReviewed by:
Patrick Zardo, Hannover Medical School, GermanyCopyright © 2022 Chen, Chuang, Lu, Hung, Liao, Tsai, Lin, Chen, Hsu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Shing Chen Y2hlbmpzQG50dS5lZHUudHc=
Speciality section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.